A MUTEIN OF HUMAN BASIC FIBROBLAST GROWTH FACTOR TGP-580
ACCELERATES COLONIC ULCER HEALING
BY STIMULATING
ANGIOGENESIS IN THE ULCER BED IN RATS
2Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
INTRODUCTION
Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease, are characterized by mucosal inflammation, erosions and ulcers, resulting in pain and hemorrhagic diarrhea. The number of patients with IBD is increasing every year in European and Asian countries, as well as in North America, and it has now become almost a global disease (1). Inflammation is a very important characteristic feature of IBD; therefore, most emphasis has been placed on ways of inhibiting it. This approach led to the development of therapeutics, and many drugs such as glucocorticoids, anti-inflammatory drugs (sulfasalazine, 5-aminosalicylic acid), immunosuppressants (cyclosporine and azathiopurine) and anti-tumor necrotizing factor α agents (infliximab and adalimumab) have been developed and used for the treatment of IBD (2). However, these drugs are not always satisfactory in the therapy of the disease because recurrence or relapse is often observed after the treatment period, although they inhibit inflammation during the treatment. In addition, some of them cause adverse events when used for a long period (2, 3). Another important approach to the treatment of IBD is to perform active healing of the damaged mucosa. However, at present, there are no drugs available for the treatment of damaged mucosa.
Natural, physiologic products of the body are often used as effective treatment modalities, but synthetic derivatives with a slightly modified form are often more effective than the natural products, for example, the natural glucorticoid cortisol versus the much more potent prednisolone or dexamethasone. Here, we present a similar, new example with a peptide growth factor. Namely, the administration of naturally occurring bFGF was effective to accelerate healing of experimental ulcers in the stomach (4), duodenum (5) and colon (6), but this peptide is acid- and pepsin-sensitive, so it rapidly degrades after oral administration. It was found that modifying only two amino acids (i.e., replacing cysteines with serines) created a stable analog of human bFGF, namely, TGP-580 (7). We reported that TGP-580 accelerated the healing of gastric and duodenal ulcers in rats (5, 8, 9). Furthermore, Hull et al. (10) reported that oral TGP-580 (0.1 mg, twice daily for 4 weeks) was very effective for the treatment of intractable gastric ulcers associated with the use of nonsteroidal anti-inflammatory drugs (NSAID) in 9 patients in an open pilot study. However, there are no reports on the effect of TGP-580 on the healing of colonic ulcers. In addition, few reported studies observed the effect of glucocorticoids on colonic ulcer healing, in spite of them being commonly used for the treatment of IBD.
Many animal models have been developed and used to study the etiology and pathogenesis of IBD (11), and we also reported that the administration of sulfhydryl alkylators, N-ethylmaleimide (NEM) and iodoacetamide, into the colon caused UC in rats similar to that in humans, causing symptoms such as mucosal damage with inflammation, diarrhea with bleeding and a decrease in body weight (12). The pathogenesis of this model of ulcerative colitis is relatively well characterized; for example, following the depletion of protective endogenous glutathione and alkylation of protein-SH groups, cell necrosis develops, resulting in colonic erosions and ulcers, followed by acute (3 – 7 days) and chronic (7 – 14 days) inflammation. During chronic inflammation, angiogenesis-dependent granulation tissue replaces the ulcerated mucosa, over which surviving epithelial cells migrate and complete the spontaneous healing (13, 14). Therefore, in the present study, we examined the effects of TGP-580 and dexamethasone, a representative glucocorticoid, on the healing of colonic ulcers induced by NEM in rats, and the results were compared with those of recombinant human bFGF (rhbFGF).
MATERIAL AND METHODS
Animals
Seven-week-old male Sprague-Dawley rats (CLEA Japan, Osaka, Japan) weighing 200 – 250 g were used. The animals were given rat chow pellets (CE-2; CLEA Japan, Osaka, Japan) and had free access to water during the experimental period, unless otherwise specified.
Experimental protocols were approved by the Animal Research Committee of the Faculty of Agriculture, Tottori University, Tottori, Japan.
Drugs
The following drugs were used: N-ethylmaleimide (NEM; Sigma, St. Louis, MO, USA), dexamethasone (Takeda, Osaka, Japan), methylcellulose and diethylether (Wako, Osaka, Japan). Both recombinant human bFGF (rhbFGF) and TGP-580 (rhbFGF mutein CS23 in which Cys 69 and Cys 87 are both replaced by serine residues) were produced using gene technology and purified in the Pharmaceutical Research Division of Takeda Pharmaceutical Co. Ltd. (Osaka, Japan) (7, 15). These peptides, monoclonal antibody 3H3 for hbFGF (MAb 3H3) and polyclonal anti-hbFGF antiserum were kindly provided by Takeda Pharmaceutical Co. Ltd. All of rhbFGF, TGP-580, MAb 3H3 and anti-rhbFGF antiserum were stored at –80°C until use. MAb 3H3 neutralizes bFGF activity; namely, 50 ng of MAb 3H3 antagonizes the proliferating activity of 1 ng of bFGF on endothelial cells of human umbilical vein origin (16). For intravenous injection, MAb 3H3 was diluted with saline, and was administered intravenously at a volume of 1 ml/kg b.w. via a tail vein. For topical administration, all of TGP-580, rhbFGF and dexamethasone were dissolved in 50 mM citrate buffer (pH7.0), and were administered intracolonally (i.c.) 7 cm from the anus via a Nelaton’s catheter at a volume of 200 µl/rat.
Induction and measurement of colonic ulcers
On the basis of our previous data on NEM-induced ulcerative colitis (12), the following conditions to produce colonic ulcers were used: under ether anesthesia 50 µl of 1% methylcellulose containing 3% NEM was introduced into the colon 6 cm from the anus via a Nelaton’s catheter in rats fasted for 24 hours. The animals were sacrificed various days (1 to 21 days) after this administration by CO2 asphyxiation. Then, the whole colon was removed with the rectum and cut longitudinally. The contents were removed by washing the tissue in saline, and the colon was spread on hard paper. The area (mm2) and severity (grade: 0, normal; 1, mucosal erosions; 2, moderate lesion; 3, deep lesion) of the mucosal damage were measured under a dissecting binocular microscope with a grid eyepiece (×10). The person measuring the lesions did not know the treatments given to the animals.
