CATIONIC PORPHYRIN-MEDIATED PHOTODYNAMIC INACTIVATION
OF CANDIDA BIOFILMS AND THE EFFECT OF MICONAZOLE
INTRODUCTION
Oral candidiasis in the form of Candida-associated denture stomatitis (CaDS) is of significant clinical concern and, is a common disease in a large percentage of denture wearers. The disease is associated with Candida adhesion and biofilm formation on the poly(methyl methacrylate) (PMMA) denture base and on epithelial surfaces. It is characterized by inflammation, chronic erythema and edema of the mucosa, particularly in areas of contact with the acrylic denture plate (1-3).
Denture trauma and pathogenic Candida infections are regarded as the main etiologic agents of CaDS. Predisposing factors of the disease include systemic diseases, immune deficiencies, impaired salivary gland function, continuous denture wearing, drug therapy (antibiotics, corticosteroids) and smoking (4-8).
Candida albicans and related species play a major role in initiating, maintaining, and aggravating the disease (7, 9). C. albicans is still considered to be the major etiologic agent of oral candidiasis. C. glabrata is the most prevalent non-albicans Candida species isolated in oral candidiasis in patients with diabetes, advanced cancer, HIV infection and patients suffering from CaDS (10-13).
CaDS is strongly associated with Candida adherence and biofilm formation on the fitting surface of dentures made of PMMA (14-16). Candida biofilms consist of a mixture of yeasts, pseudohyphae and hyphae embedded in an extracellular matrix. Higher virulence and pathogenicity of Candida biofilms are associated with up-regulation of genes related to cell adhesion, hyphal proliferation and secreted aspartyl proteinases (5, 6, 17). Candida biofilms on acrylic denture samples from patients with CaDS, examined by scanning electron microscopy, consist primarily of yeasts (blastospores), with a small number of hyphal elements. These biofilms adhere to the fitting surface of the denture, along cracks and defects of the denture acrylic (18, 19).
C. albicans biofilms are highly resistant to antifungal and antimicrobial agents, including amphotericin B, nystatin, fluconazole and chlorhexidine (18, 20, 21). This biofilm-related resistance may partially explain the high recurrence rates often observed in CaDS (15, 18, 22). The increasing resistance of C. albicans to antifungal agents has stimulated an interest in photodynamic therapy (PDT) as an alternative treatment (23-25).
PDT is a medical treatment that utilizes light of a specific wavelength to activate a photosensitizing agent (photosensitizer; PS) in the presence of oxygen. The exposure of the PS to light results in the formation of reactive oxygen species (ROS), such as singlet oxygen and free radicals, causing localized photodamage and cell death. Application of PDT in dentistry may include (i) photodynamic diagnosis (PDD) and treatment of pre-malignant/malignant lesions, and (ii) antimicrobial photodynamic therapy (aPDT) of bacterial and fungal infections (26). Several studies have investigated the fungicidal effect of aPDT against planktonic Candida, employing a variety PSs and light activating sources. Candida biofilms, germ tubes of C. albicans, and non-albicans strains have been used less frequently (24).
The azole antifungal, miconazole (Mcz), used commonly in the topical treatment of CaDS, inhibits ergosterol synthesis by targeting the lanosterol 14α-demethylase and generating toxic sterol intermediates. In addition, miconazole induces the production of ROS both in Candida planktonic cultures and in biofilms (27-29). Snell et al. (30) reported that by inducing ROS production, Mcz (25 µg/ml) increases the photoactivity of the porphyrin-based photosensitizer TMP-1363 (10 µg/ml) against planktonic C. albicans SC5314. Noteworthy, although ketoconazole also stimulates ROS production in C. albicans, it does not enhance the killing of C. albicans. As proposed by Snell et al. (30), Mcz-induced changes in the mitochondrial and plasma membranes may be potentially involved in the enhancement of the photodynamic effects of TMP-1363 on Candida cell viability. However, the effect of Mcz on TMP-aPDT of Candida biofilms has not been studied previously.
TMP-1363 at 10 or 20 µm has been shown to inactivate completely biofilms of C. albicans ATCC-10231, using a total energy dose of 43.2 J/cm2 (31). We evaluated the photodynamic activity of TMP-1363 towards mature biofilms of C. albicans, C. glabrata, C. tropicalis and C. parapsilosis, formed on denture acrylic discs, and assessed the combined action of miconazole and TMP-1363. We used Mcz and TMP-1363 at the same concentrations as those reported by Snell et al. (30) for planktonic C. albicans SC5314, to minimize the parameters in this initial study.
