POSSIBLE ROLE OF GARLIC OIL IN AMELIORATING RENAL INJURY
AFTER LIVER ISCHEMIA/REPERFUSION IN RATS
INTRODUCTION
Ischemia/reperfusion injury (I/R) is defined as cellular damage secondary to oxygen deprivation which is aggravated by the sudden restoration of blood perfusion (1). Due to the high incidence of non-alcoholic fatty liver diseases in female obese subjects, long-term complications of liver failure becomes more common despite presence of hepatoprotective activity of fibroblast growth factor-21 and omentin-1 (2). Thus, I/R is a common clinical condition such as organ transplantation, hepatectomy for cancer complications and shock (3).
In addition to acute liver injury, liver I/R causes remote organ damage such as kidney, heart, lung (4). Thus, acute kidney injury (AKI) was frequently found in patients with acute liver failure, considered as a serious clinical problem during the perioperative period increasing patients’ morbidity and mortality (5).
AKI was evident within 24 hours after liver I/R in mice and was attributed to intra-renal vasoconstriction resulted from splanchnic vasodilatation following portal hypertension (6). Also, systemic inflammatory response after I/R may be another causal factor of such remote organ damage (7).
Previously, Arduini et al. (8) reported that ischemia could impair mitochondrial antioxidant defences, thereby, enhancing the possibility of oxidative stress, and altering many enzymes such as heme oxygenase 1 (HO1). HO1 may prevent early injury in the reperfused organ, and could inhibit immune reactive cells activation, thereby ameliorating an antigen-independent I/R injury (9). Further, HO1 could be involved in mitochondrial quality control in term of mitochondrial biogenesis and autophagy (10).
In addition to cell necrosis after I/R injury, autophagy was found to play an important role in distant organ damage following warm or cold I/R (11).
Mitochondrial biogenesis, which is a crucial and dynamic process for reactive oxygen species (ROS) production in many conditions, is regulated by peroxisomal proliferator activator receptor gamma coactivator-1alpha (PGC1α) (12). In oxidative stress, there are lowered mitochondrial biogenesis and inhibited autophagy accumulating small dysfunctional mitochondria together with higher ROS production and deficient adenozyno-5’-trifosforan (ATP) production.
However, controversial studies on the status of autophagy in liver I/R are present (11, 13). So, it is important to investigate some autophagy markers in remote renal injury following liver I/R insult, and to shed more light on the associated changes in HO1 and PGC1α.
As natural antioxidant compounds are preferred to control diseases rather than synthetic ones (14), garlic (Allium sativum) has been known for its antioxidant properties (15). The four most important organosulfur compounds, suggested to be the major biological agents, are diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS) and allylmethyl trisulfide (16). Shaik et al. (17) demonstrated that diallyl sulfide could protect the liver from I/R by reducing oxidative stress through, at least in part, induction of HO1 (9).
Up to the authors’ knowledge, the reno-protective effects of garlic oil pre-treatment prior to liver I/R injury through altered HO system and mitochondrial-regulating genes in terms of biogenesis and mitophagy has not been studied before.
The aim of this study is to investigate the effects of garlic oil pretreatment prior to liver I/R on the remote renal changes in rats. Also, it investigated the possible role of garlic oil in altering some mitochondrial related gene expression in such state.
MATERIALS AND METHODS
Animals
This study was performed on 36 adult female Wistar rats, initially weighing 180 – 250 g, purchased from animal farm, Giza. The care of the experimental animals adopted the National Institutes of Health guidelines for the human use of laboratory animals. Rats were housed in animal cages (4/cage) with suitable ventilation, temperature of 22 – 25°C, normal light dark cycle and free access to food and water ad libitum in the Animal House of Medical Ain Shams Research Center, Faculty of Medicine, Ain-Shams University (MASRI) in Cairo.
After one week of acclimation, surgical procedure was run under anesthesia to avoid induction of pain in animals. Rats were not exposed to unnecessary pain or stress, and animal manipulation was performed with maximal care and hygiene.
All animal experiments were performed according to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
At the end of experiment, rats were killed by overdose of anesthesia and animal remains disposal occurred by incineration.
Rats were randomly divided into four groups (9 rats in each group):
1) Control (sham-operated group): rats were supplemented by distilled water by gavage for 2 weeks, then they were exposed to I/R procedure without clamping of blood vessels (they were served as negative control group).
2) Garlic oil-supplemented group: they received garlic oil (Sigma-Aldrich) in a dose of 5 ml/kg/day by gavage (18) for 2 weeks, thereafter; they were exposed to I/R procedure without clamping of blood vessels (served as positive controls).
3) Liver ischemia/reperfusion group: rats were exposed to liver I/R procedure.
4) Garlic oil-pretreated liver ischemia/reperfusion group: rats were pretreated with garlic oil in a similar dose to that of garlic oil supplemented group. Thereafter, they were exposed to liver I/R procedure.
Garlic oil preparation
Garlic oil is mostly prepared by steam-distillation process for medical purposes. Steam-distilled garlic oil consists of the diallyl, allylmethyl, and dimethyl mono to hexa sulfides (19).
Experimental procedures
Liver ischemia/reperfusion procedure: the overnight fasted rats were weighed and anesthetized by i.p. injection of xylazine (EPICO) (10 mg/kg) and ketamine hydrochloride (EPICO) (100 mg/kg) (20). A midline laparotomy was made using minimal dissection. Total hepatic ischemia was induced for 45 min by clamping the hepatic artery, the portal vein and the bile duct using a microvascular clamp (21). During the period of hepatic ischemia, the abdominal cavity was closed with clamps. Then, the microvascular clamp was removed allowing reperfusion of hepatic tissue for 24 hours to study the late phase of liver I/R (22). Following reperfusion, the animals received 5 ml of warmed (body temperature) normal saline intraperitoneally and the incision was closed in two layers. The ani mals subsequently recovered at room temperature (23). Thereafter, all rats were kept in metabolic cages (Courtesy of Pharmacology Department, Faculty of Medicine, Ain Shams University) during the reperfusion period to collect the urine samples for subsequent assays.
During the last hour of reperfusion period, overnight fasted rats were weighed and anaesthetized with i.p. injection of pentobarbitone (40 mg/kg b.w.). When the stage of surgical anaesthesia has been reached, judged by loss of withdrawal reflexes, an abdominal midline incision was performed. Abdominal aorta was cannulated using polyethylene catheter, blood samples were collected into serum clot activator tubes with gel. These tubes were left for 30 minutes in room temperature, then, centrifuged at 3000 rpm for 15 minutes. The separated serum samples were used for subsequent determination of renal and liver functions and total antioxidants capacity (TAC).
