NEW CATIONICALLY MODIFIED PULLULAN ATTENUATES ATHEROGENESIS AND INFLUENCES LIPID METABOLISM IN apoE-KNOCKOUT MICE
2Faculty of Chemistry, Jagiellonian University, Cracow, Poland
INTRODUCTION
Nowadays biopolymers of natural origin have found many applications in pharmaceutical and food industries and in other sectors because of their beneficial physicochemical properties (1). One of the examples of this kind of compounds is chitosan, which is a unique polymer as the only natural cationic polysaccharide (2, 3). However, synthetic polymers also have wide therapeutical potential, but they often show toxicity, poor hemocompatibility, and low biodegradability (2, 4, 5). Therefore, new biocompatible polymeric materials from natural sources are searched for, such as pullulan, which is non-mutagenic, non-toxic, non-carcinogenic, biodegradable, edible and adhesive (1, 6-8). This nonionic polysaccharide occurs in nature as a component of the cell wall of the common yeast-like fungus Aureobasidium pullulans. In the structure of the pullulan chain there are three glucose units connected with α-1,4 glycosidic bonds (maltotriose units) (9-11). Consecutive maltotriose units are connected with α-1,6 glycosidic bonds. Most metal ions, for example sodium, influence viscosity of pullulan solutions which also changes in different pH conditions (1, 8). On the other hand, change in temperature does not significantly affect pullulan solution viscosity.
Biomedical applications of pullulan and its derivatives
Pullulan is a water soluble polysaccharide and, like dextran, it can be used as a potential blood-plasma substitute (1, 12). As a plasma expander, this polymer performs best if its molecular weight is about 60 kDa. What is more, it has been reported that also pullulan modified anionically via gamma irradiation was used as a base for blood-plasma substitute (1, 12). Moreover, modified pullulan can be used in medical imaging (1, 13), as an anti-cancer drug in tumor cell targeting (1, 14), as a carrier for gene delivery (1, 15, 16) and drug delivery (1, 17-23), and in tissue engineering (1, 24, 25). Pullulan has been safely used for over 20 years in Japan as a pharmaceutical bulking agent and as a food additive (26). The allowed daily consumption of this polysaccharide is 10 g as evaluated by the Food and Drug Administration (FDA) (26).
Cationic modification of pullulan and its specificity for the liver
Pullulan is also used to deliver various substances to the liver, because of its specificity for this organ (26-28, 11). It was reported that vectors based on the cationic pullulan were applied in tumor suppressor gene delivery to the liver (26, 29). Binding affinity of the modified pullulan to liver cells was also studied both in vivo in mice and in vitro on human hepatocyte cell line HepG2 (26, 30). The cationic pullulan, obtained by reaction with GTMAC formed polyionic complexes with DNA that showed stability in plasma (26, 30). The uptake of nano-complexes with a cationic pullulan by hepatocytes, hemocompatibility of modified pullulan and in vitro transfection in studies of directional delivery of genes into liver cells were also studied (26, 30).
Polysaccharides such as pullulan and dextran after intravenous injection are delivered to the liver and can therefore be used as drug carriers (27, 31). Studies in rats have shown that the distribution of pullulan in the liver is mediated by asialoglycoprotein receptor (27, 28, 31). In vitro studies on the rat liver cells have shown that during incubation pullulan is specifically taken up by the parenchymal cells of the liver (27). Pullulan chain branching and the position of the hydroxyl groups play an important role in intracellular disposition of polysaccharides and are the cause of the differences in the distribution between the dextran and pullulan. These properties are important in view of the specific drug targeting to the liver cells using these polymers (27).
What is particularly important, in vitro studies demonstrated that the cationically modified pullulan, obtained by reaction with GTMAC, has specific affinity towards hepatocytes, leading to increased uptake of nanoplexes of derivatized pullulan by the liver cells (30). In vivo studies in BALB/c mice also confirmed uptake of the nanoplexes by hepatocytes and showed that this phenomenon is time dependent. What is more, it was observed that plasmid pullulan-based nanocomplexes were evenly distributed throughout the cytoplasm and in the region of the nucleus after internalization to HepG2 cells (30).
Contrary to the previous reports suggesting that pullulan is an indigestible carbohydrate (33- 36), it has been also shown that pullulan is slowly digested in human digestive system (33). It was found that pullulan is slowly digested in both the cooked and raw form. Referring to the conclusions of this experiment postprandial blood glucose excursion after pullulan ingestion in humans was studied (33). The decrease of incremental peak blood glucose concentration after consumption of pullulan by nondiabetic healthy adult subjects was found in comparison with a control equivalent maltodextrin challenge. There were small fluctuations in the level of glucose in the blood in 180 min period of time, suggesting that pullulan is slowly digested. It was concluded that pullulan ingestion attenuates the postprandial glycemic excursion. What is more, an increased breath hydrogen excretion and gastrointestinal intolerance symptoms after ingestion of pullulan were found, suggesting malabsorption of some pullulan portion (33).
Although it is known that pullulan is a food additive slowly digested in the gastrointestinal system of humans and that it prevents from the increase of blood glucose level, the influence of this polymer on lipid metabolism and on progression of atherosclerosis has remained unexplained so far. To show the impact of cationically modified pullulan on lipid metabolism and atherosclerosis and to characterize physicochemical properties of this polymer we have recently synthesized cationically modified pullulan (Pull-GTMAC) by covalently substituting pullulan with GTMAC. Pull-GTMAC has been administered orally to apoE-knockout mice (apoE(–/–) mice, that constitute a murine model of atherosclerosis, at a dose of 300 mg/kg b.w./day for 18 weeks and its anti-atherosclerotic activity and influence on the lipid metabolism were studied.
