ANTI-INFLAMMATORY EFFECTS OF NESFATIN-1 IN RATS WITH ACETIC
ACID - INDUCED COLITIS AND UNDERLYING MECHANISMS
INTRODUCTION
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disease (IBD) with recurrent, diffuse inflammation of the colonic and rectal mucosa, which is predominantly described by cycles of acute inflammation, ulceration and bleeding (1). The pathogenesis of disease involves an interaction between immune status, genetics, and environmental factors (2). The etiology of UC is not clearly understood, but excessive production of reactive oxygen species (ROS) by the inflamed mucosa have been suggested to contribute significantly to the development of tissue injury (3). These molecules are known to play a role not only in initiation of UC but also in progression of the disease. Furthermore, the production of these metabolites that are known to be the common mediators of inflammation which has been shown to occur in the gastrointestinal tract (4).
The possible therapeutic effects of peptides on IBD are frequently experimentally investigated. Nesfatin-1 is a recently described 82-amino-acid polypeptide (5) and nesfatin-1-containing proteins have been shown to be located in several brain areas related with feeding and metabolic regulation, including the hypothalamic paraventricular nucleus, arcuate nucleus, supraoptic nucleus, lateral hypothalamic area, and nucleus tractus solitarius in the brain stem (6, 7). In addition to the wide distribution of the peptide in the central nervous system, the messenger RNA of amino-terminal fragment was shown to be widely expressed also in the peripheral organs such as stomach, pancreas, testis, colon and adipose tissue in rodents and goldfish (8, 9). Further studies have shown 20-fold higher amino-terminal fragment mRNA expression in the rat stomach compared with the brain (10). Moreover, studies have suggested that the presence of inflammatory status might have a relation with nesfatin-1 production and release and the neurons that produce nesfatin-1 respond to peripheral inflammatory signals (11). Recently, the anti-inflammatory and gastroprotective effects of nesfatin-1 on oxidative gastric damage and (12) anti-inflammatory and anti-apoptotic effects in subarachnoid hemorrhage (SAH) were demonstrated (13).
Nesfatin-1 firstly was shown to be a potent inhibitor of both food and water intake via a leptin-independent, melanocortin receptor-dependent mechanism in paraventricular nucleus (PVN) of hypothalamus (5, 14, 15). In PVN, nesfatin-1 co-localizes most extensively with oxytocin (16, 17). Further studies have reported its close relationship with regulation of blood pressure and its increasing effect on mean arterial pressure (MAP) when injected into the lateral cerebroventricle (18). Moreover, pretreatment with the melanocortin receptor antagonist (SHU9119), and oxytocin receptor antagonists block its well-known anorexigenic, anti-dipsogenic and the hypertensive effects (14, 15, 18). Although nesfatin-1 acts on melanocortin pathway and oxytocin receptors during these effects, it is not known if it may use these receptor families for the anti-inflammatory actions or not.
Another peptide related to regulation of food intake is ghrelin, which is known as fasting hormone (19, 20). Although the effective mechanism of peptide is not clear yet, it is postulated that the increased motility, amelioration in inflammation, increased appetite, and improved colonic blood flow might have a role (21). In the literature, the anti-inflammatory role of ghrelin has been shown in variant types chronic inflammation such as colitis (20, 23). Moreover, nesfatin-1 has been co-localized with ghrelin in the stomach and hypothalamus of rats. Also, nesfatin-1 has been shown to increase expression of ghrelin (22). While ghrelin and nesfatin-1 are released from brain tissue and have common anti-inflammatory effects, it draws attention if nesfatin-1 acts on ghrelin receptors for its possible anti-inflammatory effects in colitis.
Based on the aforementioned evidence, the study was designed to examine the possible anti-inflammatory effects of nesfatin-1 in acetic acid induced colitis model and potential underlying mechanisms. Firstly, we assessed the effect of nesfatin-1 on experimental colitis model. Secondly, we tested whether nesfatin-1 effected on melanocortin pathway, oxytocin receptors or ghrelin receptors in its anti-inflammatory effect.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (250 – 300 g, n = 48) were supplied from the Marmara University Animal Center (DEHAMER; approval no: 26.2012.mar) and were housed in a temperature-controlled (22 ± 2°C) room and standardized light/dark (12/12 hour) cycles. Rats were fed with standard rat pellets and tap water ad libitum. All experimental protocols were approved by the Marmara University Animal Care and Use Committee.