Right lobe of liver and right kidney specimens, also, were dissected and were preserved at –80°C for subsequent determination of oxidative stress markers, namely MDA and mitochondrial NAD+, in addition to assay of gene expression levels of HO1, PGC1α and autophagy-related gene 7 (Atg7) in right kidney specimens.
Also, left kidney specimens from all studied groups were used for histopathological examination.
Serum samples were used for assessment of the levels of alanine transaminase (ALT), and aspartate transaminase (AST) using kinetic kits supplied by ELI TECH (France), and creatinine level was colorimeterically assayed in sera and urine samples using kits supplied by Biosystem S.A (Costa Brava, Spain). Assessment of urinary albumin was performed using colorimetric kits supplied by ELI TECH (France). TAC was performed using colorimetric kits, supplied by Biodiganostic (Egypt). All assays were performed according to the manufacturer’s instructions.
GFR was calculated by creatinine clearance over 24 hours as (U creatinine X V)/P creatinine, where U creatinine and P creatinine are urinary and sera creatinine levels, respectively, and V is urinary flow rate (24).
Evaluation of mitochondrial nicotinamide adenine dinucleotide+ in hepatic and renal tissues
Mitochondria isolation was carried out from stored frozen at –80°C hepatic and renal tissues, according to Saleh and Saleh (25). The nicotinamide adenine dinucleotide+ (NAD+) was measured after perchloric acid extraction. In the case of isolated mitochondria, 0.1 ml of 21% (v/v) perchloric acid was added to 1 mg of protein/ml suspensions. The NAD+ concentrations in the perchloric acid were measured using an alcohol dehydrogenase reaction. The reaction mixture contained 1000 µL of buffer substance (0.1 M Tris acetate, pH 8.8, and 0.5 M ethanol), 100 µL of the tissue extract neutralized, and 20 µL of alcohol dehydrogenase. The change of absorbance at 340 nm was recorded by a spectrophotometer.
Determination of liver and renal tissue malondialdehyde levels
Determination of liver and renal tissue malondialdehyde levels was performed using colorimetric kit supplied from Biodiagnostic (Egypt). Prior to dissection, both tissues were perfused with a phosphate buffered saline (PBS) solution, pH 7.4, containing 0.16 mg /ml heparin to remove any red blood cells and clots. The tissues were homogenized in 5 ml cold buffer (50 mM potassium phosphate, pH 7.5.) per gram tissue. Then, the samples were centrifuged at 4000 r.p.m for 15 minutes using cold centrifuge. The supernatant was removed and stored at –80°C till the day of assay. Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min to form thiobarbituric acid reactive product. The absorbance of the resultant pink product in each sample was measured at 534 nm against its sample blank.
RNA isolation from renal tissue and quantitative real-time PCR (qRT-PCR)
Equally weighed frozen renal tissues were homogenized in PBS, pH 7. The total RNA was extracted using TRIzol reagent (Invitrogen) (Fischer Scientific cat no 15596026), then the concentration and purity of RNA were determined by NanoDrop 1000 spectrophotometer. One µg of RNA was reverse transcribed for cDNA synthesis with QuantiTect® Reverse Transcription kit (QIAGEN, Germany, Cat no 205311). qRT-PCR was performed for HO1, PGC1α and Atg7 genes using Fast SYBR Green master mix (Qiagen Germany, Cat no. 204141), and 5 plex rotor gene ™ system (Qiagen) according to manufacturer’s instructions.
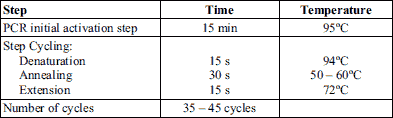
The primers for HO1 and PGC1α were designed and purchased from Macrogen, (Seoul, Korea). The primers sequence of the HO1 and PGC1α genes are displayed in Table 1. Meanwhile Atg7 was purchased from Qiagen, Germany (SG quantiTect primer; cat no. 249900; assay ID:QT0008974), also, β-actin gene, the reference gene was purchased from Qiagen, Germany (HS_ACTB_1 quantiTect primer; cat no. 249900; assay ID:QT000954 31). The cyclic conditions of QuantiTect SYBR Green PCR are shown in Table 2.

The expression of the target genes was defined based on the cycle threshold (Ct), their expression levels were calculated as 2–ΔΔ Ct after normalization to relative expression of β-actin gene (26).
Histopathological studies
The left kidney specimens (1 × 3 mm) were taken immediately after sacrifice, and were immediately dipped in 4% glutaraldehyde for two hours. Then, they were washed in phosphate buffer at pH 7.3, and were kept in the refrigerator at 4°C for 24 hours. The kidney specimens were post-fixed in 1% osmium tetraoxide for 2 hours, and then they were dehydrated in ascending grades of ethyl alcohol. Thereafter, they were cleared in propylene oxide and were finally embedded in spur premix at 60°C for 48 hours (27). Ultrathin sections were cut and mounted on grids. Contrast was enhanced in ultrathin sections by using a saturated solution of uranyl acetate in 50% alcohol followed by lead citrate. Examination by electron microscope was performed using multiple fields of interest and multiple animals in each group. The sections were examined using Philips 200 electron microscope in the Anatomy Department, Faculty of Medicine, Ain Shams University in Cairo.
Statistical analysis
The results are expressed as mean ± SE of the mean. Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA) program, version 20.0 was used to compare significance between each two groups. One-way ANOVA for difference between means of different groups was performed on the obtained results, using post-hoc test. Differences were considered significant when P ≤ 0.05. Also, two-way ANOVA for difference between means of different groups was used to demonstrate the effects of liver I/R and garlic oil supplementation on some parameters.
RESULTS
Changes in liver enzymes
As shown in Fig. 1, compared to the control rats, liver I/R group exhibited a significant rise in serum levels of ALT and AST (P < 0.001 for each). On the other hand, garlic oil-pretreated I/R group had significantly reduced serum ALT and AST levels, compared to untreated liver I/R group (P < 0.001 for each). When comparing garlic oil-pretreated liver I/R group and the controls, non-significant changes of serum ALT and AST levels were observed. Similarly, garlic oil-supplemented group and control ones had insignificant changes in liver enzymes.
