Hp infection is not only the major pathogen of the gastrointestinal tract but may also be responsible for the growth retardation, dyspeptic symptoms and reduced appetite, possibly due to inhibition of the expression and release of ghrelin, identified in the oxyntic gland area of the stomach as an endogenous ligand of growth hormone secretagogue receptor. In addition to its potent growth hormone releasing activity, ghrelin influences also appetite, energy balance, gastric motility and acid secretion (19-20).
Our preliminary findings of a higher prevalence of Hp infection and higher incidence of dyspeptic symptoms in shepherd children, as well as retardation in growth (18-22) prompted us to conduct the present study to determine the relationship between Hp infection and the serum release of appetite-affecting gastric hormones such as ghrelin, leptin and gastrin in children and adults.
Study population
We studied healthy adult subjects and school children from the same residential (Cracow) area of similar socio-economic status. Enrollment criteria for the adult group included subjects without history of malignancy, peptic ulcer, past treatment with antibiotics and bismuth salts as well as the use of non-steroidal anti-inflammatory agents or immunosuppressive therapy. Total number of 120 male healthy subjects aged from 23 to 48 yr (mean 41.5; SEM 3.2 yr) was included and divided at random into two subgroups, one with positive and another with negative UBT. We also studied 60 children (28 male and 32 female) from the same region, aged from 6 to 17 yr (mean 10.2 yr ± 2.2 yr) and divided into two subgroups, one with positive and another negative UBT. None of children had history of peptic ulcer, gastrointestinal malignancy or past treatment with antibiotics, antisecretory agents and immunosuppressive therapy. All subjects completed a detailed questionnaire under supervision of gastroenterologists regarding their demographic data, childhood and present socio-economic status, number of persons in the household, parents’ occupation, and past and present history of dyspeptic symptoms such as pain and/or discomfort in the upper abdomen, fullness, nausea and appetitive behavior as specified by Falley et al. (23).
Serum and UBT samples were collected from year 2002 to 2005. The study was reviewed, approved and supervised by the Institutional Ethical Committee for Human Research at the Medical College, Cracow, Poland.
Determination of Hp status
Hp status was assessed using our modification of the 13C-urea breath test (UBT) by placing urea, an Hp urease substrate, in special capsules (to avoid contact with oral urease containing oral bacteria) that disintegrate in the gastric lumen within about 1-2 min after swallowing. This low-dose capsulated UBT has been effectively used in over 20,000 subjects from 1994 and was found to exhibit over 95% and 97% sensitivity and specificity, as described previously (24). Briefly, two baseline breath samples were collected after an overnight fast followed by administration of capsulated 13C-urea (~ 40 mg). Post-dose breath samples were collected 10 and 20 min later. All samples were analyzed using a mass spectrometer (Heliview, Medichems, South Korea). A delta value of 2.5 over baseline was considered as positive result (17).
Blood samples were withdrawn after an overnight fast, and serum samples were separated and immediately frozen at –80 °C until determination of serum anti-Hp and anti-Cag IgG using a commercial ELISA kit (EIAGEN HP IgG, Clone Systems). As indicated by the manufacturer, the results of anti-Hp IgG > 15 AU/mL (arbitrary units) and anti-CagA > 0.3 OD (optical density) were considered seropositive, as reported previously (24,25).
Measurement of serum ghrelin, leptin and gastrin
Ghrelin concentrations were determined in serum samples using a human radioimmunoassay kit (Peninsula Lab., Inc., Bachem, San Carlos, CA) according to the manufacturer’s instruction (25-27). Serum leptin was determined using a human leptin radioimmunoassay kit (R/D Systems Inc, Minneapolis, NM) as described previously (28). Serum gastrin concentration was measured by specific RIA using highly specific antiserum (No 4562, kindly supplied by Professor Jens Rehfeld from Copenhagen University, Denmark). This antiserum recognized alpha-amidated gastrin-17 (G-17) and G-34 equally, as described previously, and has been employed in our laboratory for the last two decades at a final dilution of 1:500.000, with normal values established in healthy fasting subjects below 60 pmol/L (29). Plasma IL-8 and TNFalpha concentrations were also measured in all tested subjects by ELISA using commercially available kits (Biosource Europe SA, Belgium) in accordance with the manufacturer’s instructions (25).