Experimental design
In the beginning of the experimental period for nesfatin-1 and antagonists applications some of the rats were intracerebroventricularly (i.c.v.) cannulated under intraperitoneal (i.p.) ketamine (100 mg/kg) and chlorpromazine (0.75 mg/kg) anesthesia. Following a recovery period, the rats were divided in to two groups as control and colitis. In colitis groups, intrarectally 4% acetic acid solution (1 ml) and 10 minutes later i.c.v. nesfatin-1, 0.05 µg/5 µl, (Enzo life sciences, catalogue number: ALX-522-116-C010) (24) or vehicle (5 µl) were administered. Treatments continued for 3 days. In control group, intrarectally physiological saline solution was given. In the second part of the study, to identify the underlying effective mechanism of nesfatin-1, 5 min after colitis induction; i.c.v. atosiban, oxytocin receptor antagonist, 3 µg/rat, (Tractocile, Ferring, Switzerland) (25), SHU9119, melanocortin receptor antagonist, 0.3 nmol/rat, (Phoenix peptide, catalogue number: 043-24) (14), or GHSR-1a, ghrelin receptor antagonist, 3 nmol/rat, (BACHEM, catalogue number: H-3108.0005) (26) were administered. Following a 5 min interval nesfatin-1 administrations were made. On the fourth day, rats were decapitated, colon tissues were sampled (27). Samples from distal colon were taken and stored at –80°C for later measurements of tissue myeloperoxidase (MPO) activity, malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) levels. Formation of reactive oxygen species in colonic samples were monitored by using chemiluminescence method and luminol and lucigenin chemiluminescence measurements were made. Tissue samples were placed in 10 % formaldehyde for histological evaluation, macroscopic and microscopic scoring.
Intracerebroventricularly cannulation
The rats were anesthetized (100 mg/kg ketamine and 0.75 mg/kg chlorpromazine, i.p.) and body temperature was kept at ~37°C, using a heating pad placed under the rat. The animals were fixed in a stereotactic apparatus (Stoelting, standard stereotaxic instrument, Wood Dale, Illinois) with the head flat, and holes were drilled for bilateral implantation of cannula guides (22-gauge; Plastic Products, Roanoke, VA). The cannulas were inserted to within 1 mm above the target location (anterior/posterior [A/P], –3.30 mm; lateral [L], ± 0.0 mm; dorsal/ventral [D/V], –3.8 mm) and secured with acrylic dental cement (Croform acrylic powder and liquid) stabilized by two skull screws. A dummy cannula (Plastics One Canula C313DC) was placed in each of the guides to prevent clogging. Rats were allowed to recover for at least 5 days before experiments (28). All intracerebroventricular injections were made in a 5 µl volume over a period of 100 s using a Hamilton syringe. After each experiment, correct placement of the cannula was verified by injection of methylene blue.
Induction of colitis
After an overnight fasting, colonic inflammation was induced under light ether anesthesia by intrarectal administration of 1 ml of 4% (v/v) acetic acid in 0.9% NaCl solution (pH: 2.3) or saline (for control group) with a 8 cm long cannula. Rats were kept in Trendelenburg position for 30 seconds to prevent leakage during acetic acid and saline solution administration process. At the end of this process, 1.5 ml of phosphate buffer solution (PBS) of pH 7.4 was administered (3).
Measurement of colon myeloperoxidase activity
Since tissue myeloperoxidase (MPO) activity was shown to correlate significantly with the number of neutrophils determined histochemically, it is frequently used to estimate tissue neutrophil accumulation in the inflamed tissues (29). The method used for the assay of MPO activity was similar to that was previously described by Bradley et al. (29). Colon samples (0.2 – 0.5 g) were homogenized in 10 volumes of ice-cold potassium phosphate buffer (50 mM K2HPO4, pH 6.0) containing hexadecyltrimethylammonium bromide (HETAB; 0.5%, w/v). The homogenate was centrifuged at 12,000 g for 10 min at 4°C, and the supernatant was discarded. The pellet was then re-homogenized with an equivalent volume of 50 mm K2HPO4 containing 0.5% (w/v) HETAB and 10 mm EDTA (Sigma Chemical Co., St. Louis, MO, USA). MPO activity was assessed by measuring the H2O2-dependent oxidation of o-dianisidine. 2HCl. One unit of enzyme activity was defined as the amount of MPO present per gram of tissue weight that caused a change in absorbance of 1.0 min–1 at 460 nm and 37°C. MPO activity was expressed as U/g tissue.
Measurement of colon malondialdehyde and glutathione levels
Samples of colon tissues were homogenized in 10 volumes of ice-cold 10% trichloracetic acid in an Ultra Turrax tissue homogenizer. Homogenized tissue samples were centrifuged at 3,000 g for 15 min at 4°C. The supernatant was removed and recentrifuged at 15,000 g for 8 min. GSH measurements were performed using a modification of the Ellman procedure (30). Lipid peroxidation was quantified by measuring the formation of thiobarbituric acid-reactive substances as previously described (31). Lipid peroxide levels were expressed in terms of malondialdehyde (MDA) equivalents using an extinction coefficient of 1.56 × 105 M–1 cm–1. Results were expressed in nmol glutathione (GSH)/g tissue.
