Several factors were found to affect skeletal muscle CER metabolism. Dobrzynand Gorski (5) reported that prolonged exercise decreased the content of ceramide and the activity of neutral Mg2+-dependent sphingomyelinase (N-SMase) in rat skeletal muscles. Moreover, it was shown that acute exercise induced accumulation of ceramide metabolites: sphingosine and sphinganine in rat skeletal muscles (7). The reduction in muscle CER content was also reported in rats subjected to endurance training (10). On the other hand, in insulin resistant and diabetic humans and rodents the level of ceramide in skeletal muscles was found to be markedly increased (6, 11 - 13).
Peroxisome proliterator-activated receptors (PPARs) are ligand-activated transcription factors of the nuclear hormone receptor superfamily. Three distinct PPAR isoforms termed alpha,
There are conflicting reports in the literature on the effect of thiazolidinediones on skeletal muscle ceramide content. Lessard et al. (18) showed that administration of rosiglitazone markedly increased the content of CER in the soleus muscle of obese Zucker rats. On the contrary, treatment with troglitazone reduced skeletal muscle ceramide level in mice (19). However, the above-mentioned studies did not address the mechanism of this phenomenon. Therefore, the aim of our study was to examine the effects of PPAR
Animals and study design
The experimental protocol was approved by the Ethical Committee for Animal Experiments at the Medical University of Bialystok. Male Wistar rats (200-250 grams of body weight) were housed under controlled conditions (21 °C ± 2, 12 h light/12 h dark cycle) with unlimited access to water. The animals were divided into two groups: 1) fed ad libitum on a standard laboratory rat chow (Agropol, Motycz, Poland) containing 2.8% of fat by weight (n=20), 2) fed for three weeks on isocaloric high-fat diet containing 33.9% of fat by weight (n=20), prepared as described by Pascoe and Storlien (20). Each group was further divided into two subgroups: a) control (n=10) and b) treated daily for two weeks with a selective PPAR
Sphingomyelin and ceramide content
The samples were pulverized in an aluminum mortar precooled in liquid nitrogen. The powder was then transferred to a tube containing methanol and 0.01% butylated hydroxytoluene (Sigma) as an antioxidant. Lipids were extracted by the method of Folch. Next, ceramide and sphingomyelin were isolated by means of thin-layer chromatography (TLC) using the methods described by Yano et al. (21) and Mahadevappa et al. (22), respectively. Further analysis was performed as described, in detail, elsewhere (5). Briefly, the gel bands corresponding to the standards were scrapped of the plates and transferred into screw-cap tubes containing pentadecanoic acid (Sigma) as an internal standard. Ceramide and sphingomyelin fatty acids were then transmethylated in the presence of 14% boron trifluoride (Sigma) in methanol at 100 °C for 90 min. The fatty acid methyl esters were analyzed by means of gas-liquid chromatography. A Hewlett-Packard 5890 Series II system equipped with a double flame ionization detector and Agilent CP-Sil 88 capillary column (100 m, internal diameter of 0.25 mm) were used. The content of ceramide and sphingomyelin is presented as the sum of individual fatty acid residues.
The concentration of plasma free fatty acids
Lipids were extracted from the samples as described above and the fraction of free fatty acids (FFA) was isolated by means of TLC according to Roemen and van der Vusse (23). The gel bands corresponding to the FFA standard were scrapped of the plates and transferred into fresh tubes. FFA were then transmethylated and the content of their methyl esters was determined by means of gas-liquid chromatography as previously described in detail (24).