MATERIALS AND METHODS
Materials
Pullulan from Aureobasidium pullulans, Mw = 200 kDa (Sigma); glycidyltrimethylammonium chloride technical, ≥ 90%, GTMAC (Aldrich); sodium hydroxide G.R., NaOH (Lach:ner); RPMI Medium 1640 (1×) (Gibco); Fetal Bovine Serum (FBS) (Gibco); MTT reagent (3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, approx. 98%, TLC) (Sigma); dimethyl sulfoxide (DMSO), minimum 99.5% GC (Sigma); enoxaparin Clexane 40 mg/0.4 ml, Enoxaparinum natricum solution for injection (Sanofi Aventis); n-hexane, p. a. (Chempur, Poland), hexane Chromasol V®, for HPLC, ≥ 97.0% (GC) (N-Hexane) (Sigma-Aldrich, Germany); anhydrous sodium sulfate, Na2SO4, ≥ 99.0% (Sigma-Aldrich) dried at 100°C; acetone, p.a. (POCH, Poland); acetone p.a., CH3COCH3 (Chempur); phosphate buffered saline, 10 × concentrate, BioPerformance Certified (PBS at pH 7.4, concentrate dissolved 10 × in distilled water for using in experiments) (Sigma); Kilik cryostat embedding medium (Bio-Optica); glass microscope slides SuperFrost® (Menzel Glaser); poly-L-lysine solution 0.1% w/v in water; thimerosal 0.01% added as preservative (Sigma-Aldrich); Oil Red O (Sigma); Rneasy® Fibrous Tissue Mini Kit, (Qiagen); RevertAidTM Reverse Transcriptase (Fermentas); 10 mM dNTPs Ultrapure Mix (EURx); 5 × Reaction Buffer for M-MulVRT (Fermentas); RiboLockTM RNase Inhibitor (Fermentas); PPM24937F-200: RT2 qPCR Primer Assay for Mouse Ldlr (Qiagen); PPM02946E-200: RT2 qPCR Primer Assay for Mouse Gapdh (Qiagen); SybrGreen Jump StartTMTaq MixTM Ready for quantitative PCR, in MgCl2 buffer (Sigma); Water Biotechnology Performance Certified (Sigma-Aldrich).
Experimental animals and treatment
All animal procedures were approved by the Local Ethics Committee for the Animal Experiments at Jagiellonian University. In the carried out experiments 20 homozygous female apoE-knockout mice were used (Taconic, Denmark) with nomenclature: APOE-F B6.129P2-Apoetm1UncN11 homozygous mice at the age of seven to eight weeks, with the genetic background of C57BL/6J mice. Females were divided randomly into two groups: a control group with size of 14 mice (n = 14), not receiving the tested chemical compound in the diet, and a research group of 6 mice (n = 6) fed with diet containing Pull-GTMAC at a dose of 300 mg/kg b.w./day which has been given for 18 weeks. The mice were quarantined and fed with Labofeed H fodder on a chow diet (containing 5% fat; ‘Morawski’ Works of Feed, Poland) mixed with cationic pullulan for 18 weeks in the Animal House of the Chair of Immunology at Faculty of Medicine at Jagiellonian University Medical College in the daily cycle of light and darkness (the length of the light corresponding to the natural day length in spring and summer), in the conditions of temperature range from 21°C to 22°C and humidity of 60%. The animals were kept in ad libitum access to food and water.
Synthesis of cationically modified pullulan (Pull-GTMAC)
Nine grams of pullulan (pullulan from Aureobasidium pullulans, Mw = 200 kDa, Sigma) was dissolved in 450 ml of distilled water with constant stirring using magnetic stirrer. Then, 130 ml of glycidyltrimethylammonium chloride (GTMAC) and water mixture was added (before addition, 80 ml of GTMAC had been mixed with 50 ml of distilled water). While the mixture of pullulan and GTMAC was mixed in a 1000 ml round-bottomed flask, 20 ml of 5 M NaOH was added. Next, the reaction mixture was heated for 14 h at 80°C with constant stirring. The reaction mixture was cooled and dialyzed for 5 days against distilled water with controlling the conductivity of the water until it fell below 4 µS (microsiemens). Finally, the solution was concentrated using rotary evaporator and freeze-dried.
Chemical analysis of the cationic pullulan derivative
The zeta potential of 1 mg/ml Pull-GTMAC solution in physiological saline (0.1 M) at pH = 7.4 was measured in folded capillary cells using Zetasizer Nano-ZS (Malvern Instruments) at 25°C. In order to confirm the structure of the Pull-GTMAC, the FT-IR spectra of pullulan and the derivative were recorded using a Bruker IFS 48 526 spectrometer and the elemental analysis was performed using Vario Micro CHNS elemental analyzer (Elementar). Based on the results of elemental analysis the substitution degree of pullulan with GTMAC was calculated according to the following formula:
where:
DS is degree of substitution, mN - percentage by mass of nitrogen (N) in a Pull-GTMAC sample (mean from two elemental analyses), mC - percentage by mass of carbon (C) in a Pull-GTMAC sample (mean from two elemental analyses), MW(N) - atomic weight of nitrogen, MW(C) - atomic weight of carbon.
Cytotoxicity study on HepG2 cell line
Human liver hepatocellular carcinoma cells (HepG2 cells) were incubated with Pull-GTMAC in varied concentrations for 24 hours and 48 hours. The experiments were repeated several times (incubation of cells in 6 wells of cell culture plates for each concentration of the compound). The tests were performed on the cells of the HepG2 cell line cultured in RPMI cell culture medium with 5% (w/w) of FBS serum (fetal bovine serum). Incubation of the cells was performed with Pull-GTMAC at the following concentrations: 0, 0.1, 0.3, 1, 3, 10, 30, 100 µg/ml of cell culture medium. The solutions with given substance dissolved in NaCl2 was administered in a volume of 20 µl per one well of 96-well plate. The control sample (relative to the incubation with the cationic polysaccharide) constituted 20 µl of 0.9% NaCl-solution per well with HepG2 cells.
Procedure of incubation of HepG2 cells with Pull-GTMAC and MTT test
The cells were dropped on 96-well plates (TPP Company, Switzerland) in the number of 30,000 cells per well containing 180 µl of RPMI medium with 5% FBS. HepG2 cells were incubated for 24 and 48 hours with cationically modified pullulan in an incubator (incubator Heracell 240, Heraeus Instruments) at 37°C under an atmosphere containing 5% CO2. After 24 and 48 hours 20 µl of 0.2% MTT solution in NaClag was added to each well containing cells for 2 hours of incubation at 37°C. Next, the medium containing MTT was removed from wells and the plates were frozen and incubated at –20°C overnight. Then, the plates were stirred after adding 200 µl DMSO per each well. Absorbance from each well of plate was read at the wavelength of 562 nm on a plate reader (Epoch).