Measurement of superoxide dismutase and catalase activity in the colon
Superoxide dismutase (SOD) activity in colon samples was measured according to a previously described method (32). Briefly, measurements were performed in cuvettes containing 2.8 ml 50 mM potassium phosphate (pH = 7.8) with 0.1 mM EDTA, 0.1 mM 0.39 mM riboflavin in 10 mM potassium phosphate (pH 7.5), 0.1 ml of 6 mM o-dianisidin.2 HCl in deionized water, and tissue extract (50, 100 ml). Cuvettes with all their components were illuminated with 20-W SlylvaniaGro-Lux fluorescent tubes (Sylvania GRO-LUX F18W/GRO, Erlangen, Germany) that were placed 5 cm above and to one side of cuvettes maintaining a temperature of 37°C. Absorbance was measured at 460 nm with a Schimadzu UV-02 model spectrophotometer (Schimadzu, Tokyo, Japan). A standard curve was prepared routinely with bovine SOD (S-2515-3000 U; Sigma Chemical Co, St Louis, MO, USA) as reference. Absorbance readings were taken at 0 and 8 min of illumination and the net absorbance was calculated. The method for the measurement of catalase (CAT) activity is based on the catalytic activity of the enzyme that catalyzes the decomposition reaction of H2O2 to give H2O and O2 (33). Briefly, the absorbance of the tissue samples containing 0.4 ml homogenate and 0.2 ml H2O2 was read at 240 nm and 20°C against a blank containing 0.2 ml phosphate buffer and 0.4 ml homogenate for about 1 min. With the help of the standard graphic and considering the dilutions, the SOD activity of supernatant was calculated in the form of U/mg protein (34). Catalase measurements were expressed as U/ml/min.protein.
Measurement of colon luminol and lucigenin chemiluminescence levels
Chemiluminescence (CL) assay is a direct noninvasive method for measuring reactive oxygen species. Due to limitations, i.e., potential variability and low intensity of native CL, luminol and lucigenin can be used as enhancers. Lucigenin is more specific for superoxide radical, while luminol detects a group of reactive species such as OH, H2O2, HOCl radicals (12). Due to their high quantum efficiency after oxidation, they function as bystander-substrates for oxygenation and form high levels of excited-state products and CL, when added to an in vitro biological system. The excited electrons in these compounds revert to their ground state with the emission of energy as light (CL) and can be detected by a luminometer.
Luminescence of the colon samples was recorded at room temperature using Mini Lumat LB 9509 luminometer (EG&G Berthold, Germany) in the presence of luminol or lucigenin 0.2 mM each. All counts were obtained at 15 s intervals for 5 min and the results were expressed as area under the curve (AUC) of relative light unit (rlu) for 5 min per mg tissue. The calculation was based on the integration of the curve by the trapezoidal rule (a linear approximation) (35). Results were expressed in rlu/mg tissue.
Macroscopic scoring
On the fourth day of colitis, following decapitation the last 8 cm of the colon was excised, opened longitudinally, and rinsed with saline solution. Then, the distal colon was weighed and the mucosal lesions were scored macroscopically using the criteria outlined in Table 1 (36).

Histological evaluation
For light microscopic investigations, samples from distal colon were placed in 10% formaldehyde, dehydrated in ascending alcohol series (70%, 90%, 96% and 100%), cleared in toluene and embedded in paraffin. For each animal, four randomly taken tissue sections (5 µm) were stained with hematoxylin and eosin (H&E) and examined under an Olympus BX51 photomicroscope. All tissue sections were examined by experienced histologists who were unaware of the treatments. Histological scoring was performed by the criteria shown in Table 2 (37).

Statistical analysis
Statistical analysis was carried out using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). All data were expressed as means ± S.E.M. Groups of data were compared with an analysis of variance followed by Mann-Whitney U non-parametric tests and Student's t test. Values of P < 0.05 were regarded as significant.
RESULTS
Acetic acid application increased both macroscopic (P < 0.001) and microscopic (P < 0.001) lesion scores of colitis group significantly compared to control group (Fig. 1A and 1B). Although the increased macroscopic and microscopic lesion scores of colitis group were reduced by nesfatin-1 treatment (P < 0.01 – 0.001), atosiban application prior to nesfatin-1 treatment significantly increased both damage scores (P < 0.05 – 0.001) and GHSR-1a application prior to nesfatin-1 treatment significantly increased microscopic damage score (P < 0.01).
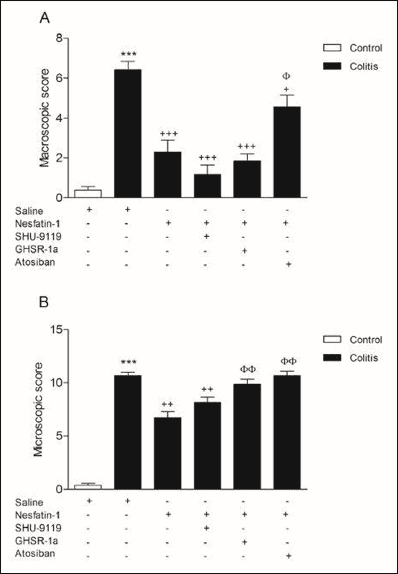 |
Fig. 1. Colonic macroscopic (A) and microscopic (B) damage scores. ***P < 0.001 compared to control group; ++P < 0.01; +++P < 0.001 compared to colitis group; jP < 0.05; φφP < 0.01 compared to nesfatin-1-treated (without any other drugs) group. |
In histologic analysis, massive epithelial loss, severe inflammatory cell infiltration, vasculitis and submucosal edema were evident in colitis group (Fig. 2B). Although a regular epithelium, moderate inflammation, vasculitis and moderate submucosal edema were observed in only nesfatin-1 or SHU9119 treated groups, severe inflammation, vasculitis and edema were prevalent in GHSR-1a or atosiban treated groups (Fig. 2C-2F).