The content of sphingosine, sphinganine and sphingosine-1-phosphate
The content of sphingosine, sphinganine and S1P was measured simultaneously by the method of Min et al. (25). Briefly, tissues were homogenized in a solution composed of 25 mM HCl and 1 M NaCl. Acidified methanol and internal standards (C17-sphingosine and C17-S1P, Avanti Polar Lipids) were added and the samples were ultrasonicated in ice-cold water for 1 min. Lipids were then extracted by the addition of chloroform, 1 M NaCl and 3 N NaOH. The alkaline aqueous phase containing S1P was transferred to a fresh tube. The residual S1P in the chloroform phase was reextracted twice with methanol /1 M NaCl (1:1, v/v) solution and then all the aqueous fractions were combined. The amount of S1P was determined indirectly after dephosphorylation to sphingosine with the use of alkaline phosphatase (bovine intestinal mucosa, Fluka). To improve the extraction yield of released sphingosine some chloroform was carefully placed at the bottom of the reaction tubes. The CHCl3 fractions containing free sphingosine and sphinganine or sphingosine liberated from S1P were washed with alkaline water (pH adjusted to 10.0 with ammonium hydroxide) and then evaporated under a nitrogen stream. The dried lipid residues were redissolved in ethanol, converted to their o-phthalaldehyde derivatives and analyzed on a HPLC system (ProStar, Varian Inc.) equipped with a fluorescence detector and C18 reversed-phase column (Varian Inc. OmniSpher 5, 4.6 mm i.d. x 150 mm). The isocratic eluent composition of acetonitrile (Merck):water (9:1, v/v) and a flow rate of 1 ml/min were used.
The activity of sphingomyelinases
The activity of neutral Mg2+-dependent and acid sphingomyelinase (N- and A-SMase, respectively) was determined as reported by Liu and Hannun (26). Briefly, the muscle homogenates were centrifuged at 1000 x g for 10 min and 50 µl of the supernatant was used for further analysis. The activity of both sphingomyelinases was measured using radiolabeled substrate, [N-methyl-14C]-sphingomyelin (Perkin-Elmer Life Sciences). In the case of N-SMase, the reaction mixture contained 100 nmol of sphingomyelin (1154 dpm/nmol) in 100 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 0.1% Triton X-100 and 5 mM dithiothreitol in a final volume of 0.2 ml. In the case of A-SMase, the assay mixture contained 100 nmol of sphingomyelin (1154 dpm/nmol) in 100 mM sodium acetate (pH 5.0), 0.1% Triton X-100 and 0,1 mM EDTA. After incubation at 37 °C for 1 h the reaction was stopped by adding 1.5 ml of chloroform:methanol (2:1 v/v), followed by addition of 0.2 ml of water. A portion of the aqueous phase was transferred to scintillation vials and counted in a liquid scintillation counter for the radioactivity of the reaction product, 14C-choline phosphate.
The activity of ceramidases
The activity of alkaline (Al-CDase) and neutral (N-CDase) ceramidase was determined by the method of Nikolova-Karakashian and Merrill (27). The activity of the enzymes was measured using radiolabeled substrate, [N-palmitoyl-1-14C]-sphingosine (Moravek Biochemicals). The tissue homogenates were centrifuged at 1000 x g for 10 min and 50 µl of the supernatant was used for the analysis. The reaction was started by the addition of supernatant to the tubes containing 20 µl of substrate mixture (50 nmol of ceramide – 2353 dpm/nmol, 2.5 mg Triton X-100, 1 mg Tween 20, 0.4 mg sodium cholate) and 130 µl of a reaction buffer. The reaction buffer contained 125 mM sucrose, 0.01 mM EDTA and 100 mM Tris-HCl (pH 7.2) or 125 mM HEPES (pH 8.0) for N-CDase and Al-CDase activity assay, respectively. After incubation at 37 °C for 1 h the reaction was stopped by adding 2 ml of basic Doyle’s solution (isopropanol:heptane:1 N NaOH, 40:10:1, v/v/v), 1.8 ml of heptane and 1.6 ml of water. Samples were then centrifuged and the upper phase was discarded. The lower phase was washed with 1.6 ml of heptane and then 1 ml of 1 N H2SO4 and 2.4 ml of heptane were added. After centrifugation, aliquots from the upper phase were transferred to scintillation vials and analyzed for the radioactivity of the reaction product, 14C-palmitate.
Protein content
Protein content was measured with BCA protein assay kit (Sigma) according to the manufacturer’s instructions. Bovine serum albumin (fatty acid free, Sigma) was used as a standard.