MATERIAL AND METHODS
Fungal strains
Seven oral Candida isolates were used throughout the study. Three strains were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA): C. albicans GDH18 (ATCC MYA-274), C. albicans UTR-14 (ATCC MYA-2732) and C. glabrata GDH1407 (ATCC MYA-275). Stock cultures were stored in liquid nitrogen, sub-cultured on Sabouraud dextrose agar (BD Difco, Sparks, MD, USA) and stored at 4°C. Four clinical isolates, C. albicans 6122/06, C. glabrata 7531/66, C. tropicalis 8122/06 and C. parapsilosis 11375/06 were isolated from patients with documented Candida-associated denture stomatitis attending the Department of Prosthetic Dentistry, Poznan University of Medical Sciences, after the patient’s informed consent had been obtained under a protocol approved by the Bioethics Committee. These patients were not on any antimycotic therapy. Swabs were collected from areas of palatal mucosa, inoculated onto Sabouraud’s medium with chloramphenicol (bioMerieux SA, Marcy-l’Etoile, France) and incubated at 37°C for 48 hours. After obtaining pure cultures, the microorganisms were identified by the commercially available test kit ID 32 C (bioMerieux SA). Stock cultures were stored at –20°C.
Candida biofilm formation
A loopful of the yeast was inoculated in Yeast Nitrogen Base medium (YNB) (BD Difco) supplemented with 100 mM glucose, and incubated overnight at 37°C. Cells were harvested, washed with PBS, and standardized to 1 × 107 cells/ml YNB-glucose. Biofilms were developed on heat-cured PMMA discs (5 mm diameter × 1.5 mm thick) that were polished with waterproof silicon carbide paper (grit p600), obtained from Hing Lung Engineering Inc. (Hong Kong, China). Discs were soaked in 70% ethanol for 15 min, dried at room temperature and submerged in the standardized Candida suspension in 48-well plates (1 disc/well). In order to maneuver the disks sterile tweezers were used to grip the disks by the sides so the top and bottom surfaces were not tampered with. The plate was placed on a titer plate shaker model 4625 (Lab-Line Instruments, Melrose, IL, USA), designated to produce an accurate 0.120-inch orbital shaking motion, and incubated for 90 min at 37°C at 75 rpm (adherence phase). Non-adherent cells were removed from the discs by washing with PBS. The discs were then transferred to a 96-well plate, submerged in YNB/100 mM glucose (1 disc/250 µl/well) and incubated for 48 hours at 37°C on the titer plate shaker at the same setting (biofilm formation phase).
Photodynamic treatment
The cationic photosensitizer 5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrin tetra tosylate (C72H66N8O12S4; mw.1363) (TMP-1363) (Frontier Scientific Inc., Newark, DE, USA) was dissolved in phosphate-buffered saline (PBS). The stock solution (5 mg/ml) was diluted in PBS to obtain the desired concentration. Miconazole (Mcz; nitrate salt; Sigma-Aldrich, St. Louis, MO, USA) was solubilized in dimethyl sulfoxide (DMSO). The stock solution (5 mg/ml) was diluted in culture medium to obtain the desired final concentration of 25 µg/ml (0.5% DMSO).
The 48-h Candida biofilms were incubated with Mcz (25 µg/ml; 52.2 µM) or without Mcz, for 2 hours at 37°C, followed by treatment with the PS, TMP-1363 (10 µg/ml; ~7.3 µM) for 30 min at 37°C. Untreated biofilms were used as controls. The values obtained for biofilms exposed to Mcz alone (for 2 h 30 min) and TMP-1363 alone (30 min) constitute the controls for the effect of Mcz on TMP-aPDT. We used Mcz and TMP-1363 at the concentrations used previously for planktonic C. albicans SC5314, to limit the number of parameters and compare the sensitivity of biofilms and planktonic microorganisms; however, we investigated seven oral Candida isolates under these conditions.
The plates were exposed to broadband visible light (350 – 800 nm) from a Dura Max light bulb (75W Med 120V A19 Cl/LL 20W; Philips Electronics North America Corporation, Andover, MA, USA), at a distance of 10 cm to the plate, for 30 min, at room temperature. The irradiance at the surface of the plate was 32.5 mW/cm2 (light dose of 58.5 J/cm2), as measured by an RD 0.2/2 radiometer with a TD probe (Optel Vision, Quebec City, Canada). Infrared radiation was minimized using a 1 cm water filter between the cell plates and the light source. Biofilms treated with TMP-1363 alone, but shielded from light, were used for the evaluation of dark toxicity. In the absence of light, TMP-1363 did not reduce the metabolic activity of Candida biofilms (data not shown).