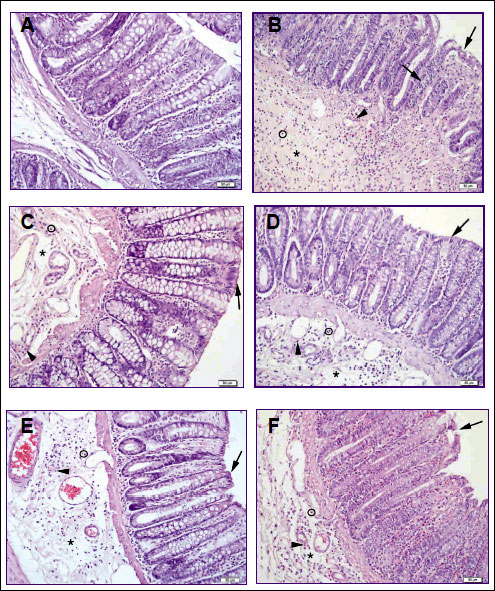 |
Fig. 2. Light microscopic examination of the colonic mucosa in experimental groups. (A) Control group, describes normal morphology, regular epithelial line and submucosa. (B) Colitis group characterized by degenerated surface epithelium (➜), severe submucosal edema (*), inflammation (o), vasculitis (►) (C) Nesfatin-1 treated colitis group, describes regular epithelial line (➜), moderate submucosal edema (*), inflammation (o), vasculitis (►). (D) SHU9119 treated colitis group, describes quite regular surface epithelium (➜), moderate submucosal edema (*), inflammation (o), vasculitis (►). (E) GHSR-1a treated colitis group, describes regular epithelial line (➜), severe submucosal edema (*), moderate inflammation (o), vasculitis (►). (F) Atosiban treated colitis group, is characterized by degenerated surface epithelium (➜), severe submucosal edema (*), inflammation (o), moderate vasculitis (►). Hematoxylin and eosin (H&E) stain. |
Colonic MPO activity, the indicator of neutrophil migration to injured tissue, showed a marked increase in colitis group compared to control group (P < 0.001) (Fig. 3A). While nesfatin-1 treatment ameliorated the increased MPO activity (P < 0.01), SHU9119 application prior to nesfatin-1 reversed its effect by increasing the neutrophil infiltration through the tissue. Additionally, MPO activity remained decreased in GHSR-1a and atosiban applicated groups suggesting that these receptors may not have a role in the effect of nesfatin-1 on neutrophil infiltration (P < 0.001) (Fig. 3A).
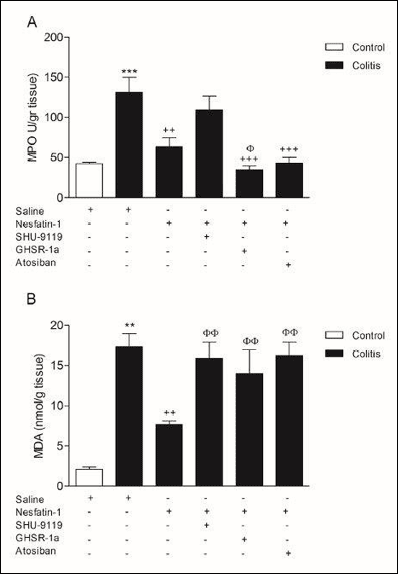 |
Fig. 3. Colonic tissue myeloperoxidase (MPO) activity (A) and malondialdehyde (MDA) levels (B). **P < 0.01, ***P < 0.001, compared to control group; ++P < 0.01, +++P < 0.001 compared to colitis group; φP < 0.05, φφP < 0.01 compared to nesfatin-1-treated (without any other drugs) group. |
As expected, a significant increase in colonic MDA level (P < 0.01) with a concomitant decrease in antioxidant GSH content (P < 0.001) was observed in the colitis group (Fig. 3B and 4A). The increase in colonic MDA level of the colitis group was reduced by nesfatin-1 treatment, underlying the lipid peroxidation inhibiting effect of nesfatin-1 (P < 0.01) (Fig. 3B). Besides, SHU9119, GHSR-1a and atosiban treatments prevented the beneficial effect of nesfatin-1 on MDA level (P < 0.01) (Fig. 3B). Although nesfatin-1 treatment did not affect the antioxidant GSH levels compared to colitis group, SHU9119 and GHSR-1a treatments increased GSH levels compared to colitis group (P < 0.01) and compared to only nesfatin-1 treated group (P < 0.05) (Fig. 4A).