Statistical analysis
All data are presented as means ± SD. Statistical comparisons were made by using two-way analysis of variance followed by Newman-Keuls test. If variances were heterogeneous among groups, Dunnett’s T3 test was used instead. p<0.05 was considered statistically significant.
General features of the experimental animals (Table 1)
Pioglitazone treatment of the control group fed on the standard chow did not produce significant alterations in weight gain. High-fat feeding of control rats increased the weight gain, however, the difference did not reach statistical significance (p<0.07). In animals fed on the high-fat diet pioglitazone administration considerably increased the weight gain.
| Table 1. Effect of pioglitazone and high-fat diet on the weight gain and plasma free fatty acid (FFA) concentration in the experimental groups of rats. |
 |
| Values are grams and nmol/ml ± SD for weight gain and plasma FFA concentration respectively (n=10). * p<0.05 vs. the control group fed standard diet, # p<0.05 vs. the control group fed high-fat diet. |
High-fat diet elevated the plasma FFA concentration in the control rats. Treatment with pioglitazone decreased the concentration of plasma FFA in animals fed both on the standard and on the high-fat diet by 44 and 31% respectively.
The content of sphingomyelin and ceramide (Fig. 1)
In control animals fed on the standard chow the content of sphingomyelin was highest in the soleus and lowest in the WG, with the content in RG in between. High-fat diet increased the level of sphingomyelin in all examined muscle types. Administration of pioglitazone reduced the content of sphingomyelin in the soleus and RG in rats fed either diet. In WG PPAR
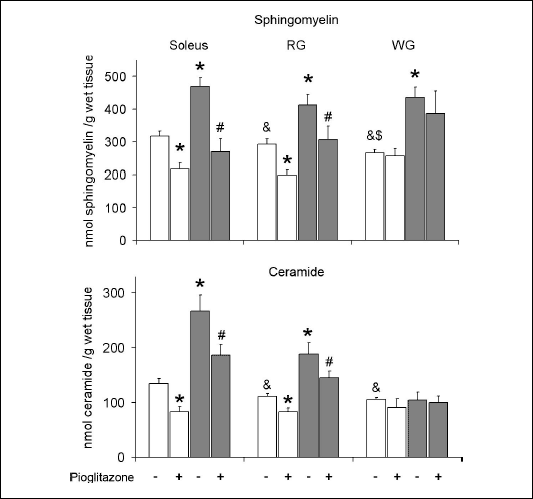 |
| Fig. 1. Effect of pioglitazone and high-fat diet on the content of ceramide and sphingomyelin in skeletal muscles. Rats were fed either the standard chow (white bars) or the high-fat diet (grey bars). Values are means ± SD, n=10. RG – red section of the gastrocnemius, WG – white section of the gastrocnemius, * p<0.05 vs. the control group fed standard diet, # p<0.05 vs. the control group fed high-fat diet, & p<0.05 vs. the respective value in the soleus, $ p<0.05 vs. the respective value in the RG. |
The content of sphinganine, sphingosine and S1P (Fig. 2)