Metabolic activity (XTT reduction assay)
Several methods have been used for quantification of Candida biofilms. Commonly used methods are based on: (i) exposure to tetrazolium salts, (ii) determination of dry weight, (iii) determination of the number of colony forming units (CFU), and (i.v.) measurement of adenosine triphosphate (ATP)-bioluminescence (32). Tetrazolium salts assays provide some of the most reproducible, accurate and widely used colorimetric assays for measuring the metabolic activity of mammalian and microbial cells. Second generation of the dyes, e.g. XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) are negatively charged and largely cell-impermeable. They form water-soluble formazans and are used in conjunction with an intermediate electron acceptor such as 1-methoxy-5-methylphenazinium methylsulfate (mPMS) or menadione, to facilitate dye reduction. A considerable amount of evidence indicates that XTT reduction occurs at the cell surface, or at the level of the plasma membrane via trans-plasma membrane electron transport (33). Ramage et al. (34) adapted the XTT assay for testing antifungal susceptibility of fungal biofilms, using a 96-well microplate platform. Chandra et al. (20) reported that this assay could be used to quantify the antifungal susceptibilities of denture-associated Candida biofilms.
Counting the number of colony forming units (CFU) from Candida biofilm was one of the earliest methods used to quantify biofilm formation. In this method, biofilm cells are scraped into the medium and the resulting suspension is inoculated on agar media plates, incubated for up to 24 – 48 hours, and the resultant CFU are counted. Data are expressed as CFU/ml and a log10 reduction compared to untreated organisms. A CFU assay has been used mostly for testing antifungal activity with planktonic Candida cells (32, 33).
In this study, metabolic activities of the biofilms were measured by the XTT reduction assay (20, 34). XTT (Sigma) is reduced into a water-soluble brown formazan product that is measured spectrophotometrically. Discs containing no Candida cells served as controls. After removal of YNB medium, 200 µl of the XTT-menadione solution 158 µl PBS, 40 µl XTT (1 mg/ml), 2 µl menadione (0.4 mM in acetone) was added to each well. After incubation at 37°C for 3 – 5 hours, 150 µl of the solution was transferred to a new well in a 96-well plate, and the optical density of the formazan product was measured at 490 nm (OD490), using a Molecular Devices Versamax microtiter plate reader (Sunnyvale, CA, USA). The metabolic activities of the biofilms were expressed as a percentage of the control OD490 (100%; untreated biofilm). The relative percentage changes then defined the antifungal efficacy of experimental conditions. The control OD490 values were in the range from 0.750 to 1.400 (average = 1.040 ± 0.180).
Statistical analysis
Statistical software (Stat ViewTM, BrainPower Inc., Calabasas, CA, USA) was used for analysis of the data. The results are expressed as mean ± standard deviation of two or three independent experiments each performed in triplicate. Statistical analysis was performed with Student’s t-test for unpaired values. A P value of < 0.05 was considered statistically significant.
RESULTS
Previous studies demonstrated that miconazole enhances the sensitivity of planktonic C. albicans SC5314 to the photodynamic activity of the porphyrin-based photosensitizer, TMP-1363; (30); but the effect of miconazole on TMP-aPDT against Candida biofilms was not investigated. Here, we evaluated whether the combined use of miconazole and TMP-aPDT enhances the photodynamic activity of TMP-1363 against Candida biofilms formed on denture acrylic by three ATCC strains and four clinical isolates. The metabolic activity of the biofilms was measured by the XTT assay. Statistical significances between control (untreated biofilm) and treated biofilm, determined by P values, are presented in Fig. 1 and Fig. 2. The treatment of biofilms with miconazole and TMP-1363, without exposure to light, did not affect metabolic activity to a greater degree than miconazole alone. For example, the viability of C. tropicalis was 71.1 ± 5.2% following treatment with miconazole alone, and 65.2 ± 4.7% after treatment with miconazole + TMP-1363 in the dark. Thus, the effects of miconazole + TMP-1363 were irradiation-dependent.
ATCC strains
TMP-1363 alone was ineffective in aPDT (TMP-PDT) against C. albicans MYA-274 and C. glabrata MYA-275, and only slightly effective against C. albicans MYA-2732 (viability: 86.4 ± 11.9% of control) (Fig. 1). Miconazole alone reduced the metabolic activity of C. albicans MYA-2732, and C. glabrata MYA-275 biofilms by approximately 25% and 45%, respectively, but had no effect on C. albicans MYA-274.