For histological observation, the preparation of colon was fixed in 10% formalin solution and embedded in paraffin. Serial sections (4 µm) of each tissue were made and stained with Azan according to conventional methods.
Measurement of bFGF concentration and content in the colonic wall
The concentration of bFGF was measured according to the method described in our previous paper (9). The animals were sacrificed by CO2 asphyxiation and the colon was removed. The colon was opened longitudinally, washed in ice-cold saline and spread flat on a piece of paper. After excess moisture was absorbed with paper, samples of the center of the ulcerated area of the colon (3 cm in length) were obtained and weighed. The tissue was minced in a test tube containing modified 50 mM Tris-HCl buffer (pH 7.6) in a volume 19 times the wet weight of the tissue. The Tris-HCl buffer contained 1.6 M NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA; Sigma, St. Louis, MO, USA), 1 mM phenylmethylsulfonyl fluoride (PMSF; Wako, Osaka, Japan), 1 mM (p-amidinophenyl)-methanesulfonyl fluoride HCl (p-APMSF; Wako) and 0.05 mM NEM. The tissue was then homogenized with a Polytron® (Kinematica, Lucerne, Switzerland) on ice. The homogenate was centrifuged for 2 min at 12,500 × g, and the supernatant was stored at –80°C until it was assayed.
bFGF concentration in the supernatant was measured by a sandwich enzyme immunoassay according to the method of Watanabe et al. (17) using 3 monoclonal antibodies (MAb 52, MAb 98 and MAb 3H3; Takeda Pharmaceutical Co. Ltd.) for human bFGF. Rat bFGF (18) was used as the standard. This method allows a bFGF concentration of more than 100 pg/ml to be measured. Thereafter, bFGF content in the ulcerated colon was calculated by multiplying the concentration of bFGF (µg/g w.w.) and the weight of the colon (g/3 cm).
Distribution of endogenous bFGF in the colon
The distribution of bFGF was determined immunohistochemically according to the method described in our previous paper (9). Either the colon from normal rats or the ulcerated colon 7 days after ulcer induction by an enema of 50 ml of 3% NEM was obtained and frozen with dry-ice/acetone after being embedded in OTC Compound® (LAB-TEK Products, Elkhart, IN, USA). Several consecutive thin sections of 12 µm from almost the center of the ulcer were prepared using a cryostat microtome and mounted on gelatin-coated glass slides. The sections were fixed in ice-cold acetone for 10 min and treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature to inactivate endogenous peroxidase. They were then immersed in 10% non-immune goat serum for 30 min, washed in 0.01 M phosphate-buffered saline (pH 7.2) and stained with polyclonal rabbit anti-rhbFGF antiserum for 2 hours at room temperature. Primary antibody preabsorbed with excess rhbFGF (50 µg/ml) for 3 hours at room temperature or phosphate-buffered saline was substituted for the primary antibody to carry out control staining. After washing in phosphate-buffered saline, bound antibody was detected using a Vectastain ABC Kit (Vector Labs, Burlingame, CA, USA). The sections were incubated for 30 min at room temperature with biotinylated goat anti-rabbit IgG and then with ABC reagent (avidin-biotin complex coupled to peroxidase) for 60 min at room temperature. To visualize the antigen-antibody complexes, the sections were then incubated for 6 min at room temperature in 3-amino-9-ethyl-carbazole (Biomeda Corp., Foster, CA, USA). The antiserum used in this experiment has cross-reactivity with rat bFGF, but not with bovine acidic FGF, mouse FGF, mouse EGF (epidermal growth factor), mouse NGF (nerve growth factor), porcine PDGF (platelet-derived growth factor), bovine insulin, human TGF (transforming growth factor)-β or human IL (interleukin)-2 (unpublished information; Takeda Pharmaceutical Co. Ltd.). In addition, when the serum reacted with a sufficient amount of rhbFGF, the section was not stained immunohistochemically.
Histological measurement of angiogenesis in the ulcer bed
Angiogenesis in the ulcer bed was measured immunohistochemically according to the method described in our previous paper (9). The colon from normal rats or the ulcerated colon 11 days after ulcer induction by an NEM enema was obtained and fixed in ice-cold acetone. After dehydration using a graded alcohol series, tissues were embedded in paraffin (Tissue Prep®; Fisher Labs, Inc., Fair Lawn, NJ, USA). Several consecutive thin sections of 4 µm from almost the center of the ulcer were prepared. The section showing the maximal length of the ulcer was chosen, and the endothelial cells of the microvessels were stained immunohistochemically using rabbit polyclonal antibodies for human factor VIII (Dako Japan Co., Kyoto, Japan). As microvessels in the ulcer bed originated from the ulcer margin and extended to the surface of the ulcer bed, photographs of two areas of the middle of both ulcer edges were taken for each animal. The length of all microvessels positively stained for factor VIII in the two areas (total 2.3 mm2) was measured in each rat under blind conditions, and mean length (mm/0.01 mm2) was used as an index of the density of microvessels.
Effect of drugs on ulcer healing
Colonic ulcers were produced by an enema of 50 µl of 3% NEM into the colon 6 cm from the anus. Two hundred microliters of TGP-580 (0.2 – 20 µg/ml), rhbFGF (0.2 – 20 µg/ml), dexamethasone (20 µg/ml) or vehicle (50 mM citrate buffer) was administered intracolonally twice daily (9:00 AM and 5 PM) for 3, 6 and 10 days starting the day after ulcer induction. The animals were sacrificed 16 hours after the final doses, and the lesions were observed macroscopically and histologically.
In this study, changes of both body weight (body weight gain) and diarrhea were also examined at 4, 7 and 11 days after ulcer induction. The diarrhea was graded according to the following criteria: 0, normal; 1, slight (wet around the anus); 2, moderate; and 3, marked (lower part of the abdomen is soiled with soft feces or rectal bleeding).
Effects of MAb 3H3 and TGP-580 on ulcer healing
Colonic ulcers were produced by an enema of 50 ml of 3% NEM into the colon 6 cm from the anus. MAb 3H3 (0.5 mg/kg), TGP-580 (0.1 mg/kg) or vehicle (saline, 1 ml/kg b.w.) was administered intravenously via a tail vein once daily for 10 days starting the day after colitis induction. The animals were sacrificed 24 hours after the final dose, and the lesions were observed macroscopically.
Statistical analysis
All data are expressed as mean ± S.E.M. Differences between groups were analyzed by Student’s t-test for paired group comparisons, or analysis of variance (Dunnett’s multiple range test) if more than two variables were considered. The significance level was set at P < 0.05.
