EFFECTS OF TADALAFIL (PDE5 INHIBITOR) AND ROFLUMILAST (PDE4 INHIBITOR) ON AIRWAY REACTIVITY AND MARKERS OF INFLAMMATION IN OVALBUMIN-INDUCED AIRWAY HYPERRESPONSIVENESS IN GUINEA PIGS
INTRODUCTION
Phosphodiesterases (PDEs) represent a superfamily of enzymes - phosphohydrolases, which are able to catalyze a hydrolysis of 3`cyclic phosphate bonds in cyclic 3`,5`adenosine and/or guanosine monophosphate (cAMP and cGMP). Each type of cells can produce several different PDE subtypes. Thus, their origin and localization is considered to be a major regulatory mechanism in controlling the concentration of cyclic nucleotides in the respective cells and tissues.
Theophylline, as a major representative of methylxanthines, plays an important role in the therapeutic management of various pulmonary diseases. However, it possesses relatively narrow therapeutic index, based on its pharmacokinetic properties. Theophylline inhibits most of the PDE subtypes in different tissues and organs, i.e. its effect is non-selective regarding the PDE inhibition (1, 2). Furthermore, theophylline action is mediated via several other mechanisms, such as antagonism of adenosine receptors, activation of histone-deacetylase, etc. (3). Simultaneous influencing of several PDEs and a broad spectrum of mechanisms of action result in serious adverse effects of theophylline. In effort to minimize these adverse effects and keeping favorable impact of the PDE inhibition, the interest of researchers has been focused on agents with more selective action, i.e. the selective PDE inhibitors.
Nowadays, the PDEs are classified into 11 super-families, assembling tens of gene products (4). Nevertheless, more than 100 of different mRNA products have been estimated (1). According to the structure of their active site, many of PDE isoforms are associated with specific body functions and various pathological conditions. Therefore, the selective PDE inhibitors can influence various functions and pathological conditions without or with limited effects on other organs or systems, and without an occurrence of considerable non-specific adverse effects (5).
There are several selective PDE inhibitors which have been successfully used in experimental and clinical studies. However, due to their participation in immune responses in the respiratory system, the isoforms PDE3, PDE4, PDE5, and PDE7 are of particular attention of pneumologists (6). Inhibition of PDE4 can effectively suppress the neutrophil-mediated inflammation, as previously confirmed for roflumilast in chronic obstructive pulmonary disease (COPD) (7, 8). More recently, efficacy of roflumilast has been proven also in allergic asthma both in experimental and clinical conditions (4, 9, 10), and therefore the use of roflumilast in asthma and asthma-COPD overlap has been considered (11, 12). On the other hand, PDE5 inhibition is widely used for therapy of erectile dysfunction, pulmonary hypertension, and other cardiovascular diseases, where relaxation of smooth muscle is of benefit (3). However, expression of PDE5 has been confirmed in several immune cells, suggesting its potential role in the allergic inflammation, as well (1, 6).
Increased intracellular levels of cAMP (caused by the inhibition of PDE4) and cGMP (caused by the inhibition of PDE5) have led to suppressed immunological responses and to the relaxation of smooth muscle (13). Therefore, we have hypothesized that the administration of selective PDE4 and PDE5 inhibitors, either alone, or in a combination, will improve the symptomatology of allergic asthma, which is usually associated with eosinophilic inflammation and airway hyperresponsiveness.
In this study, the effects of selective PDE4 inhibitor roflumilast and selective PDE5 inhibitor tadalafil were analyzed in a single or combined administration, and their actions were compared with a potent anti-inflammatory corticosteroid dexamethasone in a guinea pig model of airway hyperresponsiveness associated with eosinophilic inflammation evoked by ovalbumin.
MATERIALS AND METHODS
Animals
The study protocol was approved by the local Ethics Committee of Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Slovakia and the National Veterinary Board, Slovakia. All experiments were performed according to the guidelines for protection of animals used for scientific-educational purposes.
Healthy adult male guinea pigs (n = 48, 250 – 350 g) of TRIK outbred strain (supplied by Department of Toxicology and Laboratory Animal Breeding Station, Dobra Voda, Slovakia) were used for the study. They were kept in a faculty animal house where they underwent 7-days quarantine and acclimation. During their keeping in the animal house, they had adequate food and water ad libitum.
Animals were randomly divided into 6 groups (n = 8 in each group). One of the groups was left without sensitization and served as a non-sensitized healthy control group (Control group, naive). In the remaining five groups, the airway hyperresponsiveness was induced by repeated exposures to antigen (ovalbumin). The guinea pigs in one of the sensitized groups were given a vehicle (sterile saline) only, and served as an ovalbumin-sensitized non-treated group (Ovalbumin group). The sensitized animals in the latter four sensitized groups were treated with selective PDE5 inhibitor tadalafil (DND Pharm-Technology Co., Inc., China; Ovalbumin + Tadalafil group), with selective PDE4 inhibitor roflumilast (DND Pharm-Technology Co., Inc., China; Ovalbumin + Roflumilast group), with dexamethasone (Dexamed, Medochemie, ltd., Cyprus; Ovalbumin + Dexamethasone group) all at the doses of 1.0 mg/kg b.w.; and with the combination of tadalafil and roflumilast, both at the dose of 0.5 mg/kg b.w. (Ovalbumin + Roflumilast + Tadalafil group). All the tested drugs were administered intraperitoneally once daily for 7 consecutive days. This route of administration was chosen to prevent potential pharmacokinetic interactions from gastrointestinal tract and had been shown previously to be of the similar effectiveness as oral or inhalational administration (14).