Data analysis
Hp infection was considered present when positive results were obtained for the UBT and the anti-IgG or anti-CagA ELISA tests. The data were expressed as means ± standard error of the mean. Statistical analyses were performed using the Student t test or Tukey test after analysis of variance. A P value of less than 0.05 was considered statistically significant.
Based on UBT and anti-Hp IgG tests two groups of school children and two groups of adults were included in this study, one with negative UBT and serology Hp tests and another with positive Hp tests. Anti-CagA antibodies occurred in 58% of Hp positive adults and in 52% of Hp positive school children, but were not detectable in Hp negative subjects.
Mean basal serum ghrelin concentration in Hp positive adults was 550 ± 80 pg/ml compared to 1040 ± 100 pg/ml in age-matched Hp-negative controls, and this difference was highly statistically significant (Fig. 1). Serum basal leptin levels in Hp positive adults were significantly higher than those in Hp-negative controls. Serum basal gastrin concentrations in Hp positive adults averaged 53.6 ± 6.2 pmol/l and were significantly higher than those in the Hp-negative controls (31 ± 4.2 pmol/l). Serum ghrelin levels in Hp-infected children were about 650 ± 40 pg/ml and were markedly lower than those in Hp-negative children (1150 ± 94 pg/ml). Serum leptin was 4.3 ± 0.6 ng/ml in Hp-infected children, significantly higher than that in children without Hp infection (Fig. 1). Plasma gastrin was 47 ± 16 pmol/l in Hp-infected children and this was, significantly higher than that in Hp-negative children (28.3 ± 3.2 pmol/l).
 |
| Fig. 1. Serum ghrelin and leptin concentrations in fasted school children and adults with and without Hp infection Asterisk indicates significant (P < 0.05) change as compared to the values recorded in in Hp positive children and adults. |
Fig. 2 shows the day-time alterations in serum ghrelin and leptin in 5 Hp positive and 5 Hp negative adults. Serum ghrelin concentrations showed a marked increase after an overnight fast and also before lunch and dinner, but immediately after ingestion of meal the hormone level dramatically declined. These increments in serum ghrelin were significantly more pronounced in Hp negative than in Hp positive subjects. In contrast, serum levels of leptin showed a tendency to increase with the decline of serum ghrelin, but failed to demonstrate the meal-related alterations. The overall levels of serum leptin were significantly higher in Hp positive than in Hp negative subjects. (Fig. 2).
 |
| Fig. 2. The daily alterations in serum levels of ghrelin and leptin in adult subjects at 2 h intervals before and after breakfast, lunch and dinner with and without Hp infection. Mean of 5 determinations in 5 adult subjects. Asterisk indicates significant increase as compared to the values recorded in Hp positive subjects. |
In Hp positive adults, basal serum levels of TNF-a (36.6 ± 7.2 pg/ml) and IL-8 (38.2 ± 5.1 pg/ml) were 2-5 times higher than those in Hp-negative controls. Similarly, cytokine concentrations were significantly lower in Hp-negative than in Hp-positive children. Dyspeptic symptoms (23), including mainly discomfort centered in the upper abdomen accompanied by the lost or reduced appetite, were reported by majority (65%) of Hp-positive children, but only by 15% of Hp-negative children.
The results of this study show that Hp infection has pronounced influence on serum levels of gastric hormones involved in appetitive behavior, particularly on the serum concentrations of ghrelin and gastrin. Ghrelin exhibits marked fluctuations during the day time, reaching the peak value just before the meal and then shows the sudden decline to the nadir followed by subsequent slow increase before the next meal. These ghrelin fluctuations were more pronounced in Hp negative than in Hp positive subjects. Such a day-time alterations in serum ghrelin were tested only in adults so further studies are needed to find out whether they occur also in children. It is of interest that reduced serum levels of ghrelin were accompanied by the dyspeptic symptoms including the suppression of the appetite, observed in majority of examined Hp infected children.