Procedure of murine feces collection
After 16 weeks of apoE-knockout mice feeding with a diet containing Pull-GTMAC at a dose of 300 mg/kg b.w./day the feces were gathered at the end of 72-hour collection of stool from both research and control groups. All droppings from each group were counted. Feces from 72-hour collection were weighed, frozen and stored at –20°C. The material was collected in order to carry out the extraction of raw fat by the method of Soxhlet continuous extraction.
Extraction of fat by the method of Soxhlet continuous extraction
The extraction of the fat was carried out from a stool samples of apoE-knockout mice both from the control group fed with a feed containing no tested compound (14 animals in the group) and from the tested group fed with a feed containing cationic pullulan (6 animals in the group) for 16 weeks. Extraction was performed with n-hexane using a Soxhlet apparatus and the ratio of the weight of extracted fat and the total weight of the sample was calculated. Three extractions using the samples from the tested group and five extractions using the material from the control group were carried out. Results for each group are presented as the mean value calculated from data obtained from 3-5 measurements with the standard error of the mean (S.E.M.). The final results were obtained as the average content of raw fat (H) in a group of mice with S.E.M. and expressed as a percentage score.
Procedure of the Soxhlet continuous extraction
Stool sample was kneaded and rubbed into a homogenous mass. Next, 6 g of the prepared sample was weighed and then mixed with 6 g of anhydrous sodium sulfate and placed in an extraction thimble. Round-bottomed 100 ml volume flask was put in the heating bath (at 90 – 95°C) and 50 – 60 ml of n-hexane was poured carefully into it. The flask was connected with the Soxhlet extractor and the extraction thimble containing the sample was placed in the extraction tube of Soxhlet apparatus. The extractor was combined with the condenser tube. The sample was extracted for 5 cycles (overflows). After the extraction the solvent was evaporated using rotary evaporator. Next, 2 ml of acetone was added to the flask containing the extract and gently mixed. The flask was placed on a rotary evaporator and acetone was evaporated. The flask content was dried for about 10 minutes in a stream of an inert gas (argon or nitrogen). Then, the flask with the extract was dried for 10 minutes in a vacuum oven at about 100°C. The flask was cooled and weighed on an analytical balance with an accuracy of 1 mg. The raw fat content in the sample of feces (H) was calculated and expressed in percent. Calculations were performed according to the following formula:
where: m0 - mass of the stool sample, m1 - mass of the empty flask, m - mass of the flask containing the extract.
Procedure of tissue isolation
After feeding with feed mixed with cationically modified pullulan for 18 weeks, mice were heparinized via intraperitoneal administration of 10 mg of enoxaparin and euthanized by CO2 inhalation.
At first, the blood was taken by puncture to the right ventricle of heart. Next, the auricle of right atrium was incised. The vascular system was perfused with PBS at pH 7.4 with a puncture of the left ventricular apex at constant pressure. Then, a fragment of the liver was incised and submerged in RNA stabilizing buffer as a material for the study of gene expression. Thoracic and abdominal part of aorta and heart were purified in situ and resected. The resected heart was cut in half with a scalpel and the upper part of the heart with aortic root was dipped into cryostat gel. Then, the heart was snap-frozen and stored at –80°C. Plasma was obtained by centrifuging of blood with an acceleration of 2,000 × g for 10 min at 4°C. The plasma was stored at –80°C.
Analysis of plasma lipid profile of apoE-knockout mice
Plasma lipids analysis was made in apoE-knockout mice fed for 18 weeks with a feed containing Pull-GTMAC (6 mice). The control plasma samples were derived from the mice fed with feed containing no cationic polysaccharide in the diet (n = 6 in group).
Levels of various lipid fractions were marked (total cholesterol, LDL, HDL, triglycerides) for groups of apoE-knockout mice fed with feed containing cationic pullulan. Levels of lipid fraction expressed in mmol/l of plasma were presented as a mean ± S.E.M. for all mice in a group. The volume of plasma samples for testing was approximately 210 µl. The research was conducted in the liquid phase of the plasma in the Laboratory of Biochemistry (Department of Diagnostics, University Hospital in Cracow) using automatic biochemical analyzers: for total cholesterol the CHOD-PAP method of analysis was used on Hitachi 917 and Modular P analyzers, for HDL cholesterol - the direct enzymatic-colorimetric method by Abell-Kendall on Hitachi 917 and Modular P analysers, for cholesterol LDL (DIRECT) - the enzymatic method on Modular P analyser, for triglycerides - the GPO-PAP method on Hitachi 917 and Modular P analysers.
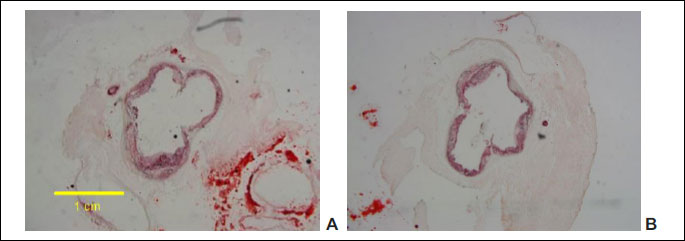
Quantification of the area of atherosclerotic plaque
The cross-sections of the aortic root was performed according to the standard protocol (37-39). At first, the series of 10 adjacent cryostat sections was cut using a cryostat (Jung cryostat CM 1800, Leica) every 100 µm from the proximal 1 mm long segment of aortic root at –20°C. The collecting of sections was started at starting point which was situated in 100-µm distance from place where there was the appearance of the aortic valves (using microscope to watch the frozen scraps up to date) and the sections were gathered at 100-µm intervals. The sections were collected on glass microscope slides (for cryostat) covered with 10 µl (per 1 glass) solution of poly-L-lysine. After air drying the sections were fixed and stained with oil red O dye (ORO), which is applied in staining of fats, triglycerides and lipoproteins. The method of fixation was based on incubating the frozen sections for 10 min in buffered 4% formalin (formaldehyde solution in phosphate buffer at pH = 7.4) and washing twice for 5 minutes in distilled water. Preparations stained with ORO were covered with cover slips in gelatin-glycerol mixture (glycerogelatin). Then, microscopic pictures of cross-sections of the aortic root were taken using Camedia 5050 digital camera (Olympus) and BX50 microscope (Olympus) with a 4 times magnifying lens. The pictures were taken under magnification of 80 times and stored as .jpeg files with resolution of 2040 × 1536 pixels. A computer morphometric image analysis was conducted in order to evaluate the area of the plaque using a LSM 5 Image Browser (Zeiss) computer program. The results for the mice were presented as the average area of atherosclerotic plaques located on 9 sections spaced about 100 µm. The mean area of atherosclerotic plaque with S.E.M. for both groups of mice was calculated (the mean of areas measured for all individuals in the group). Six mice were used in the method of cross-section of the aorta in the group receiving the feed with Pull-GTMAC and 14 animals were used in the group receiving no tested compound.