Ovalbumin-induced airway hyperresponsiveness
The sensitization of animals by ovalbumin, which causes airway reactivity changes on the immunological basis, was performed during 14 days (14, 15). The allergen (1% ovalbumin dissolved in aqua pro injectione) has caused a tissue injury and subsequent structural changes accompanied with eosinophilic inflammation (16). Ovalbumin (Sigma Aldrich, Germany) was administered on the 1st day of sensitization intraperitoneally (0.5 mL) and subcutaneously (0.5 mL), and on the 3rd day intraperitoneally (1.0 mL). On the 14th day and the 21st day, 1% ovalbumin dissolved in saline was inhaled for 1 minute using an inhalation chamber (challenge). The measurement of in vivo airway reactivity to a bronchoconstriction mediator histamine (Sigma Aldrich, Germany) followed in 5 hours after the ovalbumin challenge and in vitro measurements were performed on the following day (within 24 hours after the challenge) after killing the animal. In the treated groups, tested drugs (vehicle, tadalafil, roflumilast, and dexamethasone) were administered at a dose of 1.0 mg/kg b.w. (single treatment) or at a dose of 0.5 mg/kg b.w. (in combination therapy by roflumilast and tadalafil) intraperitoneally for seven consecutive days, starting from 15th day. The 7th dose was administered 30 minutes before the in vivo assessment and before killing the animal on the last day. Only animals with a minimum of 20% increase in the specific airway resistance after ovalbumin challenge on the 14th day were included in the further testing.
Assessment of in vivo airway reactivity
Specific airway resistance (sRaw), a sensitive marker of the airway reactivity in in vivo conditions, was measured using a double-chamber whole-body plethysmograph for small laboratory animals (HSE type 855, Hugo Sachs Electronic, Germany). The method has been previously described by Pennock et al. (17) and is based on a measurement of the time delay between the pneumotachometer signals from thoracic and nasal chambers, whereas the volume changes in the nasal and in the thoracic chambers were evaluated separately. The specific airway resistance calculated from a phase displacement between the two chambers was evaluated immediately after an inhalation (2 min) of bronchoconstricting agent (histamine at the concentration of 10–6 mol/L in saline) on the 14th day and on the 21st day of sensitization 5 hours after the challenge with ovalbumin. For comparison, the reactivity after a saline nebulization was used. During the intervals, fresh air was insufflated into the nasal chamber. The results were evaluated using a special software (HSE Pulmodyn Pennock, Hugo Sachs Elektronik, Germany). Due to the fact that the specific airway resistance can be influenced not only by a contraction of airway smooth muscle but also by mucosal edema and by changes in epithelial secretion, to avoid any pitfalls with interpretation of these data the tissue samples from lungs and trachea were analyzed also in in vitro conditions.
Evaluation of in vitro airway reactivity
After the ovalbumin challenge and eventual treatment, the animals were killed by an overdose of anesthetics: combination of tiletamine and zolazepam (ZOLETIL, at the dose of 20 mg/kg b.w.), and xylazine (XYLARIEM, at the dose of 20 mg/kg b.w. intraperitoneally), and subsequent exsanguination; trachea and lungs were excised immediately. The measurement of the in vitro airway reactivity was realized in the organ baths, multi-chamber tissue bath systems (Experimetria-ISO-09-TSZ8, Hungary). Tracheal strips (approximately 15 mm) were cut on the opposite side of a smooth muscle. Lung tissue strips (2 × 2 × 15 mm) were cut from the margin of upper lobes of the left lungs. The strips were mounted between two hooks and placed into the organ chambers containing Krebs-Henseleit buffer (NaCl 110.00 mmol/L, KCl 4.80 mmol/L, CaCl2 2.35 mmol/L, Mg2SO4 1.20 mmol/L, KH2PO4 1.20 mmol/L, NaHCO3 25.00 mmol/L, and glucose 10.00 mmol/L; in glass distilled water). The chambers were maintained at 36.5 ± 0.5°C and aerated continuously with a mixture of 95% O2 and 5% CO2 to maintain pH 7.5 ± 0.1. One of the hooks was connected to a force transducer (EXP, Experimetria, Hungary) and an amplifier (EXP CLSG-4, Experimetria, Hungary), and the tension changes were recorded online using a special computer software (SPEL Advanced Iso Sys v3.2, Experimetria, Hungary). The tissue strips were initially set to 4 grams of tension for 30 min (loading phase). Then, in each strip the tension was readjusted to a baseline value of 2 grams for another 30 min (adaptation phase). During both periods, the tissue strips were washed at 10 min intervals with the pre-warmed Krebs-Henseleit buffer. The contractile responses to cumulative doses of histamine and acetylcholine (10–8 – 10–3 mol/L, Sigma-Aldrich, Germany) were used as a marker of the in vitro airway smooth muscle reactivity after the adaptation phase had been finished. Data of the tracheal and lung tissue reactivity are shown in grams (g) of the smooth muscle tension (18).
Hematological assay
Samples of blood were taken from the heart immediately after the overdosing of animals with anesthetics during an exsanguination phase. Bronchoalveolar lavage (BAL) of the right lung was performed twice using instillation and immediate suction of pre-heated saline (37°C) at the dose of 0.01 mL/g b.w. The total leukocyte count in the blood was determined in a Burker’s chamber after staining by Turck. Differential leukocytes counts in the blood and BAL fluid were evaluated microscopically after staining by May-Grunwald/Giemsa-Romanowski and relative counts of the cells were expressed in percents (%).