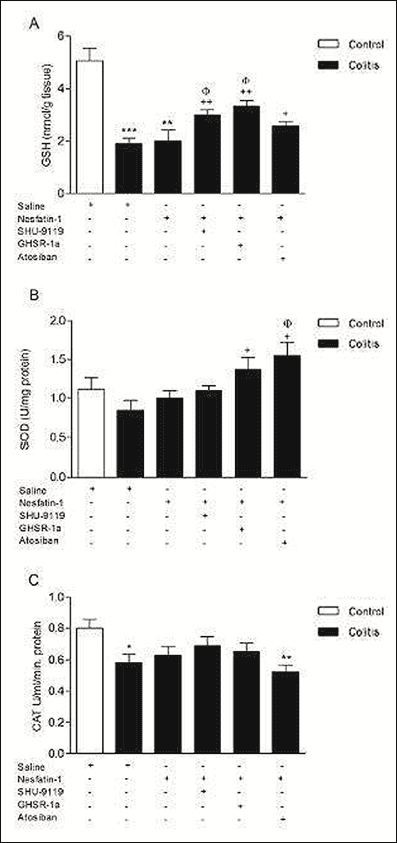 |
Fig. 4. Colonic tissue glutathione (GSH) levels (A), superoxide dismutase (SOD) (B) and catalase (CAT) (C) activities. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group; +P < 0.05, ++P < 0.01 compared to colitis group; φP < 0.05 compared to nesfatin-1-treated (without any other drugs) group. |
SOD activity was tended to decrease in colitis group (P = 0.02) and increased in GHSR-1a and atosiban treated groups compared to colitis group (P < 0.05) (Fig. 4B). Although antioxidant colonic CAT activity in the colitis group showed a decrease compared to control group (P < 0.05), it was not different than control in nesfatin-1 treated group (Fig. 4C). Furthermore CAT levels of atosiban treated group were also declined compared to control group (P < 0.01), suggesting an inhibitor effect on protective role of nesfatin-1 (Fig. 4C).
While luminol- and lucigenin-enhanced CL levels showed significant increases in the colitis group as compared with control values (P < 0.001), both parameters were decreased in nesfatin-1 treated group compared to colitis group (P < 0.05 – 0.001) (Fig. 5A and 5B). As luminol levels were tended to increase in GHSR-1a and atosiban treated groups, lucigenin levels were risen back in all treatment groups (P < 0.05 – 0.001), suggesting that the applications of GHSR-1a and atosiban prior to nesfatin-1 inhibited its protective effect and nesfatin-1 may have a role on ghrelin and oxytocin receptors in its reactive oxygen metabolite decreasing effect. (Fig. 5A and 5B).
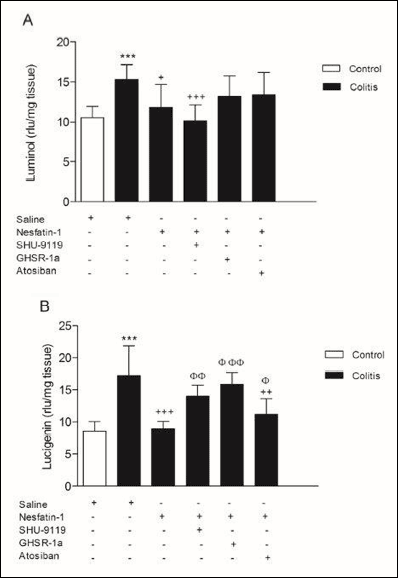 |
Fig. 5. Luminol- (A) and lucigenin- (B) enhanced tissue chemiluminescence levels. ***P < 0.001 compared to control group; +P < 0.05, ++P < 0.01, +++P < 0.001 compared to colitis group; φP < 0.05, φφP < 0.01, φφφP < 0.001 compared to nesfatin-1-treated (without any other drugs) group. |
DISCUSSION
Colitis is defined as the inflammation of the layer that covers the inner surface of the colon and a disease causing wounds with bleeding in the mucosa, and affecting the patient's quality of life. In this study, we aimed to show the colonic tissue damage induced by acetic acid application, and to demonstrate the probable recovery in the colon tissue with nesfatin-1 therapy and the possible underlying mechanism nesfatin-1 acts on.
The anti-apoptotic, neuroprotective (13), antihyperglycemic (38), anxiogenic, anorexigenic (39) effects of nesfatin-1 and in addition, the role of nesfatin-1 in delaying gastric emptying (24) and in the cardiovascular control (40) were revealed by previous studies. This is the first study that shows the anti-inflammatory effect of nesfatin-1 in an experimental colitis model by ameliorating inflammatory status and enhancing the biochemical and histological parameters. Previously, the anti-inflammatory effect of intraperitoneally administrated nesfatin-1 was shown in an acedic acid induced gastritis model by Kolgazi et al. (12). Additionally, in a recent study its essential role in healing process of chronic gastric ulcers via activating the gastric blood flow at ulcer margin and also its inducing role in the mucosal regeneration was reported (41). As the possible beneficial effects of peptides on inflammation models attrack attention and are frequently experimentally investigated, recently, the reparative and healing activity of another intraperitoneally administrated peptide such as stable gastric pentadecapeptide BPC157 supported the nature of certain endogenous peptides in amelioration of colitis (42). However, our study focuses on the central administration of nesfatin-1 and peripheral anti-inflamatory effects in a colitis model. Furthermore, neuroprotective effects of nesfatin-1 in a brain injury model induced by subarachnoid hemorrhage and improved sensitivity of central nesfatinergic system to peripheral inflammatory stimuli was reported (11, 13). Bonnet et al. conveyed the association between nesfatin-1 neurons and peripheral inflammation and pointed out activation of central nesfatin-1-expressing neurons during an inflammatory stimulus such as LPS stimulated endotoxaemia and reported that the neurons of the central nesfatinergic system were sensitive to peripheral inflammatory stimulus and were belong to a specific immunosensitive neurocircuitry (11). Similarly, peripheral inflammatory status such as colitis may stimulate central nesfatinerjic system to ameliorate the inflammatory process. Thus, we aimed to demonstrate the possible protective effect of i.c.v. administered nesfatin-1 in a colitis model. However, additional studies are required to analyze improved central nesfatin-1 activity in colitis.