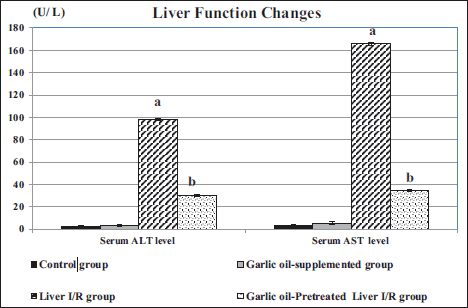 |
Fig. 1. Changes in liver functions in the different studied groups. (a): Significance by LSD at P < 0.05 from the control group; (b): Significance by LSD at P < 0.05 from liver I/R group. |
Assessment of distant effects of liver ischemia/reperfusion on the kidney
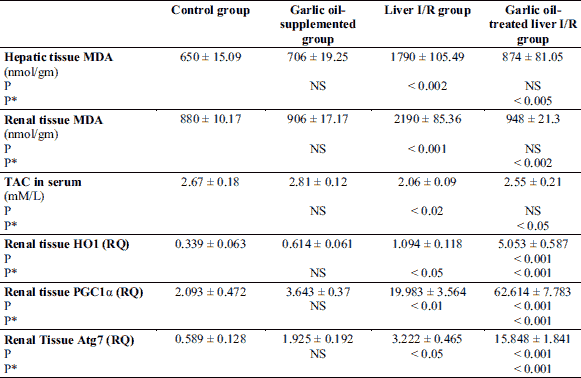
Liver I/R group exhibited significantly higher serum creatinine level compared to the control rats (P < 0.001), however, garlic oil-pretreated liver I/R had significantly lowered serum creatinine level compared to the untreated liver I/R group (P < 0.001), as shown in Fig. 2. Urinary albumin level was significantly elevated in untreated liver I/R group compared to the controls (P < 0.001). However, it was significantly reduced in garlic oil-pretreated liver I/R group compared to liver I/R group (P < 0.001). Non-significant changes in serum creatinine and urinary albumin levels were observed when comparing either garlic oil-supplemented or garlic oil-pretreated liver I/R group to the control rats, as shown in Fig. 2.
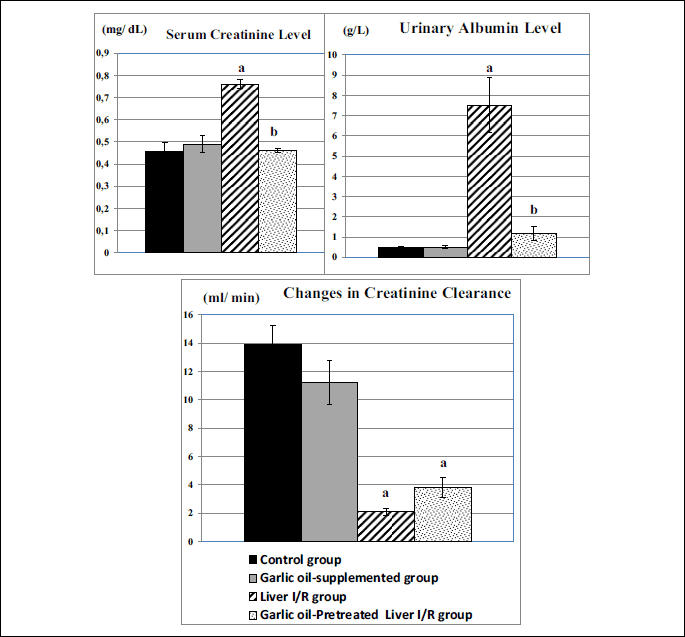
In addition, a significant reduction in creatinine clearance, as a test of glomerular filtration rate (GFR), was observed in untreated and treated liver I/R groups when each one was compared to control rats (P < 0.001 for each). However, creatinine clearance was insignificantly changed in garlic oil-pretreated liver I/R group compared to liver I/R group, as shown in Fig. 2.
Changes in oxidative stress markers
Hepatic tissue MDA and mitochondrial NAD+ levels were significantly elevated in liver I/R group compared to the control ones (P < 0.002, P < 0.001 respectively), while they were significantly lowered in garlic oil-pretreated liver I/R group compared to liver I/R group (P < 0.005, P < 0.01 respectively), despite a significantly higher hepatic tissue mitochondrial NAD+ compared to the control rats (P < 0.02). Similarly, liver I/R rats exhibited significantly higher renal tissue MDA and mitochondrial NAD+ levels compared to the control rats (P < 0.001, P < 0.01 respectively). Meanwhile, garlic oil-pretreated liver I/R group had significantly lowered renal tissue MDA and NAD+ levels compared to the untreated liver I/R group (P < 0.002 for each), in spite of a significantly higher renal tissue mitochondrial NAD+ (P < 0.02), as shown in Table 3 and Fig. 3. On the other hand, total antioxidant capacity (TAC) was significantly lower in liver I/R group than the controls (P < 0.02). Meanwhile, it was significantly elevated in garlic oil-pretreated liver I/R group compared to the untreated liver I/R rats (P < 0.05), as shown in Table 3.
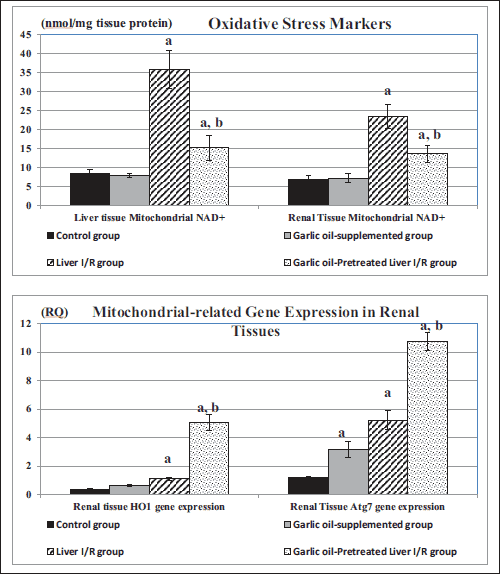 |
Fig. 3. Changes in mitochondrial NAD+ in hepatic and renal tissues and renal tissue HO1 and Atg7 gene expressions in the different studied groups. (a): Significance by LSD at P < 0.05 from the control group; (b): Significance by LSD at P < 0.05 from liver I/R group. |
Changes in mitochondrial related- gene expression in renal tissues
Liver I/R group had up-regulated HO1 gene expression in renal tissues compared to the control group (P < 0.05). Similarly, garlic oil pre-treated liver I/R group exhibited a significantly higher HO1 gene expression in the renal tissues compared to either untreated liver I/R group or control group (P < 0.001 for each), as shown in Table 3 and Fig. 3.
PGC1α, being an important factor for induction of mitochondrial biogenesis, was significantly upregulated in renal tissues in liver I/R group compared to the controls (P < 0.01). Similarly, garlic oil-pretreated liver I/R group showed significantly enhanced expression of PGC1α in renal tissues compared to either the controls or the untreated liver I/R group (P < 0.001 for each), as shown in Table 3.
On the other hand, Atg7 expression in renal tissues, being an indicator of mitophagy, was significantly elevated in the liver I/R group compared to the controls (P < 0.05). Also, it was significantly enhanced in the garlic oil-pretreated liver I/R group compared to the liver I/R group (P < 0.001), as shown in Table 3 and Fig. 3.