RESULTS
Time-dependent changes in N-ethylmaleimide-induced colonic ulcers
The administration of NEM into the colon caused severe damage in the colonic mucosa (Fig. 1A). On day 3, there was an obviously distinguishable border between normal and damaged mucosa, and the mucosa surrounding the lesions was elevated. On day 7, the colon seemed to be shortened longitudinally, distended transversely and looked like a barrel. The ulcerated mucosa was sometimes covered with a gray or white-yellow, mud-like substance, probably a product of the inflammation. This mud-like substance began to detach on day 7 and had almost disappeared by day 14 (Fig. 1A). The ulcerated area decreased with time, but was still observable on day 21. Time-dependent changes in the area and severity of the mucosal lesions are shown in Fig. 1B. The area was almost the same until 3 days after the administration of NEM; it then gradually decreased until 14 days. The severity was almost the same until day 7; it then started to decrease rapidly (Fig. 1B).
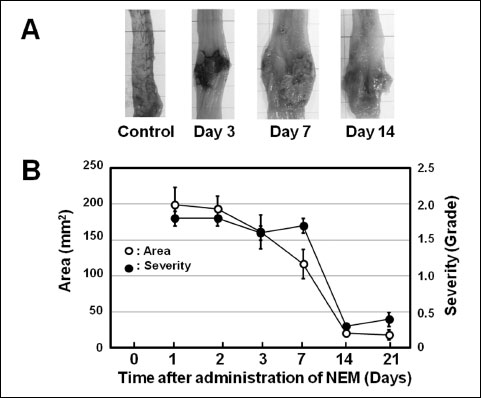 |
Fig. 1. Time-dependent changes in the colonic ulcers induced by NEM in rats. Fifty microliters of 3% NEM was introduced into the colon (i.c.) 6 cm from the anus, and the animals were autopsied 1, 2, 3, 7, 14 and 21 days later. (A): Macroscopic observation at days 0, 3, 7 and 14. (B): Time-dependent changes of both the area and the severity of the lesions. Data show the mean ± S.E.M. (n = 12). |
The weight of the normal colon was 0.21 ± 0.02 g/3 cm (n = 6). The weight of the ulcerated colon gradually increased with time and reached a maximum on day 7 (1.82 ± 0.22 g/3 cm, n = 5), after which it gradually decreased.
Time-dependent changes in bFGF concentration and content in ulcerated colon
The concentration of bFGF in the normal colon was 2.32 ± 0.06 µg/g w.w. (n = 6). It was markedly decreased the day after the administration of NEM; that is, the concentration was 1.10 ± 0.06 µg/g w.w. (n = 6, P < 0.001 vs. normal) (Fig. 2A). However, it gradually increased at day 7 and later; that is, the concentrations at day 7 and day 14 were 1.34 ± 0.13 µg/g w.w. (n = 6, P < 0.001 vs. normal) and 2.20 ± 0.11 µg/g w.w. (n = 5), respectively. At day 21, the concentration of bFGF was almost at the same level as that of normal colon (Fig. 2A).
The content of bFGF in the normal colon was 0.24 ± 0.02 µg/3 cm colon (n = 6). It gradually increased until day 5, but it had markedly increased by day 7 and continued to increase until day 21 (Fig. 2B). The contents on day 7 and day 14 were 2.89 ± 0.29 µg/3 cm colon (n = 6, P < 0.05 vs. day 0) and 3.79 ± 0.45 µg/3 cm colon (n = 5, P < 0.01 vs. day 0), respectively.
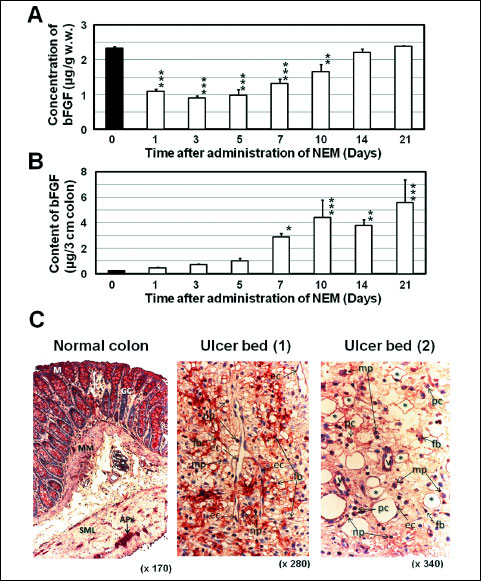 |
Fig. 2. Time-dependent changes in the concentration and content of bFGF and distribution of bFGF in ulcerated colon in the rat. (A), (B): Colonic ulcers were induced as shown in Fig. 1 and the ulcerated colon (3 cm in length) was sampled at the times indicated. The concentration of bFGF was measured by a sandwich enzyme immunoassay using 3 monoclonal antibodies for human bFGF (details are shown in the text), and the content of bFGF (µg/3 cm colon) was calculated from the concentration and the weight of the colon (3 cm). Data show the mean ± S.E.M. (n = 5 or 6, except for day 21, n = 3). * and ***: P < 0.05 and 0.001 vs. Day 0 (normal control) (Dunnett’s test). (C): The ulcerated colon was sampled 7 days after the induction of ulcers. bFGF was stained immunohistochemically (reddish area) using anti-bFGF antiserum. Localization of bFGF in the normal colon: positive responses are observed in the epithelial cells of the mucosa (M), goblet cells (GC), Auerbach’s plexus (APx), muscularis mucosae (MM), smooth muscle layers (SML) and infiltrated cells. Localization of bFGF in the ulcer bed: positive responses are observed in fibroblasts (fb), vascular endothelial cells (ec), macrophages (mp), neutrophils (np), small blood vessels (v) and plasma cells (pc). Asterisks: blood vessels in angiogenesis. |
Distribution of endogenous bFGF in the colon
In the normal colon, positive staining for bFGF was observed in the mucosal epithelial cells, goblet cells, Auerbach’s plexus, vascular wall, muscularis mucosae, smooth muscle layer and infiltrated cells (Fig. 2C). In the ulcer bed 7 days after the induction of ulcers, fibroblasts, vascular endothelial cells, macrophages, neutrophils, small blood vessels and plasma cells showed positive staining for bFGF (Fig. 2C).