The macroscopic and microscopic damage scores of colonic tissue was evaluated histopathologically and increased in rats with colon injury significantly and was consistent with the results of a previous study on an acetic acid-induced colitis model (43). Although application of nesfatin-1 ameliorated the increased scores in the colitis group, the atosiban application has reduced both macroscopic and microscopic damage inhibitory effect of nesfatin-1. This result suggests that the reducing effect of nesfatin-1 on damage scores might be via oxytocin receptors. Previously, nesfatin-1 has been shown to use the oxytocinergic signaling pathway while regulation of food intake. Similar to its well known satiety controlling effect, the interaction with oxytocin might be an intermediate pathway for nesfatin-1 in its anti-inflammatory effect (15). Although the macroscopic damage reducing effect of nesfatin-1 continued with the application of GHSR-1a, the microscopic damage reducing effect was eliminated. This situation suggests that ghrelin mediates the microscopic damage reducing effect of nesfatin-1. Similarly, in the light microscopic histological examination, the damage was continued in the groups treated with GHSR-1a and atosiban, as nesfatin-1 was not able to show healing effect in the groups treated with these antagonists prior to nesfatin-1 application.
Myeloperoxidase is an enzyme especially presented in neutrophils, and also in monocytes and macrophages in a very small amount. Therefore, MPO activity is directly proportional to the quantity of neutrophils in inflamed tissues, and measurement of MPO activity is considered to be a sensitive and quantitative index of acute intestinal inflammation (44). In our study, an increase in colonic MPO activity was observed via acetic acid administration that suggests increased neutrophil infiltration in the colon tissue following damage induction. Although there is no previous study showing the effect of nesfatin-1 on colitis, it has been shown that intraperitoneal administration of nesfatin-1 inhibited the increase in myeloperoxidase activity of the brain tissue in a SAH model (13) and also of the gastric tissue with acetic acid induced ulcer model (12). Similarly, our MPO results suggest that the application of nesfatin-1 may prevent colonic tissue damage by reducing the neutrophil infiltration. The reduction in MPO activity continued also in groups receiving antagonists, GHSR-1a and atosiban, while SHU9119 application prior to nesfatin-1 prevented this reduction. These results indicate that melanocortin receptors may mediate the effect of nesfatin-1 on neutrophil infiltration. Furthermore, it is known that alpha-melanocyte-stimulating hormone causes anti-inflammatory effects on colitis and melanocortin-1 receptor (MC-1R) has role in this anti-inflammatory function (45). In the study conducted by Maaser et al. on C57BL / 6 and MC-1R mutant mice, the protective effect of MC-1R was demonstrated in colitis model induced by dextran sodium sulfate (44). Although the relationship between tissue MPO activity and melanocortin receptors have been pointed out in previous studies, our study suggests that the effect of nesfatin-1 on MPO activity may be via melanocortin-1 receptors.
It is well known that malondialdehyde, the end product of lipid peroxidation, is detected in increasing amounts with damage (43) and in our study this damage were clearly manifested in the groups treated with acetic acid. Nesfatin-1 has been demonstrated to ameliorate the MDA levels in a few inflammatory processes (12, 13). Consistent with previous studies, in this study MDA levels were reduced with nesfatin-1 treatment, moreover were elevated with all antagonist applications. Eventually, nesfatin-1 may be effective in restoring continuity of cells by reduction of lipid peroxidation via suppression of malondialdehyde production and may play a role on ghrelin, oxytocin and melanocortin receptors in this function.
Glutathione molecule is an important antioxidant for the protection against free oxygen radicals and is oxidized with peroxidase enzyme and eliminates the hydrogen peroxide radical (30, 46). Consistent with a previous study, serious depletion in the antioxidant glutathione levels was observed in the colitis group with colonic damage (43), however in our study nesfatin-1 treatment was not able to increase antioxidant glutathione levels. According to our results nesfatin-1 may not effect on antioxidant glutathione levels during its anti-inflammatory action.
SOD is in a class of enzymes that catalyze the dismutation of the oxygen and hydrogen peroxide, and is primary protective against oxyradicals such as superoxide that is a major reactive oxygen specy in the cells (47). The effect of nesfatin-1 treatment on the activity of SOD in colitis is not known yet. According to our results, although nesfatin-1 treatment did not create significant change in SOD activity compared to the colitis group, in previous inflammatory models nesfatin-1 increased the SOD activity (12, 13). The reason for lack of significant change in SOD activity of nesfatin-1-treated group compared to colitis group may be due to the non-significant decrease in SOD levels of colitis group, although there was a tendency to decrease (P = 0.22). If the SOD levels were decreased significantly in colitis group, the levels would be increased to the control levels with the treatment by nesfatin-1. Eventually, nesfatin-1 treatment inhibited the decrease tendency that occurs in SOD levels with the colitis induction.