It was noted that garlic oil-supplemented rats had upregulated renal tissue HO1, PGC1α and Atg7 compared to the controls, though they did not reach the statistical level of significance, as shown in Table 3 and Fig. 3.
By using two-way ANOVA, it was found that garlic oil pretreatment caused significant lowering of serum creatinine and urinary albumin levels when compared to I/R operation. Also, garlic oil pretreatment significantly elevated GFR compared to I/R operation, as shown in Fig. 4. Similarly, all studied mitochondria-related gene expressions in renal tissue (HO1, Atg7 and PGC1α) were significantly enhanced by garlic oil pretreatment compared to I/R operation, as shown in Fig. 5.
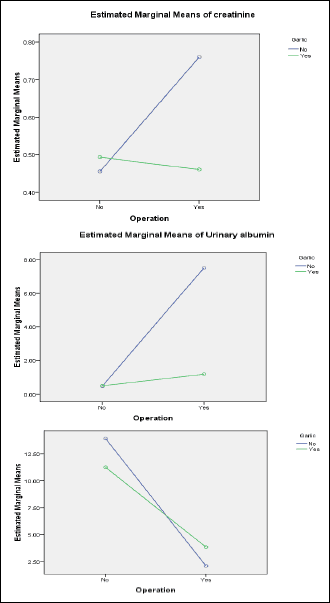 |
Fig. 4. Changes in parameters of renal function in the different studied groups using two way ANOVA to demonstrate the interaction between I/R operation and garlic oil supplementation. |
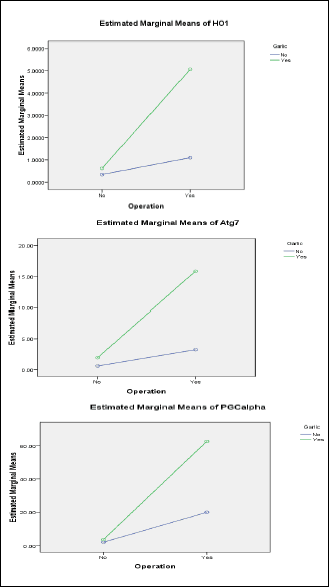 |
Fig. 5. Changes in mitochondrial gene expression in renal tissues in the different studied groups using two way ANOVA to demonstrate the interaction between I/R operation and garlic oil supplementation. |
Histological results
Electron microscopic examination of renal sections of the control group showed appearance of normal ultrastructure of the renal tubular epithelium with thin basal lamina, nuclei appeared euchromatic with marginally arranged chromatin and prominent nucleolus, mitochondria appeared elongated and spherical in shape, and brush border with apical microvilli were obvious (Fig. 6A). By further magnification, the normal ultrastructure of the renal tubular epithelium had numerous mitochondria which appeared spherical and elongated with prominent cristae. The brush border with microvilli was prominent (Fig. 6B). The ultrastructure of glomerulus in the control rats showed capillary tufts containing red blood cells, and the podocytes appeared clearly with intact foot processes (Fig. 7). Further magnification showed the normal structure of the filtration membrane; capillary fenestrations, thin glomerular basement membrane and podocyte foot processes were clearly evident (Fig. 8).
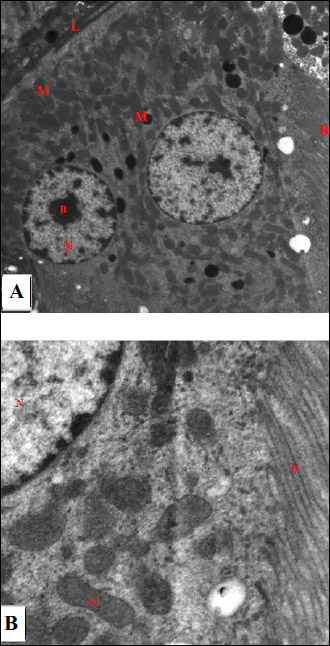 |
Fig. 6. An electromicrograph of a section of rat kidney of the control group showing: (A): the normal ultrastructure of the renal tubular epithelium. Note the normal basal lamina (L), euchromatic nuclei (N) with marginally arranged chromatin and prominent nucleolus (n), numerous mitochondria (M) elongated and spherical in shape and the brush border with apical microvilli (B). (Uranyl acetate and lead citrate ×1500); (B): Further magnification of electromicrograph A showing the normal ultrastructure of the renal tubular epithelium. Note the euchromatic nucleus (N) with marginally arranged chromatin, numerous mitochondria (M) with prominent cristea and the brush border (B) (Uranyl acetate and lead citrate × 6000). |
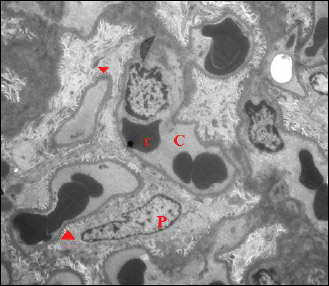 |
Fig. 7. An electromicrograph of a section of rat kidney of the control group showing normal glomerular ultrastructure. Notice capillary tuft (C) containing red blood cells (c), podocytes (P) with foot processes (arrow head). (Uranyl acetate and lead citrate × 1000). |
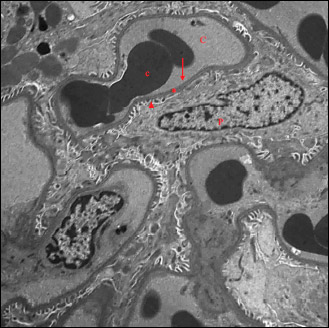 |
Fig. 8. Further magnification of the previous electromicrograph showing normal glomerular ultrastructure. Notice capillary tuft (C) containing red blood cells (c), podocytes (P) with foot processes (arrow head). Note the normal structure of the filtration membrane; capillary fenestrations (arrow), thin glomerular basement membrane (*) and podocyte foot processes (arrow head). (Uranyl acetate and lead citrate × 2000). |
Garlic supplemented group (positive control group) had the same arrangements of renal tubules and glomeruli as negative control group.
Electron microscopic examination of renal sections of liver I/R group revealed thickening of basal lamina of the renal tubular epithelium heterochromatic nuclei with peripheral clumped chromatin and indented nuclear membrane, in addition to small numerous mitochondria and cytoplasmic autophagosomes (Fig. 9A and 9B). With further magnification, degenerative changes in mitochondria appeared, some mitochondria with indistinct cristae were noticed (Fig. 10). Electron microscopic examination of the glomeruli of liver I/R group showed abnormal glomerular ultrastructure, effacement of podocyte foot processes with occluded filtration slits and congested blood capillaries (Fig. 11).