The control of food intake and body weight actually concerns the control of adipose tissue with the key role of hypothalamus, possessing several neuronal centers such as that in lateral hypothalamic nuclei considered to be “hunger” center and in ventromedial nuclei serving as the “satiety” center (Fig. 3). In addition, hypothalamic paraventricular and arcuate nuclei (ARC) are the sites of action of multiple hormones released from the gut and adipose tissue that regulate food intake and energy expenditure (Fig. 4). There are two distinct types of neurons in ARC that are important in control of food intake; (1) proopiomelanocortin (POMC) and cocaine-amphetamine-related constructs (CART) neurons activated by peripheral anorexigenic hormones and releasing a-melanocyte-stimulating hormone (alpha-MSH) in satiety center and (2) neurons activated by orexigenic peptides such as ghrelin and orexins that release the substances including neuropeptide Y (NPY), orexins and Agouti-related Peptide (AgrP) in hunger center. Arcuate nucleus integrates also afferent neural (mostly vagal) and humoral inputs such as enteropeptides including orexigenic (ghrelin and orexins) and anorexigenic peptides (cholecystokinin, polypeptide YY, glucagon-like peptide-1, oxyntomodulin, leptin and others) that exert a physiological role in governing appetite and satiety. The peripherally (gut, adipose tissue) and centrally expressed modulators of appetitive behavior act through specific receptors in the afferent (mostly vagal) nerves and hypothalamic neurons implicated in adiposity signaling and regulation of food intake (Fig. 5).
 |
| Fig. 3. Enteropeptides and neurotrasmitters involved in the suppression (anorexigenic peptides) and stimulation of appetitive behavior resulting, respectively, in cahexia and obesity. |
 |
| Fig. 4. Hypothalamic centers and peripheral adiposity, satiety and hunger signals as well as vagal afferents involved in the control of satiety and hunger in medial and lateral hypothalamic area, respectively. |
 |
| Fig. 5. Peripheral signals originating from the adipose tissue (leptin), pancreas (insulin), the stomach (ghrelin) and intestines (PYY3-36, oxyntomodulin (OXM), GLP-1, PP and GI affectine appetitive behavior through the action on the hypothalamuc or vagal nerves. |
The major gastrointestinal hormone with known orexigenic properties is ghrelin (29) which has been identified in gastric ghrelin X/A cells, now called Gr cells, characterized by large eletrodense granules of P/D type in man and A-like type in rats (31). Ghrelin is a 28 amino acid peptide, primarily released by these endocrine cells in empty stomach (Fig. 6). As shown previously and demonstrated in this report, plasma concentrations of ghrelin peak under fasting conditions before the meal and then level off after meal to a nadir to increase again after gastric emptying before next meal (32). The mechanisms of ghrelin action on appetite and food intake is believed to be primarily mediated through peripheral input at the arcuate nucleus (ARC) and further spread to the nucleus tracti solitari (NTS). Ghrelin exerts growth hormone (GH)-releasing properties (33) and is involved in the hypothalamic regulation of metabolic control and energy balance. Ghrelin serves as a ligand for growth hormone secretagogue receptors (GHS-R). The primary hypothalamic target for ghrelin are neurons in ARC that contain and release NPY and AgRP in the lateral hypothalamus and in PVN to mediate orexigenic effect in the brain (34, 35). It may also inhibit the neurons in the ARC that contain POMC-derivative a-MSH that mediate the anorexigenic effect in the ventromedial hypothalamus (36). An appetite stimulating action of ghrelin has been proven in humans (37). Clinical implications of this have been forwarded as patients with Prader-Willi syndrome exhibit greatly increased circulating levels of ghrelin accompanied by marked enhancement of appetite and obesity (38, 39). Furthermore, gastric bypass surgery for morbid obesity leads to the considerable weight loss and reduction in appetite (40, 41), pointing at a ghrelin as mediator of altered energy balance and appetitive behaviour. Peripherally in the gut, ghrelin was shown by us to stimulate gastric acid secretion and gastrin release (43) and to exhibit the gastro- and pancreato-protective activities against various irritants (43). In addition, ghrelin was reported to exert a prokinetic effect on the small bowel, where it stimulates activity front of the migrating motor complex (MMC) through cholinergic mechanisms (44). It is of interest that food desire combined with an increase of gastric acid secretion occurs after intake of ethanol at low concentration (“coctail”), which appears to enhance the overexpression of ghrelin in the oxyntic mucosa and an increase in plasma levels of this peptide, gastric motility and gastric acid secretion (45). Thus, ghrelin appears to contribute to the initiation of food intake, body mass index (BMI) and stimulation in motilin-like fashion of gastrointestinal motility.