In control animals fed on the standard chow the content of sphinganine, sphingosine and S1P was significantly lower in the WG comparing to the soleus and RG. High-fat diet increased the content of sphinganine in the soleus and did not affect its level in other examined muscle types. In rats fed on the standard chow pioglitazone did not alter the content of sphinganine in either muscle. However, in high-fat fed animals administration of PPAR
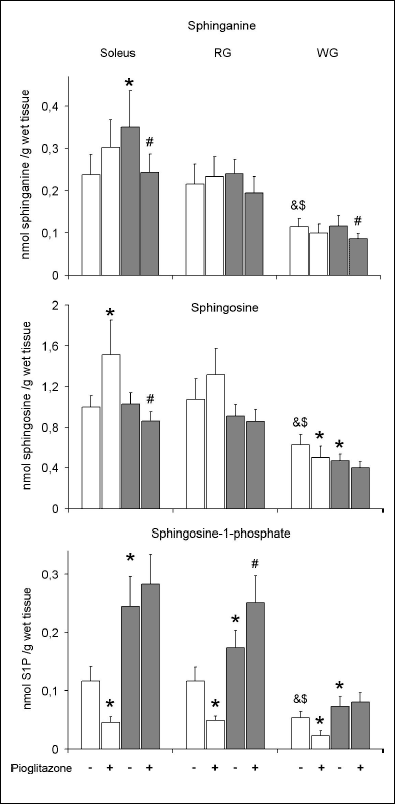 |
Fig. 2. Effect of pioglitazone and high-fat diet on the content of sphinganine, sphingosine and sphingosine-1-phosphate (S1P) in skeletal muscles. Rats were fed either the standard chow (white bars) or the high-fat diet (grey bars). Values are means ± SD, n=10. RG – red section of the gastrocnemius, WG – white section of the gastrocnemius, * p<0.05 vs. the control group fed standard diet, # p<0.05 vs. the control group fed high-fat diet, & p<0.05 vs. the respective value in the soleus, $ p<0.05 vs. the respective value in the RG. |
The activity of sphingomyelinases (Fig. 3)
In the control animals maintained on the standard chow the activity of N-SMase was significantly lower in the RG comparing to the soleus and WG. High-fat diet markedly reduced the enzyme activity in all examined muscles. Administration of pioglitazone to rats fed on the standard chow increased the activity of N-SMase in the soleus and RG. However, in the case of high-fat fed animals the PPAR
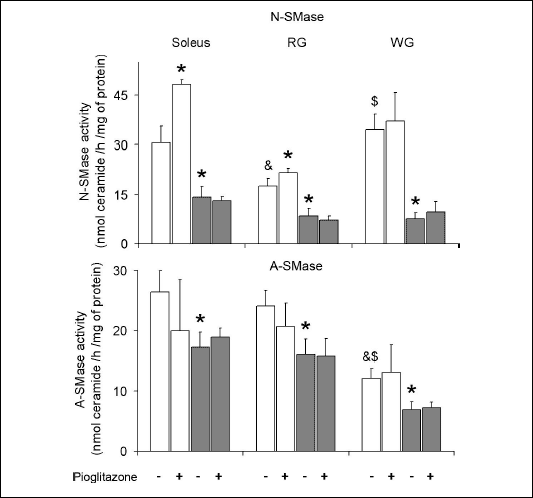 |
| Fig. 3. Effect of pioglitazone and high-fat diet on the activity of neutral (N-SMase) and acid (A-SMase) sphingomyelinase in skeletal muscles. Rats were fed either the standard chow (white bars) or the high-fat diet (grey bars). Values are means ± SD, n=6. RG – red section of the gastrocnemius, WG – white section of the gastrocnemius, * p<0.05 vs. the control group fed standard diet, & p<0.05 vs. the respective value in the soleus, $ p<0.05 vs. the respective value in the RG. |
The activity of ceramidases (Fig. 4)
The activity of Al- and N-CDase was similar in all examined muscles. High-fat diet increased the activity of N-CDase in the soleus and did not affect it in other examined muscles. Administration of pioglitazone to rats fed on the standard chow elevated the enzyme activity in the RG. In high-fat fed group the drug reduced the activity of N-CDase in the soleus and induced the opposite effect in the WG. The activity of Al-CDase in the soleus and WG was not affected by neither high-fat diet nor pioglitazone. In the RG the enzyme activity was reduced by high-fat diet and increased by administration of pioglitazone.