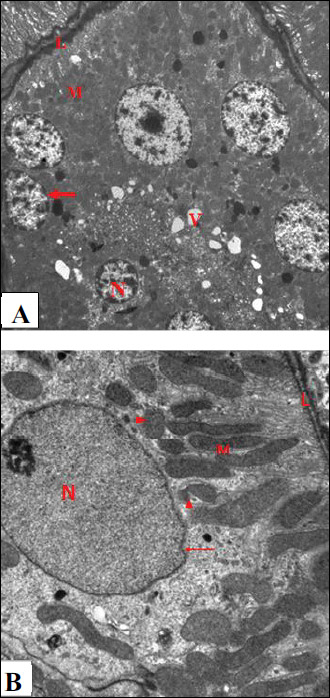 |
Fig. 9. An electromicrograph of a rat kidney section of liver I/R group showing: (A): the renal tubular epithelium with thickened basal lamina (L), heterochromatic nuclei (N) with peripheral clumped chromatin and indented nuclear membrane (arrow), numerous mitochondria (M) and vacuolization (V). (Uranyl acetate and lead citrate × 1000). (B): the renal tubular epithelium with basal lamina (L), nucleus with irregular nuclear membrane (arrow). Some mitochondria (M) show degenerative changes and indistinct cristae (arrow head). (Uranyl acetate and lead citrate × 3000). |
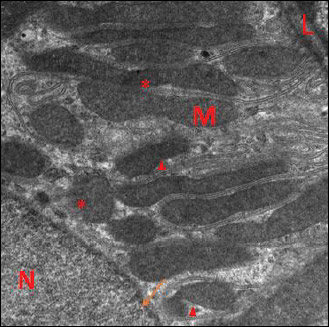 |
Fig. 10. Further magnification of the previous electromicrograph showing the renal tubular epithelium with thick basal lamina (L), nucleus with irregular nuclear membrane (arrow). Some mitochondria show degenerative changes (M) and indistinct cristae (arrow head) degenerative changes (arrow head) and indistinct cristae (*); (Uranyl acetate and lead citrate × 6000). |
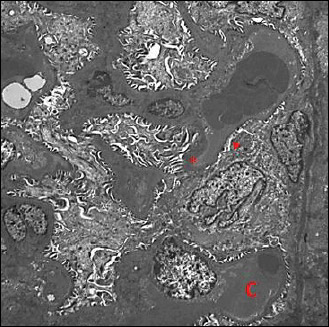 |
Fig. 11. An electromicrograph of a rat kidney section of liver I/R group showing abnormal glomerular ultrastructure. Notice the thickened basement membrane (*) and effacement of podocyte foot processes with occluded filtration slits (arrow head). Congested capillaries (C) are noticed. (Uranyl acetate and lead citrate × 1000). |
Electron microscopic examination of kidney sections of garlic oil-pretreated liver I/R group showed restoration of normal ultrastructure of the renal tubular epithelium. The nuclei appeared euchromatic with marginally arranged chromatin, and normal nuclear membrane were noticed. Numerous mitochondria appeared, which were spherical and elongated in shape, in addition the brush border with apical microvilli was present, with congested blood capillaries (Fig. 12). By further magnification, numerous mitochondria appeared elongated and spherical shape enclosed within the basal infoldings with normal appearance of mitochondrial cristae (Figs. 13 and 14) and cytoplasmic autophagosomes were seen. The normal glomerular ultrastructure in this group was observed, with appearance of the capillary tufts containing red blood cells and clearly present podocytes with normal foot processes (Fig. 15).
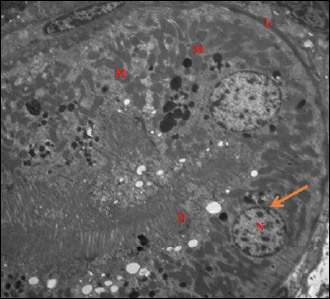 |
Fig. 12. An electromicrograph of a section of rat kidney of garlic oil-pretreated liver I/R group showing normal ultrastructure of the renal tubular epithelium, and normal basal lamina (L), euchromatic nuclei (N) and marginally arranged chromatin, normal nuclear membrane integrity (arrow), numerous mitochondria (M) spherical in shape, and the brush border with apical microvilli (B). (Uranyl acetate and lead citrate × 1500). |
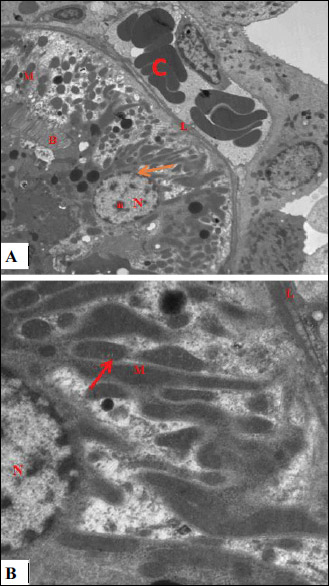 |
Fig. 13. An electromicrograph of a section of rat kidney of garlic oil-pretreated liver I/R group with further magnification showing: (A): normal ultrastructure of the renal tubular epithelium with normal of basal lamina (L), euchromatic nuclei (N) with prominent nucleolus (n) and marginally arranged chromatin, restoration of nuclear membrane (arrow), numerous mitochondria (M) which are elongated and spherical in shape enclosed within the basal infolding, and the brush border with apical microvilli (B). Note blood vessels still congested (C). (Uranyl acetate and lead citrate × 3000); (B): with further magnification, there is normal ultrastructure of the renal tubular epithelium with euchromatic nuclei (N) with marginally arranged chromatin, numerous mitochondria (M) and normal appearance of mitochondrial cristea (arrow). (uranyl acetate and lead citrate × 4000). |
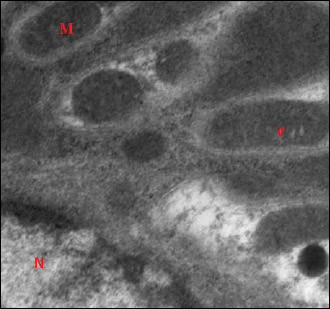 |
Fig. 14. Further magnification of the previous electromicrograph of a section of rat kidney of garlic oil-pretreated liver I/R group showing mitochondria (M) which are either elongated or spherical in shape with intact mitochondrial cristae (c). Note the nucleus (N). (Uranyl acetate and lead citrate × 4000). |
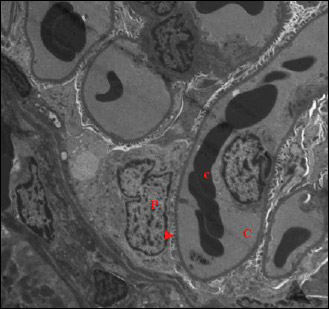 |
Fig. 15. An electromicrograph of a rat kidney section of garlic oil-pretreated liver I/R group showing gain of normal glomerular ultrastructure. Notice capillary tuft (C) containing red blood cells (c), podocytes (P) with normal foot processes (arrow head). (Uranyl acetate and lead citrate × 1500). |
DISCUSSION
This study demonstrated that liver I/R induced remote acute renal failure, renal tubular and glomerular damage and prominent oxidative stress. Pretreatment with garlic oil prior to liver I/R exhibited reno-protective and antioxidant effects.