The combination of TMP-1363 and Mcz reduced significantly (P < 0.0005) the metabolic activity of the biofilms by approximately 60%, 46% and 58%, for C. albicans MYA- 2732, C. albicans MYA-274 and C. glabrata MYA-275, respectively. For C. albicans MYA-274, neither TMP-aPDT nor miconazole alone caused cytotoxicity (Fig. 1B), but their combination caused significant cytotoxicity (P < 0.0005), suggesting a synergistic interaction. For C. glabrata MYA-275, the values of OD490 obtained for Mcz alone and TMP-aPDT + Mcz were not significantly different (P < 0.35) (Fig. 1C).
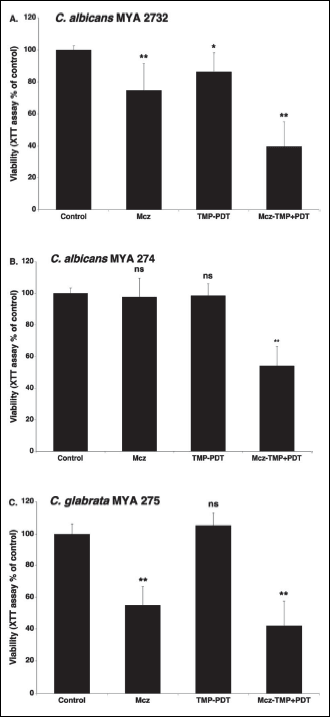 |
Fig. 1. Photodynamic activity of TMP-1363 (10 µg/ml; 7.3 µM) against Candida biofilms (ATCC isolates) in the absence and presence of miconazole (25 µg/ml; 52.2 µM): C. albicans MYA 2732 (A), C. albicans MYA 274 (B) and C. glabrata (C). The metabolic activity of the biofilms was measured by the XTT assay and expressed as a percentage of the control OD490 (untreated biofilm). The control OD490 values were in the range from 0.750 to 1.400 (average = 1.040 ± 0.180). Each value is a mean ± standard deviation (S.D.) of two or three independent experiments performed in triplicate. Asterisks indicate significant differences compared with control (untreated biofilm) (*P < 0.005, **P < 0.00005). |
Clinical isolates
Miconazole alone reduced the metabolic activity of biofilms of C. albicans 6122/06, C. glabrata 7531/06, C. tropicalis 8122/06 and C. parapsilosis 11375/06 by approximately 26%, 48%, 30% and 47%, respectively (Fig. 2). The photodynamic activation of TMP-1363 alone reduced the metabolic activity of the biofilms by approximately 40%, 35% and 30%, for C. albicans, C. tropicalis and C. parapsilosis, respectively. C. glabrata was not sensitive to TMP-aPDT, under the experimental conditions used in our study. The combined use of TMP-1363 and miconazole reduced the metabolic activity of biofilms of C. albicans, C. glabrata, C. tropicalis and C. parapsilosis, by approximately 73%, 50%, 56% and 68%, respectively. For C. glabrata 7531/06, the viabilities obtained with miconazole alone or TMP-aPDT + miconazole were not significantly different (P < 0.35). For the other clinical isolates, the combined treatment, TMP-aPDT + miconazole, was significantly (P < 0.0005) more effective than miconazole alone.