Analysis of lipid metabolism gene expression
The study of LDLR (low density lipoprotein receptor) gene expression was performed using real-time PCR method on cDNA material from the liver of apoE-knockout mice fed with a diet containing Pull-GTMAC at a dose of 300 mg/kg b.w./day for 18 weeks. RNA isolation was carried out by column-based method using RNA isolation kit for tissues. 1000 ng of isolated RNA was dissolved in RNase free water in final volume of 10.7 µl used for a sample prepared to the reverse transcription reaction. After adding to the sample 0.9 µl of 3 times diluted (in RNase-free H2O) solution of oligonucleotide primers, denaturation of RNA was performed at 72°C for 10 min in T3 Thermocycler (Biometra). Next, 8.4 µl of a reaction mixture was added, consisting of: 0.9 µl of reverse transcriptase in concentration of 200 units per µl (U/µl), 2 µl of buffer of triphosphate deoxynucleotides mixture, 4 µl of buffer for reverse transcriptase, 1.5 µl of RNase inhibitor at a concentration of 40 U/µl. The samples were incubated in a thermal cycler with the following temperature profile: annealing (hybridization of the primers): 42°C for 2 hours, elongation: 70°C for 10 min. For performing the real-time PCR reaction mixtures of sense and antisense primers were used: for murine receptor for low density lipoprotein (mouse LDLR), and for mouse glyceraldehyde-3-phosphate (mouse GAPDH) as a reference gene. Commercial main solutions of the primer mixture (primer assays) were used at a concentration of 10 µM for each of the genes. Next, cDNA was 10 times diluted in RNase-free water. To wells of 96-well plate 13 µl of reaction mixture was added, containing:
7.5 µl of a mixture containing the SYBR Green dye,
1 µl of mixture of primers (10 µM),
4.5 µl of RNase-free water.
Then, 2 µl of diluted cDNA was added to the wells in 3 replicates for each of the genes (GAPDH and LDLR). The plate was transferred to the thermocycler for real-time PCR (7900 HT fast real-time PCR System with 96 Wells Fast Block, Applied Biosystems). The following temperature profile was used in the reaction of real-time PCR: Step 1: 95.0°C for 10:00 minutes; Step 2: (40 cycles) 95.0°C for 0:30 min, 60.0°C for 1:00 min (annealing), 72.0°C for 0:45 min; Step 3: (step of dissociation): 95.0°C for 0:15 min, 60.0°C for 0:15 min, 95.0°C for 0:15 min. The results of the real-time PCR reaction was initially analyzed using the Sequence Detection Systems Version 2.4 software (Applied Biosystems). Next, analysis of the results was performed using DDCt method. The number of mice in groups for the reaction was 3 individuals in the tested group and control group. Optimized results of the real-time PCR reaction were presented as the mean value of expression change (in relative units) for 3 mice ± S.E.M.
Statistical analysis
Continuous variables (for example area of the atherosclerotic plaque) were expressed as the arithmetic mean ± S.E.M. (where S.E.M. is a standard error of the mean). The T-test was used for statistical analysis of the results. The P value < 0.05 was considered as statistically significant.
RESULTS
Chemical characterization
In the FT-IR spectrum of Pull-GTMAC the band at a wavenumber of 1470 cm–1, characteristic for methyl groups of the quaternary amine, was observed, that has not been present in native pullulan (Fig. 1). This band clearly confirmed the cationic modification (2, 40, 41). Also, as found from the elemental analysis, the modified pullulan contained 3% of nitrogen, while this element has not been present in unmodified pullulan. The corresponding degree of substitution of Pull-GTMAC was calculated to be 71.25%. The zeta potential of Pull-GTMAC at pH 7.4 was +15.2 mV (mean from three replicates).
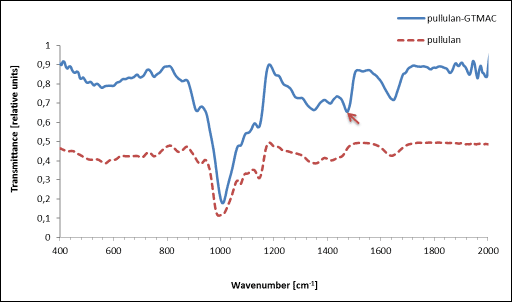 |
Fig. 1. FT-IR spectrum of pullulan (dotted line) and pullulan-GTMAC (solid line, pointer- the band characteristic for methyl groups of the quaternary amine) |
Cytotoxicity of the cationically modified pullulan
Pull-GTMAC exhibited antiproliferative properties in MTT test performed on HepG2 cell line. There was statistically significant decrease of HepG2 cells viability induced by pullulan-GTMAC at concentrations of 0.1, 0.3, 1.0, 3.0 µg/ml of cell culture medium after 24 hour incubation as well as after 48 hours of incubation at concentrations of 0.3, 1.0, 3.0 µg/ml (Fig. 2). However, cationically modified pullulan increased cell viability after 48 hour incubation at concentration of 100 µg/ml.