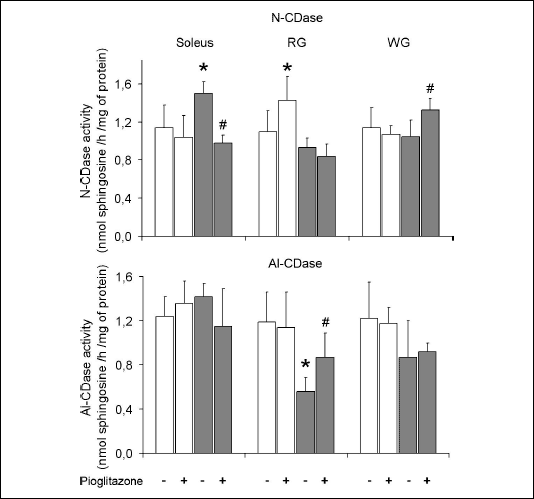 |
| Fig. 4. Effect of pioglitazone and high-fat diet on the activity of alkaline (Al-CDase) and neutral (N-CDase) ceramidase in skeletal muscles. Rats were fed either the standard chow (white bars) or the high-fat diet (grey bars). Values are means ± SD, n=6. RG – red section of the gastrocnemius, WG – white section of the gastrocnemius, * p<0.05 vs. the control group fed standard diet, # p<0.05 vs. the control group fed high-fat diet. |
The high-fat diet increased the content of sphingomyelin in all examined muscles. This effect was likely a result of reduced rate of SM degradation to ceramide, since the activity of both SMase isoforms was markedly inhibited by the high-fat diet. Increased dietary fat intake also induced the accumulation of ceramide in the soleus and RG. This is a surprising observation considering the abovementioned data on the activity of SMases. In the RG the reduced ceramide formation from sphingomyelin could be counterbalanced by the observed decrease in the activity of Al-CDase. This was not the case in the soleus where the activity of N-CDase was elevated by high-fat diet. However, the changes in the activity of ceramidases did not affect the content of sphingosine which indicates that the rate of ceramide deacylation was not altered significantly. In view of the above data, high-fat diet induced accumulation of muscle ceramide could not be explained by changes in the activity of SMases and/or CDases. Therefore, the most likely explanation of this phenomenon is the enhanced rate of ceramide synthesis de novo caused by increased lipid availability. This is supported by the increased content of sphinganine (an intermediate in de novo synthesis of CER) in the soleus and elevated concentration of plasma FFA in high-fat fed animals.
There are very few data in the literature regarding the effects of increased dietary fat intake on the activity of the examined enzymes and the content of ceramide in different tissues. Yang at al. (28) found a marked reduction in the activity of N- and A-SMase as well as N-CDase in the colonic mucosa of rats fed on a high-fat diet. On the other hand, Geelen and Beynen (29) reported an increase in the activity of N- and A-SMase in the liver of high-fat fed rats, which indicates that this response depends on the tissue type. Unfortunately there are no similar studies relating to muscles. Todd et al. (30) found that the content of ceramide in rat skeletal muscle is increased by high-fat diet, which is in line with the results of our study. Similar effect was reported in human and rat skeletal muscles after intravenous injection of lipid emulsions (31, 32). However, Lee et al. (33) did not observe changes in the level of muscle CER in rats subjected to a high-fat diet.
Our study demonstrated that pioglitazone reduced the content of ceramide in the soleus and RG irrespectively of dietary fat intake. This effect could not be attributed to the decreased rate of CER formation from sphingomyelin, since the activity of A-SMase was not affected by pioglitazone, irrespectively of the diet. Moreover, in rats fed on the standard chow the administration of PPAR
There is very few data in the literature concerning the effect of thiazolidinedione administration on CER level in muscle tissue. Our results are in line with reports showing that treatment with troglitazone reduces ceramide content in mice soleus (19) and in the heart of Zucker diabetic fatty rats (37). Similarly to us, the authors of the above studies concluded that this effect was a result of the reduction in the rate of ceramide synthesis de novo. However, it should be noted that Lessard et al. (18) showed that rosiglitazone increased the content of CER in the soleus of obese Zucker rats.
A number of studies showed that ceramide inhibits insulin-stimulated glucose uptake and GLUT4 translocation. The mechanism of this action involves inhibition of several intermediates in the insulin signaling pathway, namely insulin receptor substrate 1, phosphatidylinositol 3-kinase and Akt/protein kinase B (4). As already mentioned in the introduction, ceramide level was found to be increased in skeletal muscles of diabetic and insulin resistant humans and rodents. The results of our study indicate that decrease in the content of ceramide may be one of the mechanisms by which pioglitazone improves insulin sensitivity in skeletal muscles. The observed reduction in CER content was moderate. However, it was shown that relatively small changes in the level of this sphingolipid may significantly affect insulin signaling (4).