Effects of MAb 3H3 and TGP-580 on ulcer healing
The area and severity of the lesions in the group given vehicle (saline, 1 ml/kg, intravenously (i.v.), once daily for 10 days) were 125.9 ± 19.9 mm2 and 1.2 ± 0.1 (n = 8), respectively. The administration of MAb 3H3 (0.5 mg/kg, i.v.) markedly inhibited the healing of lesions; that is, the area and grade of the lesions were 233.8 ± 40.2 mm2 (P < 0.05 vs. vehicle) and 1.1 ± 0.2 (n = 7), respectively (Fig. 3). On the other hand, the administration of TGP-580 (0.1 mg/kg, i.v.) mildly accelerated the healing of lesions, but the effect was not significant (Fig. 3).
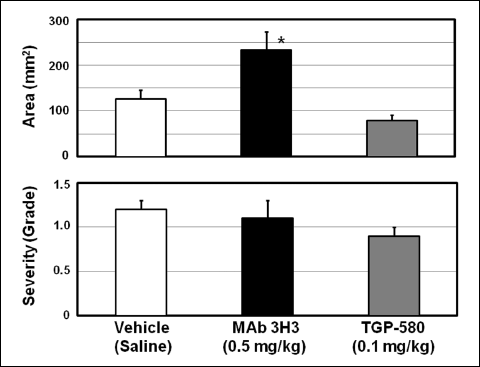 |
Fig. 3. Effects of anti-bFGF monoclonal antibody (MAb 3H3) and TGP-580 on the healing of colonic ulcers. Colonic ulcers were induced as shown in Fig. 1, and vehicle (saline 1 ml/kg), MAb 3H3 (0.5 mg/kg) or TGP-580 (0.1 mg/kg) was administered intravenously once daily for 10 days starting on the day after ulcer induction. The area and severity of lesions in the colon were examined 24 hours after the final dosing of vehicle, MAb 3H3 and TGP-580. Data show the mean ± S.E.M. (n = 7 or 8). a: P < 0.05 vs. vehicle (Dunnett’s test). |
Effects of TGP-580 and rhbFGF on ulcer healing
The area and severity of the lesions in the group given vehicle (50 mM citrate buffer, 200 ml/rat, i.c., twice daily for 10 days) were 111.3 ± 16.6 mm2 and 1.2 ± 0.1 (n = 19), respectively. The administration of rhbFGF (0.2 – 20 µg/ml) mildly and dose-dependently decreased the area and grade of lesions; that is, the effect of rhbFGF at a dose of 20 µg/ml was not significant on the area, but was significant (P < 0.001 versus vehicle) on the grade of lesions (Fig. 4A). On the other hand, TGP-580 (0.2 – 20 µg/ml) markedly and dose-dependently decreased the area and grade of lesions (Fig. 4A). The areas in the groups given TGP-580 at doses of 2 and 20 µg/ml were 67.1 ± 8.5 mm2 (n = 19, P < 0.05 versus rhbFGF and vehicle) and 50.9 ± 7.2 mm2 (n = 20, P < 0.01 versus rhbFGF and vehicle), respectively. The grades of the lesions at doses of 0.2, 2 and 20 µg/ml were 0.9 ± 0.1 (P < 0.05 versus rhbFGF and vehicle), 0.7 ± 0.1 (P < 0.01 versus vehicle) and 0.5 ± 0.1 (P < 0.001 versus vehicle), respectively. The effect of TGP-580 on the healing of colonic lesions seems to be more than 10 times stronger than that of rhbFGF (Fig. 4A). Typical macroscopic photographs of TGP-580 and rhbFGF at a dose of 20 mg/ml are shown in Fig. 4B; compared with the group given vehicle, both TGP-580 and rhbFGF decreased the ulcerated area.
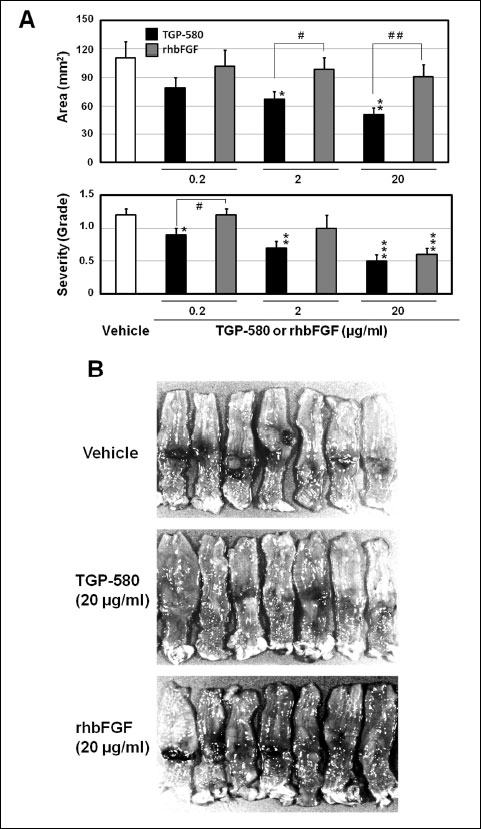 |
Fig. 4. Effects of TGP-580 and rhbFGF on the healing of colonic ulcers. Colonic ulcers were induced as shown in Fig. 1, and 200 µl of vehicle (50 mM citrate buffer), TGP-580 (0.2 – 20 µg/ml) or rhbFGF (0.2 – 20 µg/ml) was administered i.c. twice daily for 10 days starting the day after ulcer induction. The lesions in the colon were examined on day 11. (A): Dose-dependent effects of TGP-580 and rhbFGF on ulcer healing. The experiment was repeated three times (n = 6 or 7) and data were pooled. Data show the mean ± S.E.M. (n = 18 – 20). *, ** and ***: P < 0.05, 0.01 and 0.001 vs. vehicle (Dunnett’s test). # and ##: P < 0.05 and 0.01 (Student’s t-test). (B): Macroscopic observation: both TGP-580 (20 µg/ml) and rhbFGF (20 µg/ml) decreased the lesions compared with those in the vehicle group. |
Effects of TGP-580 and dexamethasone on ulcer healing
Two hundred microliters of vehicle (citrate buffer), TGP-580 (20 µg/ml) or dexamethasone (20 µg/ml) was administered i.c. twice daily for 3, 6 and 10 days, starting the day after ulcer induction. The lesions in the colon were examined 16 hours after the final dosing of TGP-580 and dexamethasone.