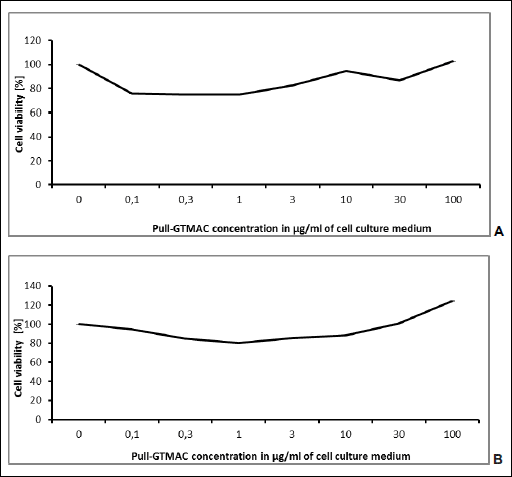 |
Fig. 2. Results of MTT test performed after 24 hour (A) and 48 hour of incubation (B) of HepG2 cells with Pull-GTMAC |
Influence of Pull-GTMAC on fat content in apoE(–/–) mice feces
It was found that under influence of Pull-GTMAC given at a dose of 300 mg/kg b.w./day the average daily mass of feces increased by 35% and the average number of droppings excreted by apoE(–/–) mouse increased by 8 pieces in relation to the control mice fed with feed without the tested compound (Fig. 3A and 3B). However, raw fat content in the feces of mice decreased by 26% in the group of apoE-knockout mice fed with the diet containing Pull-GTMAC compared to the control group of animals (average raw fat content at level of 1.415 ± 0.131% in control group versus 1.042 ± 0.035% in Pull-GTMAC treated group, T-test, P = 0.022; Fig. 3C). What is more, the average mass of fat in one piece of feces (in one dropping) decreased by 14% in the group after cationic pullulan treatment in relation to control group of mice, but this result is not statistically significant (T-test, P = 0.131). Finally, after taking into account the daily mass of feces excreted by apoE(–/–) and number of animals in group, the average mass of fat excreted per one mouse in group per day is 0.01461 g in the control group and 0.01429 g in the group treated with Pull-GTMAC. To sum up, the average mass of fat excreted daily per one mouse decreased by 2.2% under influence of Pull-GTMAC comparing to the control without statistical significance (T-test, P = 0.419).
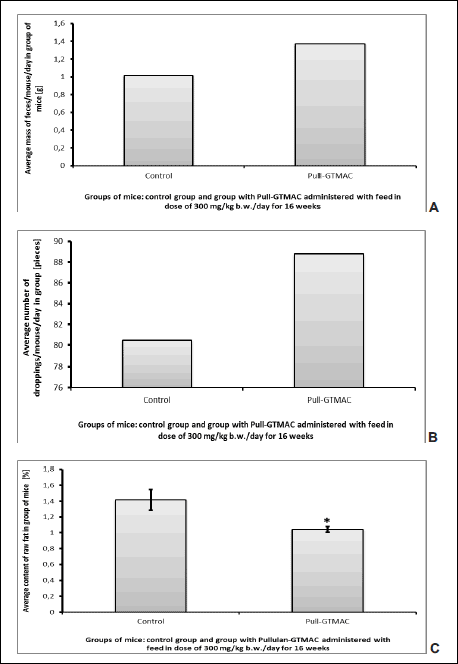 |
Fig. 3. Effect of Pull-GTMAC on fecal fat content in apoE(–/–) mice: (A) average mass of feces excreted by apoE-knockout mouse per day, (B) average number of droppings excreted by apoE-knockout mouse per day, (C) raw fat content (H) in apoE-knockout mice feces after feeding with feed containing Pull-GTMAC (mean ± S.E.M.). *P < 0.05 versus control. |
Analysis of plasma lipid profile after Pull-GTMAC treatment
No statistically significant changes in the levels of individual lipid fractions after oral administration of cationically modified pullulan (Pull-GTMAC) at a dose of 300 mg/kg b.w./day for 18 weeks were found in relation to the control group in studies on apoE-knockout mice (Fig. 4).
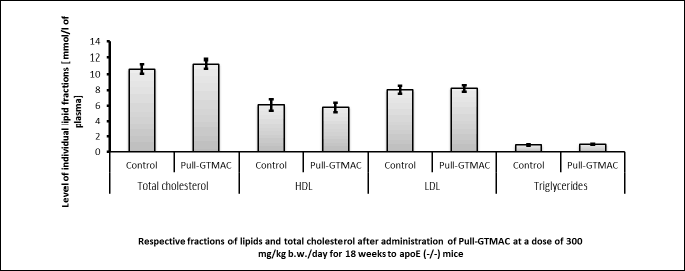
Effect of Pull-GTMAC on atherosclerotic plaque development
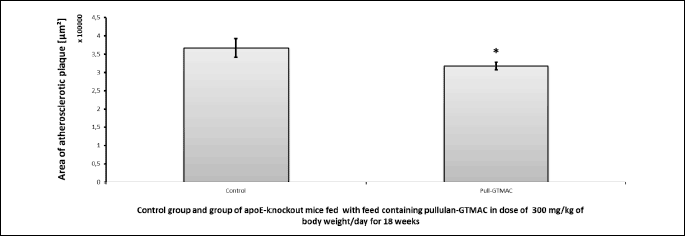
In the experiment performed in apoE-knockout mice fed with feed containing Pull-GTMAC at a dose of 300 mg/kg b.w./day for 18 weeks (using the cross-section method of aortic root imaging) a statistically significant reduction of atherosclerotic plaque under influence of cationically modified pullulan by 13% was observed compared to the control group of mice whose diet did not contain the tested compound (T-test, P = 0.047; Figs. 5 and 6). Measured lesion area was 3.67×105 ± 0.26×105 µm2 in the control group versus 3.17×105 ± 0.10×105 µm2 in Pull-GTMAC-treated group of mice. These results indicate that cationically modified pullulan attenuates atherogenesis.