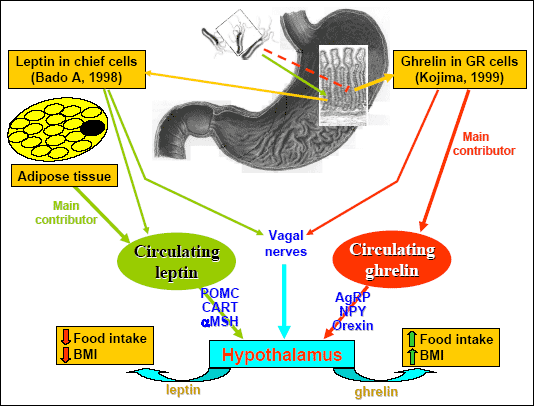 |
| Fig. 6. Ghrelin and leptin, their sites of origin and way of action on hypothalamic centers to affect food intake and BMI. The inhibitory influence of Hp infection on the release of ghrelin and stimulatory action on leptin release are also marked. |
In addition to the initiation of food intake, exogenous ghrelin decreases the release and action of leptin and vice-versa exogenous leptin reduces the plasma level of ghrelin (46). It has been proposed that leptin exerts a negative regulatory effect on the release and action of ghrelin and that increase in ghrelin induced by fasting or weight loss arises because of the diminished inhibitory input from leptin and probably also from PYY. This may implay that weight- and appetite-reducing effects of leptin are mediated not only by its direct central action on hypothalamus but also through its peripheral inhibitory effect on the release and action of ghrelin. According to our experience in rats (47), the parenteral administration of ghrelin at a dose that raised plasma hormone to the level observed under fasting conditions, significantly attenuated plasma levels of leptin, while markedly increasing food intake. Immuno-neutralization of circulating plasma ghrelin with specific IgG anti-ghrelin antibodies, caused a marked increase in plasma leptin and decreased food intake. In contrast, exogenous leptin, at the dose (10 µg/kg ip) that raised plasma leptin to the level occurring postprandially, reduced markedly plasma levels of ghrelin and attenuated food intake and these effects were reversed by the administration of specific IgG anti-leptin antibodies (47). These results clearly support the hypothesis that ghrelin negatively controls plasma release of leptin and vice-versa that leptin has a counter-regulatory influence on ghrelin release and action, though the former effect appears to be much stronger than the later. This interaction between ghrelin and leptin in control of food intake has been called “ghrelin-leptin tango”.
In addition to ghrelin, orexins A (OXA) and B (OXB), the novel neuropeptides were found to play the role in the stimulation of food intake and energy homeostasis (48). OXA has been detected in the mucosa and neuronal plexuses of the gastrointestinal tract and in the central nervous system, especially in lateral hypothalamus, the area involved in the stimulation of food intake (49, 50). Plasma levels of OXA are increased during fasting in humans (51) and are lower in obese subjects than normal-weight people (52), suggesting that peripheral OXA modulate food intake as an orexigenic peptide (53). However, intravenous infusion of OXA in humans does not appear to induce any hunger-stimulating drive in humans but increases gastric emptying.