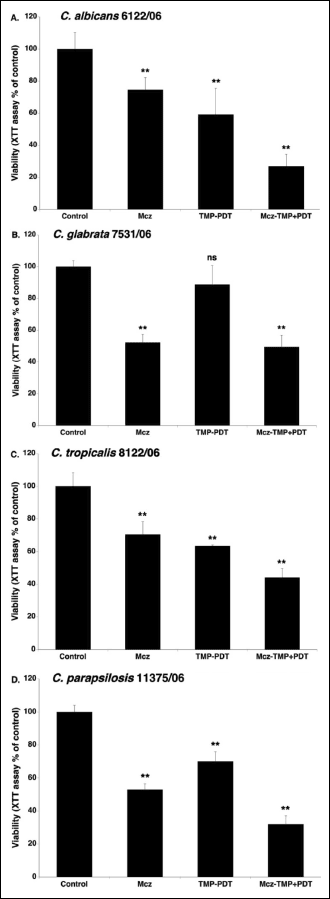 |
Fig. 2. Photodynamic activity of TMP-1363 (10 µg/ml; 7.3 µM) against Candida biofilms (clinical isolates) in the absence and presence of miconazole (25 µg/ml; 52.2 µM): C. albicans 6122/06 (A), C. glabrata 7531/06 (B), C. tropicalis 8122/06 (C) and C. parapsilosis (D). The metabolic activity of the biofilms was measured by the XTT assay and expressed as a percentage of the control OD490 (untreated biofilm). The control OD490 values were in the range from 0.750 to 1.400 (average = 1.040 ± 0.180). Each value is a mean ± standard deviation (S.D.) of two or three independent experiments performed in triplicate. Asterisks indicate significant differences compared with control (untreated biofilm) (*P < 0.005, **P < 0.00005). |
DISCUSSION
Our results indicate that responses to miconazole alone, TMP-aPDT and TMP-aPDT + miconazole are Candida strain-dependent. In some cases, miconazole has an additive effect on TMP-aPDT, and in one case it appears to be synergistic. The photodynamic activity of TMP-1363 towards Candida has been studied previously (30, 35-37). Chabrier-Rosello et al. (35) investigated C. albicans SC3153A biofilms formed on serum-coated plates, and found that the metabolic activity of the biofilms (as measured by the XTT assay) is reduced by over 90% with the use of TMP-1363 at 10 µg/ml at 2.4 J/cm2. Under the same experimental conditions, TMP-aPDT of planktonic C. albicans SC5314 and C. glabrata MRO-084-R results in 3 to 4 log reduction in yeast viability, as determined by a CFU assay. Respiratory deficient ‘petite’ mutants of these Candida strains are more sensitive to aPDT. Chabrier-Rosello et al. have suggested that intact mitochondrial function may provide an innate anti-oxidant defense against PDT-induced phototoxicity in Candida (35).
The increased sensitivity of planktonic C. albicans and C. glabrata to TMP-PDT has also been observed following pre-treatment with the cytochrome bc1 (Complex III) inhibitor, antimycin A (35). Mitra et al. (37) compared the phototoxicity of TMP-1363 against planktonic C. albicans SC5314 to methylene blue (MB)-mediated PDT. TMP-aPDT results in a greater than
3 log reduction of yeast viability determined by a CFU assay, whereas MB-aPDT is ineffective. Snell et al. (30) reported that, under similar experimental conditions with the same C. albicans strain, TMP-aPDT causes a ~1.6 log reduction in CFU, and that inducing ROS formation by miconazole, results in more effective TMP-aPDT (~2.2 log reduction in CFU).
In antifungal PDT studies, Candida biofilms have been grown on a variety of substrates: polystyrene microplates (37-44), serum-coated culture plates (35, 44), coverslips (45), cellulose nitrate membrane filters (46), PMMA discs (47), the Calgary biofilm device (48), and dentures (49). There is no agreement, however, as to which of these surfaces is the most reliable model for evaluating PDT efficiency against Candida biofilms. This project evaluated acrylic substrates and contributes additional data to support this material as an appropriate test surface.
The formation, structure and matrix composition of biofilms are highly species-dependent (17, 40, 46). C. albicans and C. tropicalis grow as yeast cells, pseudohyphae, or hyphae, and biofilms formed by these species exhibit yeast and hyphal morphology. Biofilms formed by monomeric C. glabrata contain only yeasts, whereas biofilms of C. parapsilosis are composed of both yeasts and pseudohyphae (17, 20, 50). To what extent the differences in biofilm structure and matrix composition can modify PS-mediated phototoxicity is unclear.
In the present study, Mcz alone at a concentration of 25 µg/ml (52.2 µM) exhibited antifungal activity against Candida biofilms for all strains, except C. albicans MYA 274. The resistance or tolerance of C. albicans MYA 274 to both Mcz and TMP-aPDT individually, and its sensitivity to the combination, is intriguing. Mcz has a multitude of effects on Candida, including changes in mitochondrial ultrastructure and cell membrane integrity (30). These changes, which in themselves are not sufficient to cause cytotoxicity at the Mcz concentration used, may affect the ability of the PS molecules to reach sensitive targets.
The mean reduction of metabolic activity of susceptible Candida biofilms was 36.7 ± 11.1%. Recently, we investigated the antifungal activity of miconazole (0.5 – 96 µM) against biofilms of the same Candida strains, grown on PMMA discs. Miconazole at a concentration of 25 µg/ml reduced the metabolic activity by 32.8 ± 14.1% (mean value). The minimum inhibitory concentrations of miconazole (MICs) ranged from 0.016 to 32 µg/ml, while for all Candida strains the miconazole concentrations corresponding to a reduction of metabolic activity of the biofilm by 50% was 50 µg/ml or higher (51).