Detection of markers of inflammation and oxidative stress
Standard ELISA method with commercially available kits for guinea pigs (Antibodies-online Inc., USA, Cusabio Life Sciences, USCN Life Sciences Inc., Blue Gene Biotech) was used to determine concentrations of pro-inflammatory mediators: interleukin (IL)-4, IL-5, tumor necrosis factor alpha (TNF-α), and nuclear factor kappa B (NF-κB), and the concentration of thiobarbituric acid reactive substances (TBARS) representing a marker of a lipid peroxidation (OxiSelect TBARS Assay kit, Cellbiolabs Inc.), in the lung homogenates.
Statistical analysis
Data are shown as means ± S.E.M. For statistical analysis of between-group differences, one-way ANOVA with post-hoc LSD test was used. A P < 0.05 was considered as statistically significant.
RESULTS
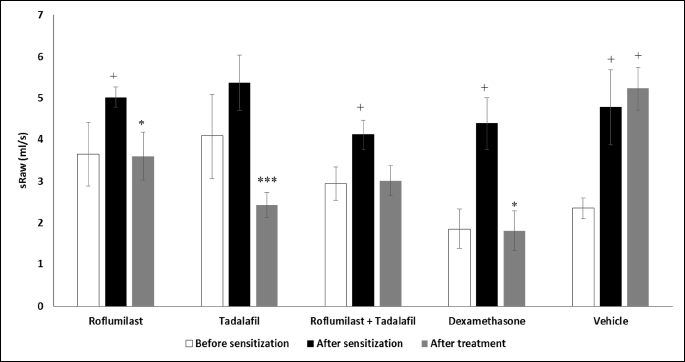
The specific airway resistance measured in the whole body double chamber plethysmograph was used as a marker of the in vivo airway responsiveness. The sensitization and challenge of guinea pigs with ovalbumin led to a significant increase in the specific airway resistance on the day 14 as well as on the day 21 (treated with vehicle). The administration of roflumilast, tadalafil, and dexamethasone at the daily doses of 1.0 mg/kg b.w. caused a significant decrease in the specific airway resistance after histamine nebulization. However, a simultaneous therapy with roflumilast and tadalafil at lower doses decreased this value only non-significantly (Fig. 1).
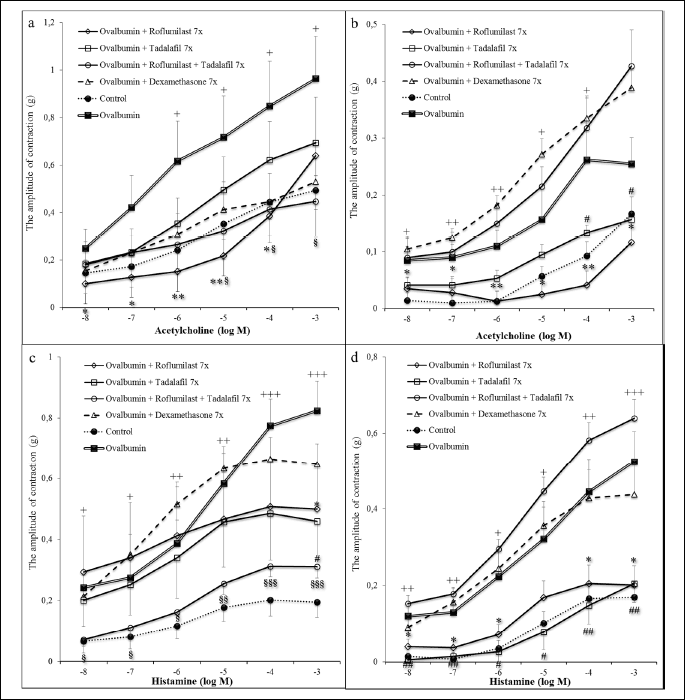
The in vitro testing of the tracheal and lung smooth muscle tissue strips contractility to cumulative doses of acetylcholine and histamine demonstrated a significant increase in the reactivity after the sensitization compared to the Control group (naive animals). The administration of roflumilast led to a suppression of contractile responses evoked by both acetylcholine and histamine in the tracheal and lung tissue. Similar decline was observed after the administration of tadalafil, however, significant changes were detected only in the lung tissue strips. Dexamethasone did not decrease significantly the in vitro airway reactivity. The combination of roflumilast and tadalafil at the doses of 0.5 mg/kg significantly suppressed the tracheal smooth muscle contractile responses evoked by cumulative doses of both acetylcholine and histamine, however, with no significant changes in the lung tissue strips (Fig. 2).
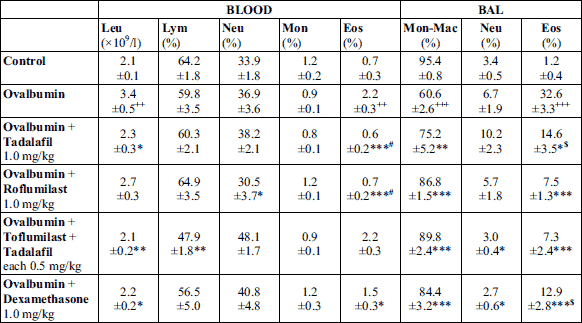
A significant increase of total leukocyte count in the blood and of differential counts of eosinophils in the blood and BAL fluid after sensitization in the non-treated Ovalbumin group confirmed a formation of eosinophilic inflammation evoked by our sensitization protocol (Table 1). The intraperitoneal administration of roflumilast, tadalafil, dexamethasone, and combination of tadalafil and roflumilast for 7 consecutive days led to a decrease in differential counts of eosinophils both in the blood and BAL fluid. The most significant decline in the differential count of eosinophils in blood and BAL fluid was observed in groups treated with tadalafil only and roflumilast only, or with roflumilast only and combination of tadalafil with roflumilast, respectively. These changes were associated with a significant increase in the number of monocytes-macrophages in the BAL fluid (Table 1).