Effect on body weight
There was no significant difference in body weight just before ulcer induction (day 0) among the groups given vehicle, TGP-580 or dexamethasone. The b.w. in the group given vehicle decreased by day 4 after ulcer induction; thereafter, it gradually increased with time. The changes in body weight on days 4, 7 and 11 were –15.5 ± 4.2, 6.5 ± 3.2 and 35.0 ± 4.6 g (n = 10), respectively. The body weight in the group given TGP-580 showed similar changes to those of the vehicle group (Fig. 5A). However, the b.w. in the group given dexametasone decreased significantly (P < 0.05 versus vehicle) by day 4, and did not recover even by day 11 (Fig. 5A).
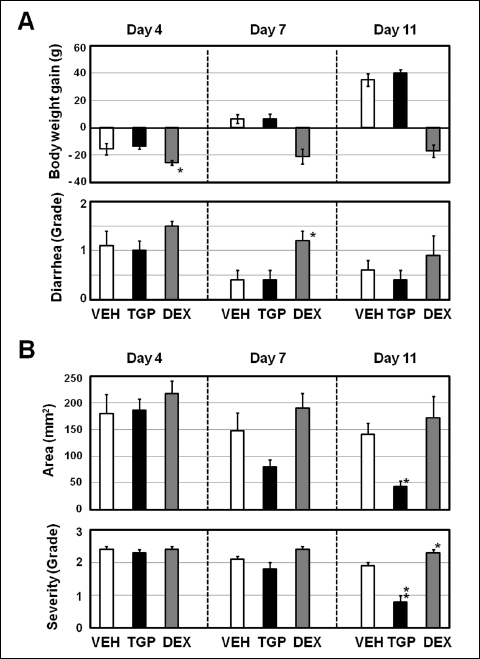 |
Fig. 5. Time-dependent effects of TGP-580 and dexamethasone on the healing of colonic ulcers. Colonic ulcers were induced as shown in Fig. 1, and 200 µl of vehicle (VEH, 50 mM citrate buffer), TGP-580 (TGP, 20 µg/ml) or dexamethasone (DEX, 20 µg/ml) was administered i.c. twice daily for 3, 6 and 10 days starting the day after ulcer induction. Changes in body weight and diarrhea (A) and the area and severity of lesions in the colon (B) were examined on days 4, 7 and 11. The experiment was repeated two times (n = 4 or 5) and data were pooled. Data show the mean ± S.E.M. (n = 8 – 10). * and **: P < 0.05 and 0.01 vs. vehicle (Dunnett’s test). |
Effect on diarrhea
The animals given vehicle showed mild diarrhea 4 days after ulcer induction, which then gradually recovered with time. The grades of diarrhea on days 4, 7 and 11 were 1.1 ± 0.3, 0.4 ± 0.2 and 0.6 ± 0.2 (n = 10), respectively. The grades in the group given TGP-580 showed similar changes to those of the vehicle group (Fig. 5A). On the other hand, the animals given dexamethasone showed higher grades than those given vehicle (P < 0.05, on day 7) (Fig. 5A).
Macroscopic observation of colonic lesions
The area and grade of lesions on day 4 in the rats given vehicle were 179.6 ± 36.1 mm2 and 2.4 ± 0.1 (n = 10), respectively. They were slightly decreased with time (day 7 and day 11), but there was no significant change among the groups (Fig. 5B). TGP-580 (20 µg/ml) did not cause any significant changes in the lesions by day 4, but gradually reduced both the area and the severity of the lesions with time (day 7 and day 11), and the area and grade of lesions on day 11 were 43.7 ± 10.0 mm2 (P < 0.05 versus vehicle) and 0.8 ± 0.2 (n = 9, P < 0.01 versus vehicle), respectively (Fig. 5B). On the other hand, dexamethasone (20 µg/ml) gradually increased both the area and the severity of the lesions, and by day 11, the severity was significantly (P < 0.05 versus vehicle) increased (Fig. 5B).
Histological observation of colonic lesions
The effects of TGP-580 and dexamethasone were confirmed by histological observation. Typical photographs of the histology on day 11 are shown in Fig. 5. Compared with the group given vehicle (Fig. 6A and 6D), TGP-580 decreased ulcer crater, increased the area of granulation tissue that consisted of numerous new blood vessels, fibroblasts depositing collagen and few mononuclear chronic inflammatory cells. The ulcerated mucosa that was replaced by granulation tissue was partially (Fig. 6B) or completely (Fig. 6E) covered by regenerating epithelial cells. However, dexamethasone did not accelerate ulcer healing, but actually delayed it (Fig. 6C).
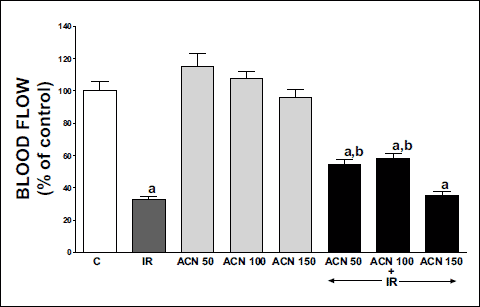 |
Fig. 6. Effects of TGP-580 and dexamethasone on the healing of colonic ulcers (histological observation, Azan staining). The photos show the histology of the ulcerated colon on day 11 from each group given vehicle (A, D), TGP-580 (B, E) or dexamethasone (C). Photos of A, B and C are from the first experiment, and those of D and E are from the second experiment. UC, ulcer crater; RM, regenerating mucosa. |
Effect on angiogenesis in the ulcer bed
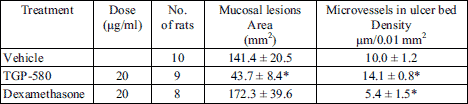
Typical photographs of immunohistochemistry on day 11 in the groups given vehicle, TGP-580 or dexamethasone are shown in Fig. 7. Microvessels in the ulcer bed were clearly stained with a reddish color. The density of microvessels in the group given vehicle was 10.0 ± 1.2 µm/0.01 mm2 (n = 10). TGP-580 significantly increased the density of microvessels in the ulcer bed and decreased the ulcerated area (Table 1). On the other hand, dexamethasone decreased the density of microvessels, and increased the ulcerated area (Table 1). The ulcerated area and the density of microvessels in each rat treated with vehicle or TGP-580 are plotted in Fig. 8. There was a good correlation between these two variables (y = –14.7x + 271.3, R2 = 0.633, where y is the area of lesions, in mm2, and x is the density of microvessels, in µm/0.01 mm2).