The main antioxidant effect of catalase is breaking down H2O2 into water and molecular oxygen. Although H2O2, is formed via SOD, is not a radical, is a precursor of OH- radical which is the most reactive free oxygen radical (47). In this study, antioxidant catalase activity was ameliorated via colitis induction, and was not significantly different than control with nesfatin-1 treatment. Similarly, catalase activity of colonic tissue was reduced in ulcerative colitis models (48, 49). Additionally, our results suggest that nesfatin-1 may inhibit the decrease in antioxidant catalase activity observed via colitis induction. Moreover, the catalase activity of the group treated with atosiban showed a trend of decrease which was not significantly different compared to the nesfatin-1-treated group and decreased significantly compared to the control group. Although the inhibitory effect of nesfatin-1 on reduction of catalase activity observed in the colitis group is not clear, the additional studies are needed to determine whether it occurs via oxytocin receptors.
In accordance with previous data, along with the significant increase of tissue myeloperoxidase activity and the alleviation of lipid peroxidation in colitis, the rise in luminol and lucigenin chemiluminescence levels point out the production of reactive oxygen metabolites in the tissue (43). The increase in luminol and lucigenin chemiluminescence were reduced in the group treated with nesfatin-1, and the reduction of luminol chemiluminescence disappeared in the groups treated with GHSR-1a and atosiban, although the reduction of lucigenin chemiluminescence inhibited by all receptor antagonists. This data suggests that the applications of GHSR-1a and atosiban inhibited the effect of nesfatin-1 and nesfatin-1 may have a role on ghrelin and oxytocin receptors in its reactive oxygen metabolite decreasing effect. As lucigenin is more specific to superoxide, nesfatin-1 may show its superoxide decreasing effect via all these antagonists especially GHSR-1a or by interfering with other pathways. Although effect of ghrelin and oxytocin receptors in luminol and lucigenin chemiluminescence levels of inflammatory processes is not known, previously reducing effect of ghrelin and oxytocin on these reactive oxygen parameters were demonstrated (50, 51). Addition to previous data, our results suggest that nesfatin-1 may use these pathways in its reactive oxygen metabolite reducing effect.
Consequently, it was observed that the oxidative damage developing in colitis model induced by acetic acid administration recovered with application of intracerebroventricular nesfatin-1 and this peptide reduced the microscopic and macroscopic damage with its anti-inflammatory effects. Our results suggest that nesfatin-1 may demonstrate these anti-inflammatory effects by preventing neutrophil infiltration to the tissue and by suppressing the free radical formation. The application of atosiban and GHSR-1a prevented the effect of nesfatin-1 on microscopic damage, lipid peroxidation, luminol and lucigenin chemiluminescence levels. At the same time, atosiban application also caused a regression in the macroscopic damage. These findings indicate that nesfatin-1 may show its anti-inflammatory and antioxidant effects on colitis via oxytocin and ghrelin receptors. Additionally, melanocortin receptor antagonist inhibited the protective effect of nesfatin-1 on MPO activity, lipid peroxidation, lusigenin chemiluminescence and glutathione levels. Although it has been shown previously that nesfatin-1 has used the melanocortin signaling pathway for its effects on food intake, this is the first study suggesting that nesfatin-1 may also use the receptors which mediate its anorexigenic effects in terms of the anti-inflammatory effects.
Acknowledgements: The authors are grateful to Dr. Serap Sirvanci for histological analysis and declare that there was no conflict of interest. This study was supported by a grant from the Marmara University Scientific Research Committee (BAPKO), SAG-C-YLP-110412-0068.
REFERENCES
- Shanahan F. Pathogenesis of ulcerative colitis. Lancet 1993; 342: 407-411.
- Karakoyun B, Uslu U, Ercan F, et al. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci 2011; 89: 402-407.
- Kasimay O, Guzel E, Gemici A, et al. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol 2006; 91: 897-906.
- Dogan Z, Ergul B, Sarikaya M, et al. The antioxidant effect of Echinacea angustifolia and Echinacea purpurea in rat colitis model induced by acetic acid. Bratisl Lek Listy 2014; 115: 411-415.
- Oh-I S, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006; 443: 709-712.
- Brailoiu GC, Dun SL, Brailoiu E, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 2007; 148: 5088-5094.
- Kohno D, Nakata M, Maejima Y, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus co-express oxytocin and vasopressin and are activated by refeeding. Endocrinology 2008; 149: 1295-1301.
- Stengel A, Tache Y. Minireview: nesfatin-1 - an emerging new player in the brain-gut, endocrine, and metabolic axis. Endocrinology 2011; 152: 4033-4038.