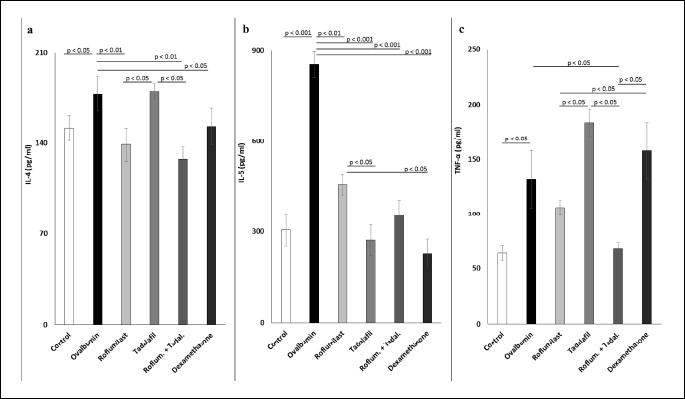
An analysis of concentrations of IL-4, IL-5, and TNF-α in the lung tissue homogenates revealed a significant elevation of these parameters in the sensitized non-treated Ovalbumin group (Fig. 3). Administration of tadalafil, roflumilast, dexamethasone, and combination of tadalafil with roflumilast at the lower doses suppressed significantly only the levels of IL-5 (Fig. 3b), with significantly stronger suppression observed after tadalafil and dexamethasone administration compared to roflumilast. On the other hand, IL-4 levels were significantly decreased only in the group treated with roflumilast, with the combination of roflumilast and tadalafil, and with dexamethasone; tadalafil did not lead to suppression of IL-4 concentration, which was significantly higher than in the groups of animals treated with roflumilast and with the combination of roflumilast and tadalafil (Fig. 3a). Only guinea pigs treated with the combination of tadalafil and roflumilast demonstrated significantly lower TNF-α levels (Fig. 3c).
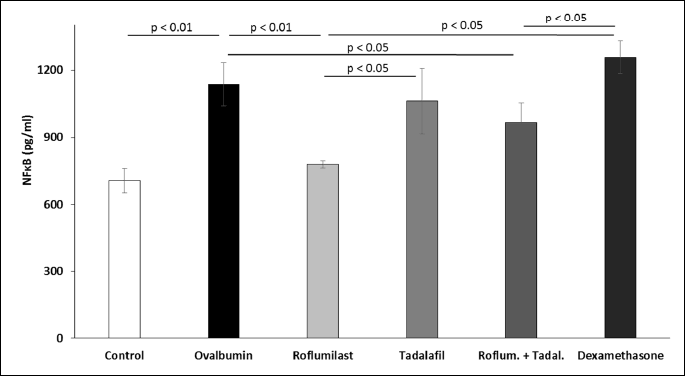
The sensitization of guinea pigs with ovalbumin was associated with elevated concentrations of NF-kB measured in the lung homogenate. Decreased levels of NF-kB were observed only in the groups treated with roflumilast alone or in the combination with tadalafil (Fig. 4).
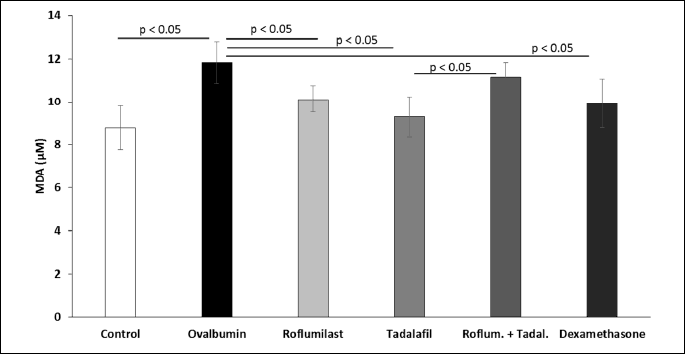
Concentrations of TBARS, a marker of lipid oxidation, in the lung tissue were increased in sensitized non-treated guinea pigs. This increase was prevented by the administration of roflumilast, tadalafil, and dexamethasone, but not by lower doses of tadalafil and roflumilast administered in the combination (Fig. 5).
DISCUSSION
Selective inhibitors of PDE (especially PDE3 and PDE4) have been extensively studied for their ability to influence the airway inflammation and hyperresponsiveness of the airway smooth muscle (19). Several clinical studies testing the second generation of PDE4 inhibitors (piclamilast, cilomilast, and roflumilast) confirmed the bronchodilating, anti-inflammatory, and antitussive effects, which were demonstrated also in the experimental conditions. Nevertheless, these promising results emphasize the necessity of testing the inhibitors of other PDE isoforms in the respiratory diseases with an inflammatory component (8, 20, 21).
In our experiments, selective PDE4 inhibitor roflumilast significantly decreased the specific airway resistance, a marker of in vivo airway reactivity, and reduced in vitro airway reactivity in both tracheal and lung tissue strips in the ovalbumin-sensitized guinea pigs, suggesting a direct bronchodilating action of roflumilast. Previously tested drug citalopram, known as a selective serotonin reuptake inhibitor used for the therapy of depression and indirectly increasing the cAMP levels (mediated probably by selective PDE4 inhibition; 22, 23), has led to a significant suppression of the airway reactivity only in in vitro conditions but not in vivo (18), whereas selective PDE4 inhibitor of the first generation, rolipram, has demonstrated potent in vivo and in vitro activity in the ovalbumin-sensitized animals (24).