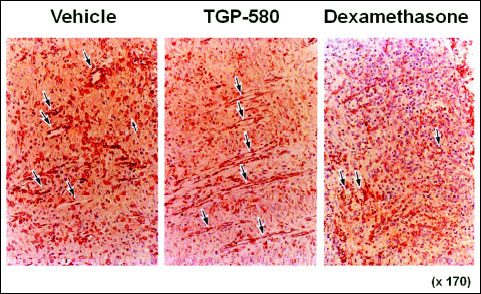 |
Fig. 7. Angiogenesis in the colonic ulcer bed in rats treated with vehicle, TGP-580 or dexamethasone. The photos show the histology of the ulcerated colon on day 11 from each group given vehicle, TGP-580 or dexamethasone. Microvessels were stained immunohistochemically (reddish area) using anti-factor VIII antiserum (ABC method). The density of microvessels (shown by the arrow) is increased in the group treated with TGP-580, but it is decreased in the group treated with dexamethasone. |
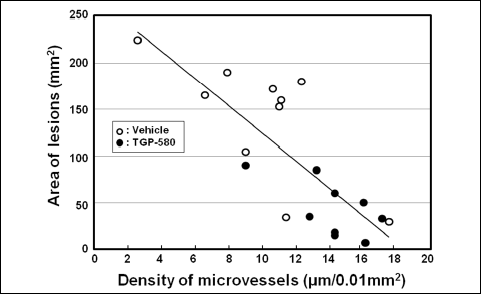 |
Fig. 8. Relationship between the lesion area and the density of microvessels in the ulcer bed. Original data were obtained from the study shown in Figs. 5B (Day 11), and each point represents the values for the TGP-580 (9 rats) and vehicle (10 rats). A good correlation between the ulcerated area (y, mm2) and the density of microvessels (x, µm/0.01 mm2) is observed (y = –14.7x + 271.3, R2 = 0.633). |
DISCUSSION
It has been reported that bFGF plays an important role in the healing of gastrointestinal ulcers in rats (4-6, 8-11) as well as in wound healing (19, 20). In the present study, we examined the role of endogenous bFGF in the healing of colonic ulcers induced by NEM in rats and obtained the following results: 1) the content of bFGF in the ulcerated colon was markedly increased with ulcer healing; 2) many cells responding to anti-bFGF antiserum were seen in the ulcer bed 7 days after ulcer formation (period of initiation of ulcer healing), and most of them were fibroblasts, vascular endothelial cells and macrophages; 3) ulcer healing was significantly inhibited by the administration of the anti-bFGF monoclonal antibody 3H3; and 4) ulcer healing was accelerated by the administration of bFGF and TGP-580. These results indicate that bFGF plays an important role in the process of colonic ulcer healing, supporting the above findings (4-6, 8, 9) on the role of bFGF in the healing of gastrointestinal ulcers.
TGP-580 was produced by site-specific mutagenesis using gene technology in which the second and third of the four cysteines within the peptide were substituted by serines. Therefore, TGP-580 is also called bFGF-CS23 (5, 8). These substitutions produced an acid-stable form of bFGF without changing its biological activities, such as its mitogenic activity on endothelial cells (7). In the present study, we compared the effects of TGP-580 and rhbFGF on the healing of colonic ulcers. Both rhbFGF and TGP-580 (0.2, 2 and 20 µg/ml, 200 µl/rat i.c., twice daily for 10 days) dose-dependently accelerated the healing of colonic ulcers. Compared with the vehicle group, rhbFGF showed a significant effect on the severity of ulcers at a dose of 20 µg/ml, which is comparable to our previous study on UC induced by iodoacetamide in rats (6). On the other hand, a significant effect of TGP-580 was observed at a lower dose of 0.2 µg/ml, suggesting that its effect on colonic ulcer healing is more than 10 times stronger than that of rhbFGF in this ulcer model. This large difference between the efficacy of rhbFGF and that of TGP-580 may be partially explained as follows: since there are many bacteria in the colon, which produce organic acids such as lactic acid, butyric acid and propionic acid, the pH of the colon is lower than 7.0. In addition, it is commonly accepted that inflammation decreases the pH (by about 0.5) of the inflamed area. As mentioned above, the biological activity (angiogenic activity) of rhbFGF is very labile under acidic conditions and almost disappears within 10 min at pH 4.0. However, the activity of TGP-580 was observed even after 30 min under these conditions (7). Therefore, it is conceivable that the biological activity of TGP-580 was maintained for longer than that of rhbFGF under acidic conditions in the ulcerated colon.
Angiogenesis in granulation tissue in the ulcer bed is thought to be critical in ulcer healing as well as in wound healing. As bFGF is one of the very active angiogenic peptides among the growth factors (21), it was expected that TGP-580 promotes neovascularization in the ulcer bed. In the present study, we examined its effect on angiogenesis in colonic ulcer bed. When TGP-580 was administered by an enema twice daily for 10 days, the density of microvessels in the ulcer bed was significantly greater than that in the vehicle group. Furthermore, there was a good correlation (R2 = 0.6331) between the density of microvessels and the healing of colonic ulcers (decrease of ulcerated area). These results suggest that TGP-580 accelerates the healing of colonic ulcers, at least in part via stimulation of angiogenesis in the ulcer bed. These results support the previous findings (4-6, 8, 9) that the angiogenic activity of TGP-580, as well as bFGF, is involved in the healing effect of the peptide on gastrointestinal ulcers.
It has been reported that peroxisome proliferator-activated receptors gamma (PPAR-γ) agonists such as rosiglitazone and troglitazone ameliorated experimental colitis induced by dextran sodium sulfate (DSS) in rats by down-regulating the pro-inflammatory cytokines and up-regulation of inflammation-limiting cytokines (22, 23). On the other hand, it has been reported that both bFGF and a PPAR-γ agonist, ciglitazone, activated mitogen-activated kinase (p38 MAPK) pathways in human umbilical vein endothelial cells (HUVEC) (24), although the effect on p42/44 MAPK was different between bFGF and ciglitazone. Considering these findings together, it will be suggested that PPAR-γ plays some role, at least in part, in the healing effect of TGP-580 on colonic ulcers in the present study.