Administration of pioglitazone also affected the content of S1P in skeletal muscles. However, the agonist induced different effects in animals fed on the standard chow (marked reduction in S1P level) and on the high-fat diet (no change or increase in S1P level). Nevertheless, we can only speculate on the mechanism of this phenomenon. A plausible explanation is that pioglitazone affected the activity of one or more of the enzymes involved in S1P metabolism, namely sphingosine kinase, sphingosine-1-phosphate lyase and sphingosine-1-phosphate phosphatase (3). Unfortunately, there is no other data in the literature regarding the effect of thiazolidinediones on the content of S1P or the activity of enzymes of its metabolism.
An interesting finding of our study is that the influence of pioglitazone on sphingolipid metabolism largely depended on the muscle type. Administration of PPAR
In summary, we found that pioglitazone reduced the content of ceramide in the oxidative muscles (soleus and red section of the gastrocnemius), but did not affect it in the glycolytic muscle (white section of the gastrocnemius) independently of dietary fat intake. This effect was likely a result of reduced rate of de novo ceramide synthesis in the oxidative muscles due to decreased availability of plasma free fatty acids. The results of our study indicate that reduction in ceramide level may be one of the mechanisms by which pioglitazone improves skeletal muscle insulin sensitivity.
Acknowledgements: This work was funded by the European Union Exgenesis project No. LSHM-CT-2004-005272, the Polish State Committee for Scientific Research grant No. 3P05B 190 22 and the Medical University of Bialystok grant No. 3-18709.
- Plutzky J. Peroxisome proliferator-activated receptors in vascular biology and atherosclerosis: emerging insights for evolving paradigms. Curr Atheroscler Rep 2000; 2: 327-335.
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 2000; 106: 847-856.
- Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci 2001; 58: 2053-2068.
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2005; 45: 42-72.
- Dobrzyn A, Gorski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. Am J Physiol Endocrinol Metab 2002; 282: E277-E285.
- Straczkowski M, Kowalska I, Nikolajuk A, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004; 53: 1215-1221.
- Dobrzyn A, Gorski J. Effect of acute exercise on the content of free sphinganine and sphingosine in different skeletal muscle types of the rat. Horm Metab Res 2002; 34: 523-529.
- Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol 2004; 89: 119-127.
- Merrill AH Jr, Nixon DW, Williams RD. Activities of serine palmitoyltransferase (3-ketosphinganine synthase) in microsomes from different rat tissues. J Lipid Res 1985; 26: 617-622.
- Dobrzyn A, Zendzian-Piotrowska M, Gorski J. Effect of endurance training on the sphingomyelin-signalling pathway activity in the skeletal muscles of the rat. J Physiol Pharmacol 2004; 55: 305-313.
- Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 1990; 265: 16880-16885.
- Gorska M, Dobrzyn A, Zendzian-Piotrowska M, Gorski J. Effect of streptozotocin-diabetes on the functioning of the sphingomyelin-signalling pathway in skeletal muscles of the rat. Horm Metab Res 2004; 36: 14-21.
- Adams JM 2nd, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004; 53: 25-31.
- Gilde AJ, Van Bilsen M. Peroxisome proliferator-activated receptors (PPARs): regulators of gene expression in heart and skeletal muscle. Acta Physiol Scand 2003; 178: 425-434.
- Stumvoll M. Thiazolidinediones - some recent developments. Expert Opin Investig Drugs 2003; 12: 1179-1187.
- Yonemitsu S, Nishimura H, Shintani M, et al. Troglitazone induces GLUT4 translocation in L6 myotubes. Diabetes 2001; 50: 1093-1101.
- Kramer D, Shapiro R, Adler A, Bush E, Rondinone CM. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism 2001; 50: 1294-1300.