PDE5 inhibition by tadalafil alone and in combination with roflumilast in this study led to the decrease of both in vivo and in vitro airway reactivity, as well. However, a significant suppression of contractile responses to the cumulative doses of acetylcholine and histamine was found in ovalbumin-sensitized animals only in the lung tissue. Similar results were observed also in the guinea pigs exposed to ovalbumin after administration of sildenafil, a short acting PDE5 inhibitor (25). Different response of the tracheal and lung tissue strips is probably caused by a different distribution of PDEs in various types of cells. Thus, more obvious effects on the in vitro airway reactivity in the lung tissue strips could be explained by a higher expression of PDE5 in the vascular smooth muscle, which is present in a higher proportion in the lung tissue compared to the tracheal tissue.
Inhibitors of PDE4 (rolipram, cilomilast, roflumilast, or piclamilast) have demonstrated a potent anti-inflammatory and immunomodulation effects with reduced adverse effects (for roflumilast represented predominantly by gastrointestinal side effects, nausea and vomiting, and unexplained weight loss) comparing to non-selective PDE inhibitors (26). Potent anti-inflammatory, antitussive and bronchodilating action has been recently demonstrated in our study with selective PDE4 inhibitor YM976 (24). Roflumilast in the present study decreased the relative counts of both neutrophils and eosinophils in the blood and of eosinophils in the BAL fluid. These results are in concordance with the study published by Kim et al. (27), who found a significant decline in the eosinophils and total white blood cells counts in mice sensitized by ovalbumin after the administration of roflumilast at the dose of 1.0 mg/kg b.w. In another in vivo study, the changes in the mobilization of neutrophils and eosinophils and/or their activation, probably due to an increase in intracellular cAMP levels after roflumilast therapy, were observed. This prevented the cells to release their content, and ameliorated the antigen-evoked contractions of the tracheal rings (28).
Selective PDE5 inhibition by the long-acting PDE5 inhibitor tadalafil in our study confirmed its effectiveness in the model of allergic inflammation. Tadalafil reduced the total leukocyte count and the relative count of eosinophils in the blood; this finding is in accordance with several previous studies (25, 29, 30).
The co-administration of two selective PDE inhibitors in our study provided significant anti-inflammatory action, i.e. decreased total leukocyte count and percentual count of lymphocytes in the blood and decreased the percentages of neutrophils and eosinophils in the BAL fluid compared to the sensitized non-treated group. Despite the fact that roflumilast and tadalafil were co-administered in the reduced doses, their combination reached even more significant decline in eosinophils count in the BAL fluid than it was observed by dexamethasone, suggesting a significant anti-inflammatory potential in this dual PDE inhibition. Similar advantages of the concomitant administration of two PDE inhibitors (PDE4 inhibitor rolipram and PDE7 inhibitor BRL50481) have been recently demonstrated by our research group (31). On the other hand, in a model of airway hyperresponsiveness in mice the pretreatment with selective PDE4 inhibitor RO-1724 in a co-administration with PDE5 inhibitor sildenafil did not show any additional benefit (29). Similarly, an earlier study published by Banner and Page (32) failed to show any significant effects of acute or chronic administration of PDE5 inhibitor in ovalbumin-induced eosinophil infiltration in guinea pigs; they suggested that PDE4 isoenzyme played a role in the control of the allergen-induced eosinophil infiltration into the airways, and indicated that a period of low dose chronic treatment with a PDE4 inhibitor or dual PDE3/4 inhibitor was necessary for eosinophil accumulation in the airways to be reduced (32). Furthermore, there are several studies suggesting benefits of selective PDE4 inhibitors and dual of PDE3/4 inhibitors such as RPL 554 and zardaverine in the therapy of bronchial asthma (33-36).
The changes in the white blood cell counts in blood and BAL fluid are tightly associated with a secretion of several cytokines. Immunohistochemical analyses of bronchial biopsies from asthma patients have indicated the mast cells as a main source of cytokines, including IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, and TNF-α (37). The secretion of IL-4 and IL-13 stimulates B-lymphocytes to an increased production of allergen-specific IgE. This is bound to receptors on mast cells and granulocytes and in case of a contact with an allergen, the rapid degranulation and release of various mediators occurs. Furthermore, IL-4 has been described as a crucial mediator responsible for a differentiation of T-cells to Th2 subtype (38).
IL-5 acts as an eosinophil-protecting and maturation factor and plays an important role in the stimulation of eosinophils in the allergic inflammation. TNF-α released from the mast cells enhances an expression of adhesion molecules (E-selectin, intercellular adhesion molecule 1 (ICAM-1)) on the endothelial cells. This enables the infiltration of leukocytes into the site of inflammation and offers an important signal for maturation and migration of dendritic cells, thus supporting the immune response. TNF-α activates alveolar macrophages and neutrophils, and together with TGF-β released from the mast cells participates in the fibrotic changes of bronchi in chronic bronchial asthma (39). In our study, roflumilast and tadalafil significantly reduced the release of IL-5, and roflumilast additionally decreased the secretion of IL-4 in the lung tissue. Roflumilast showed the tendency to decrease the concentration of TNF-α; however, without reaching the level of statistical significance. Page and Spina (6) suggested that the inhibition of PDE4 suppresses the expression of inflammatory mediators via increased intracellular concentrations of cAMP, with simultaneous suppression of TNF-α and granulocytes/monocytes colony stimulating factor (GM-CSF) secretion. The inflammation in the airways leads to the tissue remodeling due to an overexpression of matrix metalloproteases (MMP), which is induced also by TNF-α. The inhibitors of PDE4 are potent blockers of pro-MMP secretion induced by TNF-α, and therefore, may be of benefit in the diseases associated with abnormal tissue destruction and remodeling (40). In a murine model of bronchial asthma, the selective inhibition of PDE4 with roflumilast led to a significant decrease of IL-13 and IL-4 (27), that is in concordance with our data obtained from a guinea pig lung tissue.