It is now well known that bFGF stimulates the proliferation of not only fibroblasts but also epithelial and endothelial cells, thus inducing angiogenesis (25). Namely, bFGF modulates intestinal epithelial cell growth and migration, and mediates rapid epithelial repair after surface injury (26, 27). It has also been reported by Nakamura et al. (28) that bFGF-CS23 (TGP-580) promoted the re-innervation of newly formed microvessels through its effect on the Schwann cells in the gastric ulcer bed in rats. In the present study, a part of the ulcerated mucosa was already covered with regenerated mucosa in the group given TGP-580 for 10 days, although this was not observed in the group given vehicle. As bFGF has various biological activities (24-28), it is conceivable that TGP-580 may have stronger biological activities than those of the original rhbFGF, and that this may explain, in part, the stronger healing effect of TGP-580 on colonic ulcers than that of rhbFGF, in addition to its stable property to acid.
In the previous study on acetic acid-induced gastric ulcers in rats (9), TGP-580 given orally did not distribute to the normal mucosa but did selectively and highly distribute to the ulcerated mucosa. Thereafter, distribution decreased, associated with the healing of ulcers. In the present study, TGP-580 given topically into the ulcerated colon markedly accelerated the healing of ulcers, but showed a mild effect on ulcer healing when it was administered intravenously. Although we did not examine the local distribution of TGP-580 in the ulcerated colon in this study, the above findings in the gastric ulcer model and the results in the present study on ulcer healing effects of TGP-580 given topically or intravenously suggest that TGP-580 accelerates the healing of colonic ulcers by distributing topically to the ulcerated mucosa in the colon.
Prostaglandins (PG) play an important role in angiogenesis and ulcer (wound) healing (29, 30), and it has also been reported that bFGF induces angiogenesis via stimulation of PG synthesis by induction of cyclooxygenase (COX)-2 mRNA (31, 32). These findings suggest that glucocorticoids as well as NSAID delay the healing of ulcers because they inhibit the synthesis of PG by the inhibition of COX or phospholipase A2. In fact, it has been reported that the healing of gastric ulcers in rats induced by acetic acid was delayed by indomethacin (33) and dexamethasone (34, 35). Luo et al. (35) suggested that dexamethasone delayed the healing of gastric ulcers by inhibiting angiogenesis in the ulcer margin via a decrease of PG. In the present study, dexamethasone inhibited the angiogenesis in the colonic ulcer bed and delayed the healing of ulcers, supporting the findings of Luo et al. (35) in gastric ulcers. All of these findings indicate that ulceration and inflammation should be distinguished in the treatment of IBD as anti-inflammatory drugs delay ulcer healing. In other words, inflammation may play an important role in ulcer healing. This idea raises questions about the exclusive use of drugs with anti-inflammatory action for the treatment of IBD, although these drugs including dexamethasone are effective for the treatment of inflammatory responses of IBD, because of the well-known anti-inflammatory action of glucocorticoids which reduce chronic inflammation that accompanies IBD.
One of the major problems in the treatment of IBD is recurrence of the disease, as seen in other ulcerative diseases. We found that gastric ulcers cured by treatment with TGP-580 are resistant to relapse caused by indomethacin in rats (36). This was confirmed by Hull et al. (37) in 12 healthy volunteers using biopsy-induced mini-ulcers in the gastric antrum. That is, artificial gastric ulcers healed by TGP-580 (0.1 mg p.o., twice daily for 14 days) are resistant to relapse caused by indomethacin (50 mg t.i.d., for 7 days) starting just after TGP-580 treatment. These results suggest that, if the ulcerated mucosa in the colon is cured effectively by TGP-580, the likelihood of ulcer recurrence is decreased.
Colitis models induced by dextran sulfate sodium (DSS) in mice (38) and rats are often used to elucidate the pathogenesis of UC and to estimate the efficacy of drugs on this condition. Jeffers et al. (39) reported that FGF 20, a new member of the FGF family, reduced the severity of DSS-induced UC in mice. They also found that FGF 20 prevented indomethacin-induced small intestinal damage in rats. Matsuura et al. (40) reported that rectal administration of human bFGF ameliorated DSS-induced colitis as well as trinitrobenzenesulfonic acid-induced colitis in mice. The efficacy of human bFGF was also reported in a DSS-induced rat colitis model (41). These findings indicate that bFGF and its family are effective in various intestinal inflammatory models including DSS-induced UC, suggesting the effectiveness of TGP-580, a mutein of human bFGF, for the treatment of IBD.
The present study confirmed that endogenous bFGF plays an important role in the healing of colonic ulcers and also revealed that TGP-580 actively accelerates colonic ulcer healing by a new mode of action, namely, the stimulation of angiogenesis in the ulcer bed. Therefore, we conclude that TGP-580 enema is a promising option for the treatment of IBD.
Acknowledgements: The authors express our sincere thanks to Prof. J. Folkman, Harvard Medical School, Boston, MA, USA, who first proposed doing this study, but unfortunately passed away before the study was completed. We are greatly indebted to N. Aida, K. Hina and Y. Kawano, students at Tottori University, Tottori, Japan, for their technical assistance, and to Drs. S. Ebara and M. Hirose, Meiji University of Integrated Medicine, Kyoto, Japan, for valuable advice on histological studies. We are also grateful to Drs. A. Shino, K. Takami and H. Watanabe, Pharmaceutical Research Division of Takeda Pharmaceutical Co. Ltd., Osaka, Japan, for their valuable discussions, and Takeda Pharmaceutical Co. Ltd. for kind support in this study.
Conflict of interests: None declared.
REFERENCES
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46-54.
- Domenech E. Inflammatory bowel disease: current therapeutic options. Digestion 2006; 73 (Suppl 1): 67-76.
- Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenterol Dietol 2010; 56: 233-243.
- Konturek SJ, Brzozowski T, Majka J, et al. Fibroblast growth factor in gastroprotection and ulcer healing: interaction with sucralfate. Gut 1993; 34: 881-887.
- Folkman J, Szabo S, Stovroff M, McNeil P, Li W, Shing Y. Duodenal ulcer. Discovery of a new mechanism and development of angiogenic therapy that accelerates healing. Ann Surg 1991; 214: 414-425.
- Paunovic B, Deng X, Khomenko T, et al. Molecular mechanisms of basic fibroblast growth factor effect on healing of ulcerative colitis in rats. J Pharmacol Exp Ther 2011; 339: 430-437.