The different Candida biofilms responded differently to TMP-mediated aPDT. Biofilms of ATCC strains and C. glabrata 7531/06, were not sensitive to TMP-aPDT, whereas the metabolic activity of the remaining three clinical isolates was reduced by 64.2 ± 5.5% (mean value). Chabrier-Rosello et al. (36) reported that TMP-aPDT reduces the metabolic activity of C. albicans SC3153A biofilms, measured by the XTT assay, by over 90%. In their study, biofilms were grown on serum-coated culture plates, in RPMI 1640 medium, which induces hyphal forms that appear to be more susceptible to PDT than blastospores (50). The differences in experimental conditions render difficult a direct comparison with our results.
Biofilms of both C. glabrata strains were sensitive to the antifungal effect of miconazole, but were not susceptible to TMP-aPDT. It has been reported that C. glabrata biofilms are less susceptible to Photogem-mediated aPDT but not to curcumin-mediated aPDT. The observed discrepancies in the susceptibility to aPDT can be attributed to different experimental conditions, such as the C. glabrata strains, surfaces (cellulose nitrate membrane filters versus polystyrene) and medium (tryptic soy broth versus RPMI) (40, 46). The better adherence of C. glabrata to different surfaces, because of its smaller size and higher hydrophobicity, and co-adhesion between closely apposed yeasts reported by Luo and Samaranayake (52), may limit the contact area between C. glabrata cells and the PS.
Variations in the sensitivity of Candida biofilms to TMP-aPDT, observed in our study confirmed a notion that investigations of the antifungal effect of PDT should not be performed with the use of only one Candida strain (38, 40, 46, 53). Junqueira et al. (42) have examined the phototoxicity of cationic nanoemulsions of zinc-phthalocyanine (ZnPc) for biofilms (polystyrene) of thirty-nine Candida strains isolated from the oral cavity, among them C. albicans, C. glabrata, C. tropicalis, C. dubliniensis and C. parapsilosis. After the treatment, Candida cells were scraped off and seeded onto Sabouraud dextrose agar plates and yeast viability was determined by a CFU assay. A mean reduction of 0.45 ± 0.12 log (64.5%) was achieved for Candida biofilms. The lowest reduction was observed for C. albicans (0.35 log; 55.3%) and C. glabrata (0.33 log; 53.3%). Andrade et al. (38) and Dovigo et al. (40) have investigated curcumin-mediated aPDT against planktonic and biofilm cultures of C. albicans, C. glabrata, C. dubliniensis and C. tropicalis. For planktonic cultures, a 4 log reduction can be achieved at 20 µM curcumin. For biofilms, grown on polystyrene in RPMI medium, 40 µM curcumin reduces cell viability by 85 – 94%, 85 – 89%, 85% and 73% for C. albicans, C. glabrata, C. dubliniensis and C. tropicalis, respectively. Thus, Candida biofilms appear to be less susceptible to photo-inactivation when compared with planktonic cells. Similar results were reported by Dovigo et al. (46) for Photogem-mediated aPDT, and by Costa et al. (48) for rose Bengal- and erythrosine-mediated aPDT.
In conclusion, in biofilms formed on dental acrylic, the effect of Mcz alone as well as TMP-aPDT in the absence and presence of Mcz is dependent on the Candida strains. C. glabrata biofilms are sensitive to Mcz, but not to TMP-aPDT, whereas C. albicans MYA 274 biofilms are resistant to Mcz and TMP-aPDT, but sensitive to TMP-aPDT + Mcz. Our observations stress the significance of investigating more than one isolate before drawing conclusions regarding Candida sensitivity to aPDT. Despite the limitations of this study, in which only seven Candida strains were tested under in vitro conditions, the results suggest that a combination of miconazole and TMP-aPDT can be used for the treatment of Candida biofilms both on dentures and on oral surfaces with denture stomatitis. Further studies are necessary to address the differences observed in the sensitivity of Candida biofilms to aPDT, as well as to establish optimal conditions of aPDT and Mcz concentrations.
Regarding the eventual clinical treatment of denture stomatitis, it may be of interest to explore the combined use of natural anti-inflammatory agents with aPDT and/or Mcz. Based on the study by Parvu et al. (54), adjunctive administration of Allium schoenoprasum extracts may be considered as a host-modulatory therapy in patients with oral mucosa inflammatory diseases.