The higher liver enzymes in liver I/R group, herein, are similar to Atef et al. (28), and could be attributed to the effect of reperfusion injury in early and late phases. During the early phase, the first 2 hours after reperfusion, Kuppfer cells are activated (29), in addition to enhancement of the production of ROS and cytokines, namely tumor necrosis factor-α and interleukin-1 (30). While in the late phase of reperfusion, the activated neutrophils release more ROS and more proteases causing extensive oxidative stress (31). Thus, the use of 24 hours period as reperfusion period, herein, was to demonstrate distant effects of I/R injury by the intense oxidative stress of the late stage of reperfusion. Fully unexpected, the elevated mitochondrial NAD+ in liver tissues in liver I/R group, in this study, could be explained according to Jaeschke (32) to the existent oxidative stress. It was suggested that oxidative stress might induce the formation of membrane permeability transition pores and the breakdown of the mitochondrial membrane potential causing cellular death (33).
The normalized liver enzymes in sera of garlic oil-pretreated liver I/R rats, herein, are in accordance to Sener et al. (34). This protection could be due to the garlic extract’s ability to preserve I/R-induced liver damage, through anti-inflammatory and immune modulatory effects (35). Also, the observed hepatoprotective effects of garlic oil were manifested, herein, as lowering of MDA and mitochondrial NAD+ levels in the liver tissues.
Additionally, liver I/R group, herein, exhibited renal impairment manifested as higher serum creatinine level, albuminuria, and reduced GFR compared to the controls. These findings are in accordance to Jochmans et al. (36). This remote acute kidney injury could be due to the systemic inflammation provoked by liver I/R (37). In support, cytokines and growth promoting factors were elevated in impaired lymph flow from the liver in rats (38). The released inflammatory mediators could affect endothelial cell adhesion molecules as P selectin and ICAM-1 in the kidney (39). Also, this renal damage in the untreated liver I/R, herein, could be due to the oxidative stress, which was evident by higher renal tissue MDA and mitochondrial NAD+, and lowered TAC.
Meanwhile, HO1 gene expression in renal tissues was upregulated in liver I/R group, similar to Tanaka et al. (37). This effect could be explained by ROS-induced enhanced stress gene expression, as an attempt to control the coexistent oxidative stress (40). This finding could be, also, attributed to the ability of inducible isoform of HO (HO1) to regulate inflammation. Thus, overexpressed HO1 in oxidative stress could scavenge peroxy radicals, and might inhibit lipid peroxidation (41). Also, HO1has anti-inflammatory effects through release of carbon monoxide (CO), by its catabolic action, thereby mediating anti-apoptotic effects (42). Therefore, these known cytoprotective effects of HO1 limited the severity of renal impairment, herein (43).
Further, the histopathological renal changes present in liver I/R group, herein, could explain the present renal impairment. The lowered GFR could be attributed to the observed thickening of the glomerular basement membrane, and to effacement of podocyte foot processes occluding filtration slits, while albuminuria could be due to the observed tubular damage in these rats in the form of damaged microvilli of the brush border of the renal tubular epithelium, in agreement with Han et al. (44). In line, Lee et al. (45) demonstrated that liver I/R in mice developed renal morphological changes in the form of focal proximal tubular cell necrosis, cytoplasmic autophagosomes, dilated lumina and granular cast formation. On the contrary, Polat et al. (46) found no overt renal injury under light and electron microscopy after liver I/R. However, Behrends et al. (22) demonstrated that severe hepatic ischemia resulted in a moderate impairment of renal function in rats without renal inflammatory response and morphological renal damage. This discrepancy could be due to the age of the used animals, the duration of ischemia and/or the reperfusion period.
Another possible explanation of deranged renal morphology and impaired renal function in liver I/R group, herein, is mitochondrial dysfunction according to Kubli and Gustafsson (47). These rats had mitochondrial degenerative changes and minimal mitochondrial cristal damage in renal tissues, similar to Ates et al. (48). These mitochondrial changes could be mediated by oxidative injury and apoptosis (49). In support, microelements such as zinc protected against gentamicin-induced acute renal failure (50).
Another mitochondrial biogenesis-related gene, PGC1α, was higher in renal tissues of liver I/R group, herein. It increases mitochondrial DNA synthesis, and a key regulator of mitochondrial functions, oxygen consumption and ATP production, in addition to stimulating mitochondrial biogenesis (51).
Interestingly, examination of renal tissues by electro-microscope, herein, revealed presence of autophagosomes, formation of a double-membrane vesicle by autophagy. These autophagosomes could engulf cytoplasmic components, and deliver them to lysosomes for degradation (52). Also, the upregulated Atg7 gene expression in renal tissue in liver I/R group, herein, could result from higher ROS production by I/R injury (53). Atg7 was found to induce autophagy which may be selective to mitochondria, known as mitophagy (54). Thus, liver I/R induced mitophagy in renal tissues as a protection mechanism by removing and recycling of damaged mitochondria (55).
On the other hand, garlic oil pretreatment, herein, exerted reno-protective effects in accordance to Elkhoely and Kamel (56). This protection could be mediated by enhancing antioxidant defense and reducing the inflammatory cytokines tissue levels, as well as, inhibition of apoptosis by diallyl sulfide, a natural organosulfur compound in garlic. The anti-inflammatory properties of garlic extracts occur through reducing the actions of proinflammtory cytokines and nitric oxide overproduction, and cyclooxygenase-2 expression (57). Also, garlic oil pretreatment, herein, caused a near normal renal tubular and glomerular ultrastructure, which could explain the decline in albuminuria and serum creatinine levels in addition to the non-significant rise of GFR in garlic oil-pretreated liver I/R group. Similarly, Bagheri et al. (58) demonstrated that garlic pretreatment 24 hour and 15 minutes before renal I/R in rats prevented renal functional and histological injuries mediated by the antioxidant potentials of garlic.