- Zhang AQ, Li XL, Jiang CY, et al. Expression of nesfatin-1/ NUCB2 in rodent digestive system. World J Gastroenterol 2010; 16: 1735-1741.
- Stengel A, Goebel M, Yakubov I, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 2009; 150: 232-238.
- Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation 2009; 6: 27.
- Kolgazi M, Cantali-Ozturk C, Deniz R, et al. Nesfatin-1 alleviates gastric damage via direct antioxidant mechanisms. J Surg Res 2015; 193: 111-118.
- Ozsavci D, Ersahin M, Sener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery 2011; 68: 1699-1708.
- Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol 2009; 297: R330-R336.
- Maejima Y, Sedbazar U, Suyama S, et al. Nesfatin-1- regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 2009; 10: 355-365.
- Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 2008; 156: 563-579.
- Palasz A, Krzystanek M, Worthington J, et al. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides 2012; 46: 105-112.
- Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 2010; 298: R1642-R1647.
- Stengel A, Tache Y. Interaction between gastric and upper small intestinal hormones in the regulation of hunger and satiety: ghrelin and cholecystokinin take the central stage. Curr Protein Pept Sci 2011; 12: 293-304.
- Konturek PC, Brzozowski T, Engel M, et al. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol 2009; 60: 41-47.
- Deboer MD. Use of ghrelin as a treatment for inflammatory bowel disease: mechanistic considerations. Int J Pept 2011; 2011: 189242.
- Kerbel B, Unniappan S. Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. J Neuroendocrinol 2012; 24: 366-377.
- Kasimay O, Iseri SO, Barlas A, et al. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res 2006; 36: 11-19.
- Stengel A, Goebel M, Wang L, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology 2009; 150: 4911-4919.
- Asad M, Shewade DG, Koumaravelou K, Abraham BK, Vasu S, Ramaswamy S. Effect of centrally administered oxytocin on gastric and duodenal ulcers in rats. Acta Pharmacol Sin 2001; 22: 488-492.
- Sibilia V, Torsello A, Pagani F, et al. Effects of hexarelin against acid-independent and acid-dependent ulcerogens in the rat. Peptides; 2004: 25: 2163-2170.
- Tahan G, Aytac E, Aytekin H, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg 2011; 54: 333-338.
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. San Diego, Academic Press, 1986.
- Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78: 206-209.
- Aykac G, Uysal M, Yalcin AS, Kocak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology 1985; 36: 71-76.
- Casini AF, Ferrali M, Pompella A, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol 1986; 123: 520-531.
- Mylroie AA, Collins H, Umbles C, Kyle J. Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 1986; 82: 512-520.
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105: 121-126.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969; 244: 6049-6055.
- Haklar G, Ulukaya-Durakbasa C, Yuksel M, Dagli T, Yalcin AS. Oxygen radicals and nitric oxide in rat mesenteric ischemia-reperfusion: modulation by L-arginine and N-nitro-L-arginine methyl ester. Clin Exp Pharmacol Physiol 1998; 25: 908-912.
- McCafferty DM, Sharkey KA, Wallace JL. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol 1984; 266: G560-G567.
- Gue M, Bonbonne J, Fioramonti J, et al. Stress induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol 1997; 272: G84-G91.
- Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun 2010; 391: 1039-1042.
- Colmers WF. Less fat with nesfatin-1. Trends Endocrinol Metab 2007; 18: 131-132.
- Mimee A, Smith PM, Ferguson AV. Nesfatin-1 influences the excitability of neurons in the nucleus of the solitary tract and regulates cardiovascular function. Am J Physiol Regul Integr Comp Physiol 2012; 302: R1297-R304.
- Szlachcic A, Majka J, Strzalka M, et al. Experimental healing of preexisting gastric ulcers induced by hormones controlling food intake ghrelin, orexin-A and nesfatin-1 is impaired under diabetic conditions. A key to understanding the diabetic gastropathy? J Physiol Pharmacol 2013; 64: 625-637.
- Klicek R, Kolenc D, Suran J, et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol 2013; 64: 597-612.
- Kolgazi M, Uslu U, Yuksel M, Velioglu-Ogunc A, Ercan F, Alican I. The role of cholinergic anti-inflammatory pathway in acetic acid-induced colonic inflammation in the rat. Chem Biol Interact 2013; 205: 72-80.
- Fabia R, Rajab AR, Willen R, Marklund S, Andersson R. The role of transient mucosal ischemia in acetic acid-induced colitis in the rat. J Surg Res 1996; 63: 406-412.
- Maaser C, Kannengiesser K, Specht C, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut 2006; 55: 1415-1422.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
- Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn's disease. World J Gastroenterol 2013; 19: 6540-6547.
- Kuralay F, Yildiz C, Ozutemiz O, et al. Effects of trimetazidine on acetic acid-induced colitis in female Swiss rats. J Toxicol Environ Health A 2003; 66: 169-179.
- Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol 2013; 19: 5633-5644.
- Iseri SO, Sener G, Yuksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol 2005; 187: 399-406.
- Tugtepe H, Sener G, Biyikli NK, et al. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept 2007; 140: 101-108.
A c c e p t e d : July 1, 2015