In other murine model of bronchial asthma, an inhaled administration of roflumilast was effective in the suppression of IL-4, IL-5, TNF-α, IgE specific for ovalbumin, and white blood cell count in the BAL fluid. However, its combination with a corticosteroid fluticasone propionate was even more potent, suggesting perspectives of the combined use of PDE4 inhibitors with other anti-inflammatory or bronchodilating drugs (41). Nevertheless, information on the effectiveness of selective PDE4 inhibitors co-administered with other drugs (corticosteroids, or bronchodilating drugs) in asthma regarding a potential dose reduction in maintaining, or even improving their effects is still insufficient (41). In this study, the effects of co-administration of two different selective PDE inhibitors at the reduced doses were evaluated. Roflumilast combined with tadalafil decreased concentrations of all three measured inflammatory mediators, suggesting a potential of this dual inhibition. Patel et al. (10) have recently demonstrated that selective PDE4 inhibition (cilomilast, piclamilast, and rolipram), but not PDE3 inhibition (cilostamid, cilostazol, and milrinon), enhanced the anti-inflammatory action of long-acting β2-agonists (formoterol). This effect is mainly related to the decreased production of TNF-α and subsequently of IL-8 (CXCL-8), due to increased intracellular levels of cAMP in the smooth muscle cells after activation of β2-receptors and induction of mitogen-activated protein (MAP) kinase phosphatase (MKP-1) (10).
Induction of inflammatory genes in the bronchial asthma and COPD is stimulated also by oxidation stress. Redox-sensitive transcription factor NF-κB as an important element in a wide spectrum of inflammatory pathways regulating the activity of cytokines, chemokines, and adhesion molecules (e.g. TNF-α, GM-CSF, IL-8, IL-2 and IL-6, adhesion protein, E-selectin, ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1)) (42) is regulated by a reduction/oxidation potential, i.e. it is influenced by an imbalance between oxidants (reactive oxygen species (ROS)) and antioxidants (43). In our experiments, NF-κB present in the lung homogenate was suppressed only in the guinea pigs treated with roflumilast alone or in combination with tadalafil, demonstrating the superior role of PDE4 on its expression.
In this study, we decided to measure concentrations of thiobarbituric acid reactive substances (TBARS), representing one of the markers of lipid peroxidation. The reason for this analysis was based on the hypothesis that ovalbumin-sensitization could increase the oxidation stress (44). We found that the activation of eosinophils and neutrophils in the ovalbumin-sensitized guinea pigs elevated concentrations of TBARS in the lung homogenate, proving an overproduction of ROS. These data are in concordance with other authors demonstrating increased concentrations of markers of oxidation stress in bronchial asthma patients (45, 46). Oxidation stress enhances pro-inflammatory responses via activation of redox-susceptible transcription factors, e.g. NF-κB and activation protein (AP)-1 (47). ROS stimulate PDEs with subsequent decrease of cAMP and increase in Ca2+ signaling in the inflammatory cells. Therefore, antioxidant action of PDE inhibitors may prevent the increase of Ca2+ levels in the macrophages and suppress the production of TNF-α (48). In our study, the administration of roflumilast and tadalafil caused a drop in the measured concentrations of TBARS. However, an expected decrease in TBARS was not observed in the group treated by a combination of these two drugs in reduced doses, indicating a dose-dependent antioxidant potential of selective PDE4 and PDE5 inhibitors.
Our data suggest that both of the tested agents (roflumilast and tadalafil) can be used in diseases associated with allergic inflammation, which was in this study mimicked by the ovalbumin sensitization. Furthermore, the patients may benefit from their synergistic effect, as only a half dose was used for their simultaneous administration, and thus the potentially observed adverse effects of PDE4 inhibitors could be minimized. The concept of dual PDE inhibition has been previously described in the literature and new dual PDE inhibitors (e.g. PDE3/4, PDE4/5, and PDE4/7) are intensively studied for their anti-inflammatory action (24, 49). It is important to mention that increased intracellular concentrations of cAMP and suppression of calcium signaling may prevent proliferation of airway smooth muscle (50).
In our experiments, dexamethasone as a long-acting corticosteroid proved a strong anti-inflammatory activity, as well. Another corticosteroid (fluticasone) administered intranasally has demonstrated potent local anti-inflammatory effects without damage of epithelial cells, confirming benefits of corticosteroid use in inflammatory airway diseases (51). The effects of dexamethasone in our study were compared with roflumilast and tadalafil at the doses of 1.0 mg/kg b.w. as well as with the combination of the PDE inhibitors at half-reduced doses. These doses were lower than in previous experiments (52) for better comparison and demonstrating the efficacy with decreased risk of adverse effects. Despite the dose reduction, all of the agents demonstrated significant bronchodilating and anti-inflammatory effects, including action on circulating leukocytes, and lung tissue levels of cytokines and NF-κB. The difference between the action of roflumilast and tadalafil can be explained by different doses necessary to reach the same effect, as confirmed by a previous study of Takeda et al. (53). They found that both roflumilast and tadalafil were (after a single dose) potent modulators of allergen-induced alterations in tracheal smooth muscle responsiveness in mice, with higher dose of tadalafil necessary. Furthermore, they confirmed a potential of their co-administration at lower doses (0.1 mg/kg of roflumilast and 2 mg/kg of tadalafil). In contrary to that study, we used the 7-days lasting administration of these drugs, with potentially stronger action both on airway reactivity and inflammatory mediators. The doses of roflumilast and tadalafil used in our experiments were chosen in order to compare the results of roflumilast and tadalafil at the same doses in guinea pigs (54). We are aware of the fact that the pharmacological effects were not maximal based on the previous study by Sawamura et al. (55) in rats; however, the effects of tadalafil in that study were evaluated on vascular smooth muscle (pulmonary hypertension). Similarly, higher doses of roflumilast (5 mg/kg) have been used in the study by Forkuo et al. (56), who demonstrated attenuation of the asthma-like phenotype promoted by β2-agonists by PDE4 inhibition induced by roflumilast or rolipram in phenylethanolamine N-methyltransferase-knockout mice. In our study, the effects of roflumilast and tadalafil on TNF-α and NF-κB were more obvious than those of dexamethasone. These observations could be explained by the limited duration of the therapy, or by the dose of dexamethasone used in our experiments, which was lower than in other experiments (52, 57, 58). Nevertheless, the antioxidant activity of roflumilast and tadalafil was comparable to dexamethasone at the same doses.