The higher expression of HO1 in renal tissues of garlic oil-pretreated liver I/R rats, herein, is in accordance to Seetharaman et al. (57). The HO system was suggested to exert three major functions in I/R injury: antioxidant effects; maintenance of microcirculation; and modulatory effects upon the cell cycle. The antioxidant functions depend on heme degradation, oxygen consumption and the production of biliverdin/ferritin via iron accumulation, while tissue microcirculation is preserved by the vasodilator CO production. Thus, the higher HO1 gene expression in renal tissues prevented the early injury during reperfusion period, and inhibited the function of activated immune cells according to Katori et al. (9).
In line, Seetharaman et al. (57) reported that garlic oil-induced up-regulation of HO1gene expression in activated macrophages proved the anti-inflammatory effects of garlic extract. Thus, it could be suggested that upregulated HO1 in renal tissues, herein, had many cytoprotective effects, herein, through its anti-inflammatory, anti-apoptotic and anti-proliferative actions (59).
Similar to Hull et al. (60), upregulated HO1 gene expression in garlic oil-pretreated rats, herein, was, also, combined with higher expression of PGC1α, to enhance mitochondrial biogenesis in renal tissues denoted by the numerous and near-normal mitochondrial structure in these rats. Higher HO1 expression enhances mitochondrial biogenesis by increasing the expression of nuclear respiratory factor 1 (NRF1) and its co-activator PGC1α, thereby, upregulating the mitochondrial transcription factor A expression, allowing the replication of the mitochondrial DNA.
Furthermore, the association of enhanced expressions of Atg7 and HO1 expression agree with Wang et al. (61), thus HO1 exerted such renal cytoprotection by stimulating autophagy. Therefore, it could be suggested that garlic oil affects mitochondrial quality by positively activating the expression of autophagy protein, Atg7, in renal tissues. In support, Allavena et al. (62) demonstrated a significant transcriptional induction of Atg7 genes and the related genes coding for proteins involved in the autophagic pathway, in ovarian endometriosis. Also, they suggested that these proteins, being recruited in an increased autophagic flux, could undergo a more rapid turnover, afforded by the autophagic process itself or by different proteolytic mechanisms.
Meanwhile, the oxidative stress was limited in garlic oil-pretreated liver I/R group, herein, evidenced by higher TAC, lowered oxidative stress markers in renal tissues, and preserved renal mitochondrial structure. These antioxidant effects could be, also, explained by the upregulated PGC1α in renal tissues. In support, biogenesis of mitochondria could modulate the elevated ROS production by electron transport chain under ischemic conditions (63). Also, Sanchez-Ramos et al. (64) reported that PGC1α has a regulatory role on expression of antioxidant genes and ROS production in the liver, explaining the hepatocyte tolerance to I/R. In line, Funk and Schnellmann (65) found that PGC1α exerted protective role against ischemic injury of cardiac, renal and central nervous system.
This study is a novel study in demonstrating the ability of garlic oil to alter the mitochondrial morphological changes in renal tissues induced by liver I/R, and normalized renal tissue mitochondrial NAD+ level. Garlic oil attenuated liver I/R-induced renal impairment through enhancing the expression of HO1, improvement of mitochondrial quality by enhancing some genes responsible for mitochondrial biogenesis and autophagy in renal tissue, namely Atg7 and PGC1α.
It is worth noting that the animals used in the current study were female rats, as female patients survived better than males after liver transplantation or hepatocellular carcinoma resection (45). Another possible cause of the choice of female gender, herein, is the more protection of female rats against the injurious effects of liver I/R than male rats (66). This gender-specific response is due to effective cytokine/chemokine regulation as an inflammatory response to hepatic I/R (67).
From the aforementioned results, it could be concluded that garlic oil exerted cyto-protective effects on liver I/R-induced renal injury, and this protection could be mediated by HO1 enzyme, as well as, modulating mitochondrial related genes, namely Atg7 and PGCa1.
Funding: This study was funded by the authors themselves only.
Conflict of interests: None declared.
REFERENCES
- Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011; 98: 1188-1200.
- Waluga M, Kukla M, Zorniak M, et al. Fibroblast growth factor-21 and omentin-1 hepatic mRNA expression and serum levels in morbidly obese women with non-alcoholic fatty liver disease. J Physiol Pharmacol 2017; 68: 363-374.
- Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 2005; 79: 505-514.
- Sun H, Zou S, Candiotti KA, et al. Octreotide attenuates acute kidney injury after hepatic ischemia and reperfusion by enhancing autophagy. Sci Rep 2017; 7: 42701. doi: 10.1038/srep42701
- Arroyo V, Fernandez J, Gines P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis 2008; 28: 81-95.
- Lee HT, Park SW, Kim M and D’Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest 2009; 89: 196-208.
- Schepke M. Hepatorenal syndrome: current diagnostic and therapeutic concept. Nephrol Dial Transplant 2007; 22 (Suppl. 8): viii2-viii4. doi: 10.1093/ndt.gfm656
- Arduini A, Mezzetti A, Porreca E, et al. Effect of ischemia and reperfusion on antioxidant enzymes and mitochondrial inner membrane proteins in perfused rat heart. Biochim Biophys Acta 1988; 970: 113-121.
- Katori M, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. A novel strategy against ischemia and reperfusion injury: cytoprotection with heme oxygenase system. Transpl Immunol 2002; 9: 227-233.
- Turkseven S, Drummond G, Rezzani R, et al. Impact of silencing HO 2 on EC SOD and the mitochondrial signalling pathway. J Cell Biochem 2007; 100: 815-823.
- Evankovich J, Zhang R, Cardinal JS, et al. Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am J Physiol Gastrointest Liver Physiol 2012; 303: G189-G198.
- Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 2003; 299: 896-899.
- Kang JW, Cho HI, Lee SM. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion. Cell Physiol Biochem 2014; 33: 23-36.
- Craig W, Beck L. Phytochemicals: health protective effects. Can J Diet Pract Res 1999; 60: 78-84.
- Morihara N, Sumioka I, Ide N, Moriguchi T, Uda N, Kyo E. Aged garlic extract maintains cardiovascular homeostasis in mice and rats. J Nutr 2006; 136: 777S-781S.
- Nicastro HL, Ross SA, Milner JA. Garlic and onions: their cancer prevention properties. Cancer Prev Res 2015; 8: 181-189.
- Shaik IH, George JM, Thekkumkara TJ, Mehvar R. Protective effects of diallyl sulfide, a garlic constituent, on the warm hepatic ischemia-reperfusion injury in a rat model. Pharm Res 2008; 25: 2231.
- Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. in vivo evidence of hepato-and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci 2009; 5: 249-255.
- Lawson LD, Bauer R. Garlic: a review of its medicinal effects and indicated active compounds. In: Phytomedicines of Europe. Chemistry and Biological Activity, 1998. Series 69 1. Washington DC: American Chemical Society; pp. 176-209.