Influence of cationically modified pullulan on expression of lipid metabolism gene (LDLR) in the liver
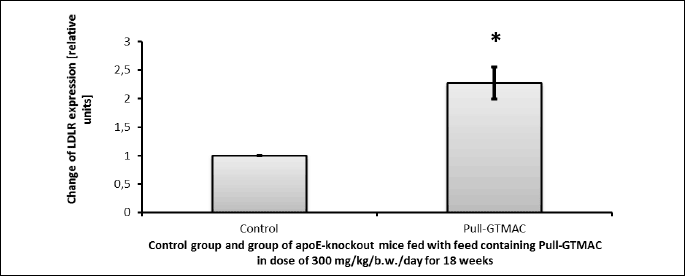
It was shown that cationically modified pullulan has a significant impact on the expression of gene involved in lipid metabolism in in vivo studies in a murine model of atherosclerosis. It is noted that Pull-GTMAC at a dose of 300 mg/kg/b.w./day administered orally for 18 weeks caused a statistically significant increase of mRNA level of LDLR (upregulation of LDLR gene) in apoE-knockout mice livers in relation to the control group of mice (T test, P = 0.025; Fig. 7).
DISCUSSION
In order to study the early stages of atherogenesis animal models were used, such as homozygous apolipoprotein E-deficient mouse (apoE-knockout mouse) which spontaneously develops atherosclerosis (42). The aim of the research described in this article was to characterize the physicochemical properties and to examine the impact of a cationically modified pullulan (Pull-GTMAC) on lipid metabolism and atherogenesis in murine model of atherosclerosis (apoE-knockout mice) after oral administration. On the basis of the experiments it was shown that the cationic pullulan inhibits atherosclerotic plaque development and influences the amount of fat excreted with the feces. However, the mechanism of Pull-GTMAC acting on lipid metabolism remains unclear. It was previously shown, that pullulan is internalized inside hepatocytes via asialoglycoprotein receptor (ASGPR) mediated endocytosis (27) and that pullulan modified with GTMAC has specific affinity towards hepatic cells (30). What is especially interesting, the galactose-specific ASGPR binds and internalizes lipoprotein (a) (a highly atherogenic plasma lipoprotein) and is involved in in vivo catabolism of this molecule (43). It was also shown that atherosclerosis is an inflammatory disease (42), and ASGPR are the receptors involved in regulation of the immune system (44). It is believed that activation of asialoglycoprotein receptors by Pull-GTMAC and internalization of this polysaccharide into hepatic cells may influence the lipid metabolism and thus cause inhibition of atherogenesis.
We have also shown that cationically modified pullulan increased the mass of apoE(–/–) mice excreted feces and daily number of droppings. The high molecular weight unmodified pullulan (high-MW pullulan, MW = 100,000 Da) shows similar effect in dogs after oral administration twice daily with diet containing 30% of the tested polysaccharide and 15% fat (45). Dogs fed with high-MW pullulan had higher fecal scores (indicating looser stools) and fecal output than dogs after maltodextrin, γ-cyclodextrin or low molecular weight native pullulan (low-MW pullulan, MW = 6300 Da) treatment. On the other hand, dogs consuming low-MW pullulan, γ-cyclodextrin or maltodextrin had higher dry mass of excreted feces than those treated with high-MW pullulan (45). What is interesting, it was also presented that C57BL/6j mice fed with a high fat diet (60% energy from fat and 0.03% w/w cholesterol) had by about 48% higher level of fecal total lipids than mice on a chow diet (46). Probably, Pull-GTMAC decreased raw fat content in apoE(–/–) mice feces which became close to normal level in normal mice. It has been previously reported that both apoE-knockout mice being on a chow diet and C57Bl/6N mice on a high fat western diet had elevated level of total serum lipids in relation to wild-type mice on a chow diet (47, 48). It is also possible that after Pull-GTMAC treatment mass of apoE(–/–) mice feces increased because it underwent compensatory regulations in relation to considerable decrease of fat in the stool.
In this article it has also shown that cationically modified pullulan did not exhibit the lipid-lowering effect in the serum but despite that it had antiatherogenic properties by reducing an antiatherogenic plaque development. Thus, it can be assumed that the mechanism of anti-atherogenic action is independent on the effect of this compound on serum lipids profile. It is believed that between the chains of Pull-GTMAC and cholesterol molecules there are present (in certain specific areas) an electrostatic interactions between the –N(CH3)3+ groups of Pull-GTMAC and the OH groups of cholesterol as same as in interactions between cationically modified chitosan substituted by GTMAC (chitosan HTCC) and cholesterol (based on molecular simulation conjectures) (2, 49).
What is more, based on research conducted under this publication it seems that pullulan modified with glycidyltrimethylammonium chloride is a nontoxic polymer. Although it exhibited antiproliferative properties in studies on HepG2 cells (liver cancer cell line) but it was a weak effect (up to 25% of decrease of cell viability after 24 hour incubation) and occurred only in certain concentrations of Pull-GTMAC (for example: in concentration of 1 µg/ml of cell culture medium). It seems to be also a safe compound to oral administration because the tested animals have eaten Pull-GTMAC with feed for 18 weeks and they did not indicate any distressing symptoms connected with condition of digestive system in comparison to the control group of mice. On the other hand, the studies on HepG2 cancer cells indicated an anticancer potential of cationically modified pullulan. But, to explain particular mechanism of anticancer activity of this compound further research should be done to determine whether the drug acting is dependent on the cell cycle (and phase-specific) or not. What is more, it seems that pullulan chains could also create pseudo-micelles with cholesterol molecules. Possibly, a similar effect may also include fatty acids and their salts as in the case of cationically modified chitosan (2).
Pull-GTMAC influenced also an expression of lipid metabolism gene. The results of the real-time PCR reaction have indicated that the Pull-GTMAC caused statistically significant increase of mRNA level for LDL receptor in the apoE(–/–) mice liver after administration at a dose of 300 mg/kg/b.w./day for 18 weeks. It is thought that cationically modified pullulan, internalized by hepatocytes via asialoglycoprotein receptors, can influence on lipid metabolism by regulation of lipid metabolism genes expression and thus it shows antiatherogenic action. Perhaps the mechanism of action of this polymer is similar to the effects caused by statins. It was reported that after 48 hours of in vitro incubation of HepG2 cells with lovastatin an expression of HMG-CoAR (3-hydroxy-3-methylglutaryl-CoA reductase) was decreased and the expression of LDLR was increased, as well as under influence of eicosapentaenoic acid (50).
To our knowledge, this is the first report that describes the effect of cationically modified pullulan (Pull-GTMAC) on atherogenesis and its influence on level of fecal lipid in apoE-knockout mice model of atherosclerosis. We showed that cationic pullulan (Pull-GTMAC) has antiatherogenic potential and influences on lipid metabolism regulating lipid metabolism gene expression. However, particular mechanism of this polymer action still remains unclear and requires further research.