In conclusion, the selective inhibitors of PDE5 (tadalafil) and PDE4 (roflumilast) led to the suppression of airway reactivity and markers of inflammation in the model of ovalbumin-induced eosinophilic inflammation associated with the airway hyperresponsiveness, which was comparable to the effects of dexamethasone at the same dose. The results suggest a potential benefit of PDE5 and PDE4 inhibitors in the therapy of allergic bronchial asthma or asthma-COPD overlap, whereas their combination seems to be one of the promising possibilities. However, further testing is necessary to confirm these expectations.
Acknowledgements: Authors thank to K. Jesenska for technical assistance.
This study was supported by VEGA grants 1/0260/14, 1/0255/18, and 1/0356/18, Slovak Research and Development Agency under the contract number APVV-0305-12 and APVV-17-0052, and by the project “Biomedical Center Martin” (ITMS code: 26220220187) - the project is co-financed from EU sources.
Conflict of interests: None declared.
REFERENCES
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev 2006; 58: 488-520.
- Urbanova A, Kertys M, Simekova M, et al. Bronchodilator and anti-inflammatory action of theophylline in a model of ovalbumin-induced allergic inflammation. Adv Exp Med Biol 2016; 935: 53-62.
- Mokry J, Mokra D. Immunological aspects of phosphodiesterase inhibition in the respiratory system. Respir Physiol Neurobiol 2013; 187: 11-7.
- Kawamatawong T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J Thorac Dis 2017; 9: 1144-1154.
- Barnes PJ. Frontrunners in novel pharmacotherapy of COPD. Curr Opin Pharmacol 2008; 8: 300-307.
- Page CP, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol 2012; 12: 275-286.
- Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011; 163: 53-67.
- Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast-a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2010; 23: 235-256.
- Meltzer EO, Chervinsky P, Busse W, et al. Roflumilast for asthma: efficacy findings in placebo-controlled studies. Pulm Pharmacol Ther 2015; 35 (Suppl): S20-S27.
- Patel BS, Prabhala P, Oliver BG, Ammit AJ. Inhibitors of phosphodiesterase 4, but not phosphodiesterase 3, increase b2-agonist-induced expression of antiinflammatory mitogen-activated protein kinase phosphatase 1 in airway smooth muscle cells. Am J Respir Cell Mol Biol 2015; 52: 634-640.
- Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol 2013; 6: 197-219.
- Bateman ED, O’Byrne PM, Buhl R, Rabe KF. Roflumilast for asthma: weighing the evidence. Pulm Pharmacol Ther 2015; 35 (Suppl): S1-S3.
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol 2006; 147 (Suppl. 1): S252-S257.
- Medvedova I, Prso M, Eichlerova A, Mokra D, Mikolka P, Mokry J. Influence of roflumilast on airway reactivity and apoptosis in ovalbumin-sensitized Guinea pigs. Adv Exp Med Biol 2015; 838: 11-18.
- Franova S, Nosalova G, Pechanova O, Sutovska M. Red wine polyphenolic compounds inhibit tracheal smooth muscle contraction during allergen-induced hyperreactivity of the airways. J Pharm Pharmacol 2007; 59: 727-732.
- Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 2007; 56: 331-340.
- Pennock BE, Cox CP, Rogers RM, Cain WA, Wells JH. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol Respir Environ Exerc Physiol 1979; 46: 399-406.
- Mokry J, Mokra D, Nosalova G, Beharkova M, Feherova Z. Influence of selective inhibitors of phosphodiesterase 3 and 4 on cough and airway reactivity. J Physiol Pharmacol 2008; 59 (Suppl. 6): 473-482.
- Chung KF. Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol 2006; 533: 110-117.
- Giembycz MA. Life after PDE4: overcoming adverse events with dual-specificity phosphodiesterase inhibitors. Curr Opin Pharmacol 2005; 5: 238-244.
- Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast - an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005; 366: 563-571.
- Rogoz R, Dziedzicka-Wasylewska M. Comparison of the effects of antidepressant drugs on the level of cAMP in the rat striatum and nucleus accumbens septi. Pol J Pharmacol 1996; 48: 481-487.
- Mombereau C, Gur TL, Onksen J, Blendy JA. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int J Neuropsychopharmacol 2010; 13: 321-334.
- Mokry J, Urbanova A, Medvedova I, et al. Effects of Selective Inhibition of PDE4 by YM976 on airway reactivity and cough in ovalbumin-sensitized guinea pigs. Adv Exp Med Biol 2016; 921: 61-70.
- Toward TJ, Smith N, Broadley KJ. Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways disease. Am J Respir Crit Care Med 2004; 169: 227-234.
- Karish SB, Gagnon JM. The potential role of roflumilast: the new phosphodiesterase-4 inhibitor. Ann Pharmacother 2006; 40: 1096-1104.
- Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodelling in a murine model of chronic asthma. Clin Exp Allergy 2016; 46: 754-763.
- Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. in vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 2001; 297: 280-290.
- Clayton RA, Dick CA, Mackenzie A, et al. The effect of selective phosphodiesterase inhibitors, alone and in combination, on a murine model of allergic asthma. Respir Res 2004; 5: 4.
- Al Qadi-Nassar B, Bichon-Laurent F, Portet K, Tramini P, Arnoux B, Michel A. Effects of L-arginine and phosphodiesterase-5 inhibitor, sildenafil, on inflammation and airway responsiveness of sensitized BP2 mice. Fundam Clin Pharmacol 2007; 21: 611-620.
- Mokry J, Joskova M, Mokra D, Christensen I, Nosalova G. Effects of selective inhibition of PDE4 and PDE7 on airway reactivity and cough in healthy and ovalbumin-sensitized guinea pigs. Adv Exp Med Biol 2013; 756: 57-64.
- Banner KH, Page CP. Acute versus chronic administration of phosphodiesterase inhibitors on allergen-induced pulmonary cell influx in sensitized guinea-pigs. Br J Pharmacol 1995; 114: 93-98.
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 2005; 365: 167-175.
- Venkatasamy R, Spina D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br J Pharmacol 2016; 173: 2335-2351.
- Matera MG, Page C, Cazzola M. PDE inhibitors currently in early clinical trials for the treatment of asthma. Expert Opin Investig Drugs 2014; 23: 1267-1275.
- Franciosi LG, Diamant Z, Banner KH, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med 2013; 1: 714-727.
- Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol 2001; 79: 149-153.
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454: 445-454.
- Djukanovic R. Asthma: a disease of inflammation and repair. J Allergy Clin Immunol 2000; 105 (2 Pt 2): S522-S526.
- Martin-Chouly CA, Astier A, Jacob C, Pruniaux MP, Bertrand C, Lagente V. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci 2004; 75: 823-840.
- Murad HA, Habib HS, Rafeeq MM, Sulaiman MI, Abdulrahman AS, Khabaz MN. Co-inhalation of roflumilast, rather than formoterol, with fluticasone more effectively improves asthma in asthmatic mice. Exp Biol Med (Maywood) 2017; 242: 516-526.
- Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015; 5: 1266-1283.
- Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 2008; 10: 799-811.
- Fatani SH. Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma 2014; 51: 578-584.
- Guo CH, Chen PC, Hsia S, Hsu GS, Liu PJ. The relationship of plasma aluminum to oxidant-antioxidant and inflammation status in asthma patients. Environ Toxicol Pharmacol 2013; 35: 30-38.
- Al-Afaleg NO, Al-Senaidy A, El-Ansary A. Oxidative stress and antioxidant status in Saudi asthmatic patients. Clin Biochem 2011; 44: 612-617.
- Mokra D, Drgova A, Pullmann R, Calkovska A. Selective phosphodiesterase 3 inhibitor olprinone attenuates meconium-induced oxidative lung injury. Pulm Pharmacol Ther 2012; 25: 216-222.
- Brown DM, Hutchison L, Donaldson K, MacKenzie SJ, Dick CA, Stone V. The effect of oxidative stress on macrophages and lung epithelial cells: the role of phosphodiesterases 1 and 4. Toxicol Lett 2007; 168: 1-6.
- Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc American Thor Soc 2005; 2: 326-333.
- Song X, Zhang Y, Wang H, et al. Stereoselectivity of tradinterol’s inhibition on proliferation of airway smooth muscle cells induced by acetylcholine through suppressing Ca(2+) signalling. J Physiol Pharmacol 2016; 67: 363-375.
- Trybus E, Krol T, Obarzanowski T, Trybus W, Kopacz-Bednarska A, Obarzanowski M. Cytological assessment of the epithelial cells of the nasal mucous membrane after local fluticasone therapy. J Physiol Pharmacol 2015; 66: 139-147.
- Lowe AP, Thomas RS, Nials AT, Kidd EJ, Broadley KJ, Ford WR. Route of administration affects corticosteroid sensitivity of a combined ovalbumin and lipopolysaccharide model of asthma exacerbation in Guinea pigs. J Pharmacol Exp Ther 2017; 362: 327-337.
- Takeda K, Shiraishi Y, Domenico J, et al. Combined effects of the phosphodiesterase type 4 (PDE4) inhibitor, roflumilast, and the PDE5 inhibitor, tadalafil, in allergen-induced airway hyperresponsiveness (AHR). Am J Crit Care Med 2009; 179: A5717.
- Urbanova A, Medvedova I, Kertys M, et al. Dose dependent effects of tadalafil and roflumilast on ovalbumin-induced airway hyperresponsiveness in guinea pigs. Exp Lung Res 2017; doi: 10.1080/01902148.2017.1386735
- Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A. Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci 2009; 111: 235-243.
- Forkuo GS, Kim H, Thanawala VJ, et al. Phosphodiesterase 4 inhibitors attenuate the asthma phenotype produced by b2-adrenoceptor agonists in phenylethanolamine N-methyltransferase-knockout mice. Am J Respir Cell Mol Biol 2016; 55: 234-242.
- Murad HA, Hasanin AH. The anti-inflammatory effects of 1,1 dimethyl-4-phenylpiperazinium (DMPP) compared to dexamethasone in a guinea pig model of ovalbumin induced asthma. Eur Rev Med Pharmacol Sci 2014; 18: 2228-2236.
- Mahajan SG, Mehta AA. Suppression of ovalbumin-induced Th2-driven airway inflammation by b-sitosterol in a guinea pig model of asthma. Eur J Pharmacol 2011; 650: 458-464.
A c c e p t e d : September 25, 2017