- Yeboah MM, Xue X, Duan B, et al. Cholinergic agonists attenuate renal ischemia- reperfusion injury in rats. Kidney Int 2008; 74: 62-69.
- Sehirli O, Ozel Y, Dulundu E, Topaloglu U, Ercan F, Sener G. Grape seed extract treatment reduces hepatic ischemia-reperfusion injury in rats. Phytother Res 2008; 22: 43-48.
- Behrends M, Hirose R, Park YH, et al. Remote renal injury following partial hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg 2008; 12: 490-495.
- Behrends M, Hirose R, Serkova NJ, et al. Mild hypothermia reduces the inflammatory response and hepatic ischemia/reperfusion injury in rats. Liver Int 2006; 26: 734-741.
- Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 2001; 281: F887-F899.
- Saleh NK, Saleh HA. Protective effects of vitamin E against myocardial ischemia/reperfusion injury in rats. Saudi Med J 2010; 31: 142-147.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real- time quantitative PCR and the 2–DD CT method. Methods 2001; 25: 402-408.
- Hayat M. Principle and Techniques of Electron Microscopy. Vol. 1. Van Nostrano Reinhold ltd. 1989, pp. 24-74.
- Atef Y, El-Fayoumi HM, Abdel-Mottaleb Y, Mahmoud MF. Quercetin and tin protoporphyrin attenuate hepatic ischemia reperfusion injury, role of HO-1. Naunyn-Schmiedeberg’s Arch Pharmacol 2017; 390: 871-881.
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991; 260: G355-G362.
- Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of pro-inflammatory cytokines and enhanced activation of nuclear factor kappa B. Ann Clin Lab Sci 2001; 31: 383-390.
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol 2000; 15: 718-724.
- Jaeschke H. Xanthine oxidase-induced oxidant stress during hepatic ischemia-reperfusion: are we coming full circle after 20 years? Hepatology 2002; 36: 761-763.
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol 1997; 272: C1286-C1294.
- Sener G, Sehirli O, Ipci Y and Ercan F. Aqueous garlic extract alleviates ischaemia-reperfusion-induced oxidative hepatic injury in rats. J Pharm Pharmacol 2005; 57: 145-150.
- Durak I, Kavutcu M, Aytac B, et al. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J Nutr Biochem 2004; 15: 373-377.
- Jochmans I, Meurisse N, Neyrinck A, Verhaegen M, Monbaliu D, Pirenne J. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transplant 2017; 23: 634-644.
- Tanaka Y, Maher JM, Chen C and Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol 2007; 71: 817-825.
- Beck B. Transforming growth factor-b1 and tumor necrosis factor-a concentration in serum during disturbed lymph flow from a liver in rats. J Physiol Pharmacol 2017; 68: 91-98.
- Molitoris BA, Sandoval R, Sutton TA. Endothelial injury and dysfunction in ischemic acute renal failure. Crit Care Med 2002; 30: S235-S240.
- Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal 2002; 4: 749-758.
- Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 2004; 6: 841-849.
- Berberat PO, A-Rahim YI, Yamashita K, et al. Heme oxygenase- 1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis 2005; 11: 350-359.
- Seifi B, Kadkhodaee M, Delavari F, Mikaeili S, Shams S, Ostad SN. Pretreatment with pentoxifylline and N-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren Fail 2012; 34: 610-615.
- Han SJ, Jang H, Seu SY, et al. Hepatic ischemia/reperfusion injury disrupts the homeostasis of kidney primary cilia via oxidative stress. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 1817-1828.
- Lee CC, Chau GY, Lui WY, et al. Better post-resectional survival in female cirrhotic patients with hepatocellular carcinoma. Hepatogastroenterology 2000; 47: 446-449.
- Polat C, Tokyol C, Kahraman A, Sabuncuoglu B, Yilmaz S. The effects of desferrioxamine and quercetin on hepatic ischemia-reperfusion induced renal disturbance. Prostaglandins Leukot Essent Fatty Acids 2006; 74: 379-383.
- Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 2012; 111: 1208-1221.
- Ates E, Genc E, Erkasap N, et al. Renal protection by brief liver ischemia in rats1. Transplantation 2002; 74: 1247-1251.
- Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int 2004; 66: 514-517.
- Teslariu O, Pasca AS, Mititelu-Tartau L, et al. The protective effects of zinc in experimental gentamicin induced acute renal failure in rats. J Physiol Pharmacol 2016; 67: 751-757.
- Andersson U, Scarpulla RC. PGC-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol Cell Biol 2001; 21: 3738-3749.
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10: 458-467.
- Ding W, Yin X. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 2012; 393: 547-564.
- Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2006; 2: 39-46.
- Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol 2011; 32: 275-281.
- Elkhoely A, Kamel R. Diallyl sulfide alleviates cisplatin-induced nephrotoxicity in rats via suppressing NF-kB downstream inflammatory proteins and p53/Puma signalling pathway. Clin Exp Pharmacol Physiol 2017; 45: 591-601.
- Seetharaman R, Park SY, Yoon MK, et al. Heme oxygenase-1-mediated anti-inflammatory effect of a novel garlic compound in RAW264. 7 murine macrophages. Hortic Environ and Biotechnol 2014; 55: 148-157.
- Bagheri F, Gol A, Dabiri S and Javadi A. Preventive effect of garlic juice on renal reperfusion injury. Iran J Kidney Dis 2011; 5: 194-200.
- McDaid J, Yamashita K, Chora A, et al. Heme oxygenase-1 modulates the allo-immune response by promoting activation-induced cell death of T cells. FASEB J 2005; 19: 458-460.
- Hull TD, Boddu R, Guo L, et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight 2016; 1: e85817.
- Wang Y, Shen J, Xiong X, et al. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One 2014; 9: e98834. doi: 10.1371/journal.pone.0098834
- Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E. Autophagy is upregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase-1. Fertil Steril 2015; 103: 1244-1251.
- Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011; 93: 884S-8890.
- Sanchez-Ramos C, Tierrez A, Fabregat-Andres O, et al. PGC-1alpha regulates translocated in liposarcoma activity: role in oxidative stress gene expression. Antioxid Redox Signal 2011; 15: 325-337.
- Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 2013; 273: 345-354.
- Lu P, Liu F, Wang CY, et al. Gender differences in hepatic ischemic reperfusion injury in rats are associated with endothelial cell nitric oxide synthase-derived nitric oxide. World J Gastroenterol 2005; 11: 3441-3445.
- Crockett ET, Spielman W, Dowlatshahi S, He J. Sex differences in inflammatory cytokine production in hepatic ischemia-reperfusion. J Inflamm 2006; 3: 16. doi: 10.1186/1476-9255-3-16