Acknowledgments: The authors thank Drs. Tomasz Goslinski and Jaroslaw Piskorz (Poznan University of Medical Sciences, Poland) for helpful discussions. This work was supported in part by Research Pilot Project Award 03-Activity 085 (S. Gebremedhin) from the Arthur A. Dugoni School of Dentistry.
Conflict of interests: None declared.
REFERENCES
- Arendorf TM, Walker DM. Denture stomatitis: a review. J Oral Rehabil 1987; 14: 217-227.
- Majewski S, Loster BW, Macura AB, et al. Application of a diagnostic-therapeutic procedure using implant-supported dental prosthesis as a preventive therapy for candidiasis of upper gastrointestinal tract in complete denture users. J Physiol Pharmacol 2008; 59 (Suppl. 5): 39-46.
- Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J 1998; 185: 380-384.
- Mech F, Wilson D, Lehnert T, Hube B, Thilo Figge M. Epithelial invasion outcompetes hypha development during Candida albicans infection as revealed by an image-based systems biology approach. Cytometry A 2014; 85: 126-139.
- Cavalcanti YW, Morse DJ, da Silva WJ, et al. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling 2015; 31: 27-38.
- Karczewska E, Wojtas I, Sito E, et al. Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J Physiol Pharmacol 2009; 60 (Suppl. 6): 33-39.
- Barbeau J, Seguin J, Goulet JP, et al. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 51-59.
- Dorocka-Bobkowska B, Budtz-Jorgensen E, Wloch S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J Oral Pathol Med 1996; 25: 411-415.
- Dorocka-Bobkowska B, Konopka K. Susceptibility of Candida isolates from denture-related stomatitis to antifungal agents in vitro. Int J Prosthodont 2007; 20: 504-506.
- Shulman JD, Rivera-Hidalgo F, Beach MM. Risk factors associated with denture stomatitis in the United States. J Oral Pathol Med 2005; 34: 340-346.
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J 1998; 43: 160-166.
- Dorko E, Jenca A, Pilipcinec E, Danko J, Svicky E, Tkacikova L. Candida-associated denture stomatitis. Folia Microbiol (Praha) 2001; 46: 443-446.
- Li L, Redding S, Dongari-Bagtzoglou A. Candida glabrata, an emerging oral opportunistic pathogen. J Dent Res 2007; 86: 204-215.
- Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology 2010; 98: 15-25.
- Douglas J. Candida biofilms and their role in infection. Trends Microbiol 2003; 11: 30-36.
- Gocke R, Gerath F, von Schwanewede H. Quantitative determination of salivary components in the pellicle on PMMA denture based material. Clin Oral Investig 2002; 6: 227-235.
- Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilm of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol 2009; 47: 681-689.
- Chandra J, Kuhn DM, Mukhrejee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 2001; 183: 5385-5394.
- Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role of Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98: 53-59.
- Chandra J, Mukherjee PK, Leidich SD, et al. Antifungal resistance of Candidal biofilms formed on denture acrylic in vitro. J Dent Res 2001; 80: 903-908.
- Konopka K, Dorocka-Bobkowska B, Gebremedhin S, Duzgune N. Susceptibility of Candida biofilms to histatin 5 and fluconazole. Antonie van Leeuwenhoek 2010; 97: 413-417.
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 3. Treatment of oral candidosis. Aust Dent J 1998; 43: 244-249.
- Biel MA. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol Biol 2010; 635: 175-194.
- Dai T, Fuchs BB, Coleman JJ, et al. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol 2012; 3: 1-16.
- Pereira Gonzales F, Maisch T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol 2012; 116: 1-10.
- Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dental Res 2007; 86: 694-707.
- Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents. Future Med Chem 2014; 6: 77-90.
- Kobayashi D, Kondo K, Uehara N. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob Agents Chemother 2002; 46: 3113- 3117.
- Vandenbosch D, Braeckmans K, Nelis HJ, Coenye T. Fungicidal activity of miconazole against Candida spp. biofilms. J Antimicrob Chemother 2010; 65: 694-700.
- Snell SB, Foster TH, Haidaris CG. Miconazole induces fungistasis and increases killing of Candida albicans subjected to photodynamic therapy. Photochem Photobiol 2012; 88: 596-603.
- Beirao S, Fernandes S, Coelho J, et al. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem Photobiol 2014; 90: 1387-1396.
- Mukherjee PK, Zhou G, Munyon R, Ghannoum MA. Candida biofilm: a well-designed protected environment (review). Med Mycol 2005; 43: 191-208.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 2005; 11: 127-152.