It seems to be very important to check the effect of cationically modified pullulan in another than apoE-knockout mice model such as, for example, female mice model with targeted replacement of the endogenous mouse ApoE gene with human ApoE4 or ApoE3 gene with a C57Bl/6 background (51, 52). The genotypes differ from one another in proinflammatory response. The mice with ApoE4 genotype had a more advanced inflammation than mice with ApoE3 genotype due to a high production of 5-oxoeicosatetraenoic acid in ApoE4 carriers (51-53). Although the apoE-knockout mice model is well known and widely used rodent model of atherosclerosis (54-56), in the model of transgenic mice that carry human alleles the cardiovascular risk can also be rated (51, 57). It is interesting that in the comparative studies the ApoE4 allele was posed a greater cardiovascular risk than the ApoE3 allele (51, 57). The higher lipid accumulation in hepatocytes isolated from both transgenic mice strains carrying the human alleles than in wild-type controls was also observed (51). Furthermore, after incubation of primary culture hepatocytes, isolated from the transgenic strains of mice with acetaminophen (APAP) it was found that mice with ApoE3 and ApoE4 alleles showed higher susceptibility to acute APAP in vitro toxicity than wild-type mice (51). Comparing the two tested transgenic genotypes, the individuals carrying ApoE3 allele were more prone to injury. On the other hand, it was also shown that the expression of Bcl-2, an antiapoptotic protein, was downregulated in isolated hepatocytes after treatment with APAP but the decline was more pronounced in ApoE4 and WT than in ApoE3 cells (51).
Acknowledgements: Authors JS and RK acknowledge the financial support from the grant from National Science Centre, Poland (NCN) No 2014/13/N/NZ7/00255 for years 2015-2017.
Conflict of interests: None declared.
REFERENCES
- Prajapati VD, Jani GK, Khanda SM. Pullulan. An exopolysaccharide and its various applications. Carbohydr Polym 2013; 95: 540-549.
- Stefan J, Lorkowska-Zawicka B, Kaminski K, Szczubialka K, Nowakowska M, Korbut R. The current view on biological potency of cationically modified chitosan. J Physiol Pharmacol 2014; 65: 341-347.
- Riva R, Ragelle H, Des Rieux A, Duhem N, Jerome C, Preat V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv Polymer Sci 2011; 244: 19-44.
- Itaka K, Ishii T, Hasegawa Y, Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials 2010; 31: 3707-3714.
- Moreau E, Domurado M, Chapon P, Vert M, Domurado D. Biocompatibility of polycations: in vitro agglutination and lysis of red blood cells and in vivo toxicity. J Drug Target 2002; 10: 161-173.
- Kimoto T, Shibuya T, Shiobara S. Safety studies of a novel starch, pullulan: chronic toxicity in rats and bacterial mutagenecity. Food Chem Toxicol 1997; 35: 323-329.
- Shingel KI. Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr Res 2004; 339: 447-460.
- Tsijisaka Y, Mitsushashi M. Pullulan. In: Industrial Gums. Polysaccharides and Their Derivatives, Whistler RL, BeMiller JN (eds.), San Diego Academic Press, 1993; pp. 447-460.
- Bulman SE, Coleman CM, Murphy JM, Medcalf N, Ryan AE, Barry F. Pullulan: a new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res Ther 2015; 6: 34. doi: 10.1186/s13287-015-0011-7
- Leathers TD. Biotechnological production and applications of pullulan. Appl Microbiol Biotechnol 2003; 62: 468-473.
- Rekha MR, Sharma CP. Pullulan as a promising biomaterial for biomedical applications: a perspective. Trends Biomater Artif Organs 2007; 20: 116-121.
- Shingel KI, Petrov PT. Behaviour of γ-ray-irradiated pullulan in aqueous solutions of cationic (cetyltrimethylammonium hydroxide) and anionic (sodium dodecyl sulfate) surfactants. Colloid Polym Sci 2002; 280: 176-182.
- Nobuyuki M, Takao E, Michiko O, Yasuhiko I, Kazunari A. Hybrid nanogels with physical and chemical cross-linking structures as nanocarriers. Macromol Biosci 2005; 5: 710-716.
- Scomparin A, Salmosa S, Bersani S, Satchi-Fainaro R, Caliceti P. Novel folated and non-folated pullulan bioconjugates for anticancer drug delivery. Eur J Pharm Sci 2011; 42: 547-558.
- Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Liver targeting of plasmid DNA by pullulan conjugation based on metal coordination. J Control Release 2002; 83: 287–302.
- Gupta M, Gupta AK. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J Control Release 2004; 99: 157-166.
- Wooram P, Kyoung SK, Byoung-chan B, Young-Heui K, Kun N. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur J Pharm Sci 2010; 40: 367-375.
- Ajay G, Mona G. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005; 26: 1565-1573.
- Kim EJ, Cho SH, Yuk SH. Polymeric microspheres composed of pH/temperature sensitive polymer complex. Biomaterials 2001; 22: 2495-2499.
- Kurkuri MD, Aminabhavi TM. Poly (vinyl alcohol) and poly (acrylic acid) sequential interpenetrating network pH-sensitive microspheres for the delivery of diclofen sodium to the intestine. J Control Release 2004; 96: 9-20.
- Fundueanu G, Constantin M, Ascenzi P. Preparation and characterization of pH- and temperature-sensitive pullulan microspheres for controlled release of drugs. Biomaterials 2008; 29: 2767-2775.
- Fundueanu G, Constantin M, Mihai D, et al. Pullulan-cyclodextrin microspheres. A chromatographic approach for the evaluation of the drug - cyclodextrin interactions and the determination of the drug release profiles. J Chromatogr B Analyt Technol Biomed Life Sci 2003; 791: 407-419.
- Mocanu G, Mihai D, Le Clerf D, Picton L, Muller G. Synthesis of new associative gel microspheres from carboxymethyl pullulan and their interactions with lysozyme. Eur Polym J 2004; 40: 283-289.