- Seno M, Sasada R, Iwane M, et al. Stabilizing basic fibroblast growth factor using protein engineering. Biochem Biophys Res Commun 1988; 151: 701-708.
- Szabo S, Folkman J, Vattay P, Morales RE, Pinkus GS, Kato K. Accelerated healing of duodenal ulcers by oral administration of a mutein of basic fibroblast growth factor in rats. Gastroenterology 1994; 106: 1106-1111.
- Satoh H, Shino A, Sato F, et al. Role of endogenous basic fibroblast growth factor in the healing of gastric ulcers in rats. Jpn J Pharmacol 1997; 73: 59-71.
- Hull MA, Cullen DJ, Hudson N, Hawkey CJ. Basic fibroblast growth factor treatment for non-steroidal anti-inflammatory drug associated gastric ulceration. Gut 1995; 37: 610-612.
- Beeken WL. Experimental inflammatory bowel disease. In: Inflammatory Bowel Disease, Kirsner JB, Shorter RG (eds.). Philadelphia, Lea & Febiger 1988, pp 37-49.
- Satoh H, Sato F, Takami K, Szabo S. New ulcerative colitis model induced by sulfhydryl blockers in rats and the effects of antiinflammatory drugs on the colitis. Jpn J Pharmacol 1997; 73: 299-309.
- Sandor Z, Nagata M, Kusstatscher S, Szabo S. New animal model of ulcerative colitis associated with depletion of glutathione and protein SH alkylation. [abstract] Gastroenterology 1994; 106: A766.
- Tolstanova G, Deng X, French SW, et al. Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Lab Invest 2012; 92: 9-23.
- Seno M, Iwane M, Sasada R, Moriya N, Kurokawa T, Igarashi K. Monoclonal antibodies against human basic fibroblast growth factor. Hybridoma 1989; 8: 209-221.
- Hori A, Sasada R, Matsutani E, et al. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res 1991; 51: 6180-6184.
- Watanabe H, Hori A, Seno M, et al. A sensitive enzyme immunoassay for human basic fibroblast growth factor. Biochem Biophys Res Commun 1991; 175: 229-235.
- Kurokawa T, Seno M, Igarashi K. Nucleotide sequence of rat basic fibroblast growth factor cDNA. Nucleic Acids Res 1988; 16: 5201.
- McGee GS, Davidson JM, Buckley A, et al. Recombinant basic fibroblast growth factor accelerates wound healing. J Surg Res 1988; 45: 145-153.
- Broadley KN, Aquino AM, Woodward SC, et al. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest 1989; 61: 571-575.
- Folkman J, Klagsbrun M. Angiogenic factors. Science 1987; 235: 442-447.
- Celinski K, Dworzanski T, Fornal R, et al. Comparison of anti-inflammatory properties of peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone in prophylactic treatment of experimental colitis. J Physiol Pharmacol 2013; 64: 587-595.
- Annese V, Rogai F, Settesoldi A, et al. PPARg in inflammatory bowel disease. PPAR Res 2012; 2012: 620839.
- Kiec-Wilk B, Grzybowska-Galuszka J, Polus A, Pryjma J, Knapp A, Kristiansen K. The MAPK-dependent regulation of the Jagged/Notch gene expression by VEGF, bFGF or PPAR gamma mediated angiogenesis in HUVEC. J Physiol Pharmacol 2010; 61: 217-225.
- Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev 1987; 8: 95-114.
- Paimela H, Goddard PJ, Carter K, et al. Restitution of frog gastric mucosa in vitro: effect of basic fibroblast growth factor. Gastroenterology 1993; 104: 1337-1345.
- Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology 1994; 106: 1254-1262.
- Nakamura M, Oda M, Inoue J, et al. Effect of basic fibroblast growth factor on reinnervation of gastric microvessels. Possible relevance to ulcer recurrence. Dig Dis Sci 1995; 40: 1451-1458.
- Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med 1983; 172: 214-218.
- Takeuchi K, Tanigami M, Amagase K, Ochi A, Okuda S, Hatazawa R. Endogenous prostaglandin E2 accelerates healing of indomethacin-induced small intestinal lesions through upregulation of vascular endothelial growth factor expression by activation of EP4 receptors. J Gastroenterol Hepatol 2010; 25 (Suppl 1): S67-S74.
- Kage K, Fujita N, Oh-hara T, Ogata E, Fujita T, Tsuruo T. Basic fibroblast growth factor induces cyclooxygenase-2 expression in endothelial cells derived from bone. Biochem Biophys Res Commun 1999; 254: 259-263.
- Tessner TG, Muhale F, Schloemann S, Cohn SM, Morrison A, Stenson WF. Basic fibroblast growth factor upregulates cyclooxygenase-2 in I407 cells through p38 MAP kinase. Am J Physiol Gastrointest Liver Physiol 2003; 284: G269-G279.
- Wang JY, Yamasaki S, Takeuchi K, Okabe S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology 1989; 96: 393-402.
- Luo JC, Shin VY, Liu ES, et al. Non-ulcerogenic dose of dexamethasone delays gastric ulcer healing in rats. J Pharmacol Exp Ther 2003; 307: 692-698.
- Luo JC, Shin VY, Liu ES, et al. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol 2004; 485: 275-281.
- Satoh H, Asano S, Maeda R, et al. Prevention of gastric ulcer relapse induced by indomethacin in rats by a mutein of basic fibroblast growth factor. Jpn J Pharmacol 1997; 73: 229-241.
- Hull MA, Knifton A, Filipowicz B, Brough JL, Vautier G, Hawkey CJ. Healing with basic fibroblast growth factor is associated with reduced indomethacin induced relapse in a human model of gastric ulceration. Gut 1997; 40: 204-210.
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694-702.
- Jeffers M, McDonald WF, Chillakuru RA, et al. A novel human fibroblast growth factor treats experimental intestinal inflammation. Gastroenterology 2002; 123: 1151-1162.
- Matsuura M, Okazaki K, Nishio A, et al. Therapeutic effects of rectal administration of basic fibroblast growth factor on experimental murine colitis. Gastroenterology 2005; 128: 975-986.
- Kojima T, Watanabe T, Hata K, Nagawa H. Basic fibroblast growth factor enema improves experimental colitis in rats. Hepatogastroenterology 2007; 54: 1373-1377.
A c c e p t e d : September 30, 2015