- Ramage G, Wande VK, Vickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 2001; 45: 2475-2479.
- Chabrier-Rosello Y, Foster TH, Mitra S, Haidaris CG. Respiratory deficiency enhances the sensitivity of the pathogenic fungus Candida to photodynamic treatment. Photochem Photobiol 2008; 84: 1141-1148.
- Chabrier-Rosello Y, Giesselman BR, De Jesus-Andino FJ, Foster TH, Mitra S, Haidaris CG. Inhibition of electron transport chain assembly and function promotes photodynamic killing of Candida. J Photochem Photobiol B 2010; 99: 117-125.
- Mitra S, Haidaris CG, Snell SB, Giesselman BR, Hupcher SM, Foster TH. Effective photosensitization and selectivity in vivo of Candida albicans by meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate. Lasers Surg Med 2011; 43: 324-332.
- Andrade CA, Ribeiro APD, Dovigo LN, et al. Effect of different pre-irradiation times on curcumin-mediated photodynamic therapy against planktonic cultures and biofilms of Candida spp. Arch Oral Biol 2013; 8: 200-210.
- Costa AC, de Campos Rasteiro VM, Pereira CA, et al. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol 2011; 56: 1299-1305.
- Dovigo LN, Pavarina AC, Carmello JC, Machado AL, Brunetti IL, Bagnato VS. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg Med 2011; 43: 927-934.
- Junqueira JC, Jorge AO, Barbosa JO, et al. Photodynamic inactivation of biofilms formed by Candida spp., Trichosporon mucoides, and Kodamaea ohmeri by cationic nanoemulsion of zinc 2,9,16,23-tetrakis(phenylthio)-29H, 31H-phthalocyanine (ZnPc). Laser Med Sci 2012; 27: 1205-1212.
- Gonzales FP, Felgentrager A, Baumler W, Maisch T. Fungicidal photodynamic effect of a twofold positively charged porphyrin against Candida albicans planktonic cells and biofilms. Future Microbiol 2013; 8: 785-797.
- Rossoni RD, Barbosa JO, de Oliveira FE, de Oliveira LD, Jorge AO, Junqueira JC. Biofilm of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med Sci 2014; 29: 1679-1684.
- Chabrier-Rosello Y, Foster TH, Perez-Nazario N, Mitra S, Haidaris CG. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrobial Agents Chemother 2005; 49: 973-980.
- Ribeiro AP, Andrade MC, da Silva J de F, et al. Photodynamic inactivation of planktonic cultures and biofilms of Candida albicans mediated by aluminum-chloride-phthalocyanine entrapped in nanoemulsions. Photochem Photobiol 2013; 89: 111-119.
- Dovigo LN, Pavarina AC, Mima EG, Giampaolo ET, Vergani CE, Bagnato VS. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses 2011; 54: 123-130.
- Mantareva V, Angelov I, Kussovski V, Dimitrov R, Lapok L, Wohrle D. Photodynamic efficacy of water-soluble Si(IV) and Ge(IV) phthalocyanines towards Candida albicans planktonic and biofilm cultures. Eur J Med Chem 2011; 46: 4430-4440.
- Costa AC, Rasteiro VMC, Pereira CA, Rossoni RD, Junqueira JC, Jorge AO. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2012; 55: 56-63.
- Mima EG, Pavarina AC, Ribeiro DG, Dovigo LN, Vergani CE, Bagnato VS. Effectiveness of photodynamic therapy for the inactivation of Candida spp. on dentures: in vitro study. Photomed Laser Surg 2011; 29: 827- 833.
- Kucharikova S, Tournu H, Lagrou K, Van Dijck P, Bujdakova H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J Med Microbiol 2011; 60: 1261-1269.
- Gebremedhin S, Dorocka-Bobkowska B, Prylinski M Konopka K, Duzgunes N. Miconazole activity against Candida biofilms developed on acrylic discs. J Physiol Pharmacol 2014; 65: 593-600.
- Lou G, Samaranayake LP. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS 2002; 110: 601-610.
- Grinholc M. Comments on ‘Biofilms of Candida albicans serotypes A and B differ in their sensitivity photodynamic therapy’ [letter to Editor]. Laser Med Sci 2015; 38: 2221.
- Parvu AE, Parvu M, Vlase L, Miclea P, Mot AC, Silaghi-Dumitrescu R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J Physiol Pharmacol 2014; 65: 309-315.
A c c e p t e d : October 31, 2016