- Na K, Lee DH, Hwang DJ, Park HS, Lee KH, Bae YH. pH-sensitivity and pH dependent structural change in polymeric nanoparticles of poly (vinyl sulfadimethoxine)-deoxycholicacidconjugate. Eur Polym J 2006; 42: 2581-2588.
- Gao J, Yu J, Wang W, Chang L, Tian R. Graft copolymerization of starch - an initiated by potassium permanganate. J Appl Polym Sci 1998; 68: 1965-1972.
- Bishwambhar M, Suneetha V, Kalyani R. The role of microbial pullulan, a biopolymer in pharmaceutical approaches: a review. J Appl Pharm Sci 2011; 1: 45-50.
- Tanaka T, Fujishima Y, Hanano S, Kaneo Y. Intracellular disposition of polysaccharides in rat liver parenchymal and nonparenchymal cells. Int J Pharm 2004; 286: 9-17.
- Kaneo Y, Tanaka T, Nakano T, Yamaguchi Y. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J Control Release 2001; 70: 365-373.
- Jo J, Yamamoto M, Matsumoto K, Nakamura T, Tabata Y. Liver targeting of plasmid DNA with a cationized pullulan for tumor suppression. J Nanosci Nanotechnol 2006; 6: 2853 - 2859.
- Rekha MR, Sharma CP. Blood compatibility and in vitro transfection studies on cationically modified pullulan for liver cell targeted gene delivery. Biomaterials 2009; 30: 6655-6664.
- Davis BG, Robinson MA. Drug delivery systems based on sugar-macromolecule conjugates. Curr Opin Drug Discov Devel 2002; 5: 279-288.
- Lee M, Kim SW. Polymeric gene carriers. Pharmaceutical News 2002; 9: 407-415.
- Wolf BW, Garleb KA, Choe YS, Humphrey PM, Maki KC. Pullulan is a slowly digested carbohydrate in humans. J Nutr 2003; 133: 1051-1055.
- Nakamura S. Pullulan [in Japanese]. J Synth Org Chem Jpn 1984; 42: 584-588.
- Okada K, Yoneyuama M, Mandai T, Aga H, Sakai S, Ichikawa T. Digestion and fermentation of pullulan. J Jpn Soc Nutr Food Sci 1990; 43: 23-29.
- Oku T, Yamada K, Hosoya N. Effect of pullulan and cellulose on the gastrointestinal tract of rats [in Japanese]. Nutr Diets 1979; 32: 235-241.
- Jawien J, Gajda M, Wolkow P, Zuranska J, Olszanecki R, Korbut R. The effect of montelukast on atherogenesis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 2008; 59: 633-639.
- Jawien J, Gajda M, Olszanecki R, Korbut R. BAY × 1005 attenuates atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 2007; 58: 583-588.
- Jawien J, Gajda M, Rudling M, et al. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Invest 2006; 36: 141-146.
- Kaminski K, Szczubialka K, Zazakowny K, Lach R, Nowakowska M. Chitosan derivatives as novel potential heparin reversal agents. J Med Chem 2010; 53: 4141-4147.
- Kaminski K, Plonka M, Ciejka J, et al. Cationic derivatives of dextran and hydroxypropylcellulose as novel potential heparin antagonists. J Med Chem 2011; 54: 6586-6596.
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol Mech Dis 2006; 1: 297-329.
- Hrzenjak A, Frank S, Wo X, Zhou Y, van Berkel T, Kostner GM. Galactose-specific asialoglycoprotein receptor is involved in lipoprotein (a) catabolism. Biochem J 2003; 376: 765-771.
- Saunier B, Triyatni M, Ulianich L, Maruvada P, Yen P, Kohn LD. Role of the asialoglycoprotein receptor in binding and entry of hepatitis C virus structural proteins in cultured human hepatocytes. J Virol 2003; 77: 546-559.
- Spears JK, Karr-Lilienthal LK, Grieshop CM, Flickinger EA, Wolf BW, Fahey GC. Pullulans and g-cyclodextrin affect apparent digestibility and metabolism in healthy adult ileal cannulated dogs. J Nutr 2005; 135: 1946-1952.
- Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010; 24: 4948-4959.
- Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol 2004; 55: 503-517.
- Desmarchelier Ch, Dahlhoff Ch, Keller S, Sailer M, Jahreis G, Daniel H. C57Bl/6 N mice on a western diet display reduced intestinal and hepatic cholesterol levels despite a plasma hypercholesterolemia. BMC Genomics 2012; 13: 84. doi: 10.1186/1471-2164-13-84.
- Parra-Barraza H, Burboa MG, Sanchez-Vazquez M, Juarez J, Goycoolea FM, Valdez MA. Chitosan-cholesterol and chitosan-stearic acid interactions at the air-water interface. Biomacromolecules 2005; 6: 2416-2426.
- Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Synergic effect of eicosapentaenoic acid and lowastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis 2010; 9: 135. doi: 10.1186/1476-511X-9-135.
- Mezera V, Kucera O, Moravcova A, et al. Comparison of acetaminophen toxicity in primary hepatocytes isolated from transgenic mice with different apolipoprotein E alleles. J Physiol Pharmacol 2015; 66: 863-873.
- Graeser AC, Boesch-Saadatmandi C, Lippmann J, et al. Nrf2-dependent gene expression is affected by the proatherogenic apoE4 genotype-studies in targeted gene replacement mice. J Mol Med (Berl) 2011; 89: 1027-1035.
- Stachowska E, Maciejewska D, Ossowski P, et al. Apolipoprotein E4 allele is associated with substantial changes in the plasma lipids and hyaluronic acid content in patients with nonalcoholic fatty liver disease. J Physiol Pharmacol 2013; 64: 711-717.
- Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 2004; 24:1006-1014.
- Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol 2011; 2011: 497841. doi: 10.1155/2011/497841
- Kus K, Wisniewska A, Toton-Zuranska J, Olszanecki R, Jawien J, Korbut R. Significant deterioration of antiatherogenic efficacy of nebivolol in a double (apolipoprotein E and endothelial nitric oxide synthase) knockout mouse model of atherosclerosis in comparison to single (apolipoprotein E) knockout model. J Physiol Pharmacol 2014; 65: 877-881.
- Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 442-448.
A c c e p t e d : September 9, 2016