- Lessard SJ, Lo Giudice SL, Lau W, et al. Rosiglitazone enhances glucose tolerance by mechanisms other than reduction of fatty acid accumulation within skeletal muscle. Endocrinology 2004; 145: 5665-5670.
- Planavila A, Alegret M, Sanchez RM, Rodriguez-Calvo R, Laguna JC, Vazquez-Carrera M. Increased Akt protein expression is associated with decreased ceramide content in skeletal muscle of troglitazone-treated mice. Biochem Pharmacol 2005; 69: 1195-1204.
- Pascoe WS, Storlien LH. Inducement by fat feeding of basal hyperglycemia in rats with abnormal beta-cell function. Model for study of etiology and pathogenesis of NIDDM. Diabetes 1990; 39: 226-233.
- Yano M, Kishida E, Muneyuki Y, Masuzawa Y. Quantitative analysis of ceramide molecular species by high performance liquid chromatography. J Lipid Res 1998; 39: 2091-2098.
- Mahadevappa VG, Holub BJ. Chromatographic analysis of phosphoinositides and their breakdown products in activated blood platelets/neutrophils. In Chromatography of Lipids in Biomedical Research and Clinical Diagnosis, A Kuksis (ed). Amsterdam, Elsevier, 1987, pp. 225-265.
- Roemen TH, van der Vusse GJ. Application of silica gel column chromatography in the assessment of non-esterified fatty acids and phosphoglycerides in myocardial tissue. J Chromatogr 1985; 344: 304-308.
- Nawrocki A, Gorski J. Effect of plasma free fatty acid concentration on the content and composition of the free fatty acid fraction in rat skeletal muscles. Horm Metab Res 2004; 36: 601-606.
- Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 2002; 303: 167-175.
- Liu B, Hannun YA. Sphingomyelinase assay using radiolabeled substrate. Methods Enzymol 2000; 311: 164-167.
- Nikolova-Karakashian M, Merrill AH Jr. Ceramidases. Methods Enzymol 2000; 311: 194-201.
- Yang L, Mutanen M, Cheng Y, Duan RD. Effects of fat, beef and fiber in diets on activities of sphingomyelinase, ceramidase and caspase-3 in rat colonic mucosa. Med Princ Pract 2002; 11: 150-156.
- Geelen MJ, Beynen AC. Consumption of olive oil has opposite effects on plasma total cholesterol and sphingomyelin concentrations in rats. Br J Nutr 2000; 83: 541-547.
- Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP. Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 2006 (in press).
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002; 51: 2005-2011.
- Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002; 277: 50230-50236.
- Lee JS, Pinnamaneni SK, Eo SJ, et al. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 2006; 100: 1467-1474.
- Cabrero A, Jove M, Planavila A, Merlos M, Laguna JC, Vazquez-Carrera M. Down-regulation of acyl-CoA oxidase gene expression in heart of troglitazone-treated mice through a mechanism involving chicken ovalbumin upstream promoter transcription factor II. Mol Pharmacol 2003; 64: 764-772.
- Carley AN, Semeniuk LM, Shimoni Y, et al. Treatment of type 2 diabetic db/db mice with a novel PPARgamma agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab 2004; 286: E449-E455.
- Ye JM, Dzamko N, Cleasby ME, et al. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 2004; 47: 1306-1313.
- Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 2000; 97: 1784-1789.
- Clore JN, Li L, Rizzo WB. Effects of fructose and troglitazone on phospholipid fatty acid composition in rat skeletal muscle. Lipids 2000; 35: 1281-1287.
- Hallakou S, Foufelle F, Doare L, Kergoat M, Ferre P. Pioglitazone-induced increase of insulin sensitivity in the muscles of the obese Zucker fa/fa rat cannot be explained by local adipocyte differentiation. Diabetologia 1998; 41: 963-968.
- Kawamura T, Yoshida K, Sugawara A, et al. Regulation of skeletal muscle peroxisome proliferator-activated receptor gamma expression by exercise and angiotensin-converting enzyme inhibition in fructose-fed hypertensive rats. Hypertens Res 2004; 27: 61-70.