In the present study, we attempted to determine the relationship between Hp infection and two crucial peptides, ghrelin and leptin, which are known to play an important role in the regulation of appetite, body mass index (BMI) and food intake (47), in the groups of healthy school children and healthy adults. We were particularly interested in the influence of Hp infection on ghrelin release into the circulation both under basal and postprandial conditions, since previous studies showed that stomach is the major source of circulating ghrelin (45, 46). It was reported that plasma ghrelin concentration is markedly decreased following gastrectomy (54), but evidence to date shows conflicting results for the influence of Hp infection with or without eradication therapy on gastric ghrelin, and, in overall, the subject remains controversial (55, 56). For instance, Nwokolo et al. (55) reported that the plasma ghrelin level increased after Hp eradication, suggesting a possible link between Hp infection and ghrelin secretion in the post-treatment stomach. In contrast, another study revealed a lack of significant difference in plasma ghrelin levels between Hp-positive and Hp-negative patients of similar age and body mass index (BMI) (56). Recent reports by Isomoto et al. (57) and Ozawa et al. (58) indicate that Hp infection actually inhibited the release of gastric ghrelin into the circulation, but again, Hp eradication in their studies had no effect on plasma ghrelin levels in subjects that underwent anti-Hp therapy and were successfully cured. Reduced expression of mRNA for preproghrelin and a concurrent fall in plasma ghrelin levels were also observed in Hp-infected Mongolian gerbils, the most appropriate experimental model for studying various aspects of Hp-associated gastric cancer pathogenesis (59).
Our previous study in the Polish shepherd population of the Tatra Mountains documented for the first time that serum ghrelin concentration is significantly decreased in Hp-positive shepherds and their children as compared to that in Hp-negative counterparts (60). Moreover, we found that Hp eradication in the same shepherds resulted in a profound increase in plasma ghrelin levels, indicating that Hp, in fact, exhibits an inhibitory effect on ghrelin release into the circulation and that removal of this bacteria from the stomach using triple eradication therapy based on a proton pump inhibitor results in an enhancement of circulating plasma ghrelin (60). The importance of the inhibitory influence of Hp infection on circulating ghrelin is supported by our recent finding (60) that ghrelin content in the gastric corpus mucosa, a major source of this hormone in the stomach, was significantly higher than that in gastric antrum and that this corpus ghrelin concentration was markedly suppressed in Hp-infected shepherds before eradication therapy, whereas anti Hp-eradication therapy counteracted the inhibitory effect of Hp on gastric ghrelin content. Our present comparative results obtained from age-matched children and adults without or with Hp infection confirm and extent the above mentioned data obtained from shepherds and their children and strongly support the conclusion that Hp infection strongly suppresses plasma ghrelin secretion while enhancing the plasma leptin response. Our findings are in keeping with previous reports by Nwokolo et al. (55), Isamoto et al. (57) and Tatsagouchi et al. (61) that Hp suppresses gastric ghrelin, as reflected in their studies by a marked decrease in the number of immunoreactive ghrelin neuroendocrine cells in gastric corpus and by a subsequent fall in plasma ghrelin levels observed in Hp-infected subjects. Moreover, our present study as well as Hp-eradicated patients in other reports (55, 57, 60 - 62) demonstrate an increase in gastric ghrelin, which may contribute to the ghrelin-induced increase in appetite and weight gain observed following successful bacteria eradication. This notion is supported by the observation that Hp infection, which negatively influenced plasma ghrelin dynamics, was shown to correlate in a positive manner with the BMI of Hp infected patients (57). It is of interest, however, that leptin levels were not significantly affected in Hp-infected adults, confirming the observation by Isomoto et al. (57) that leptin levels exhibit a positive correlation with BMI irrespective of Hp status as the primary contributor of circulating leptin is the adipose tissue. In another report, plasma leptin concentration was increased in Hp-positive subjects (63). In agreement with this latter report, we found that in Hp-infected children and adults, serum leptin concentrations were significantly increased, suggesting that the stimulatory effects of Hp on leptin release is independent on the age of examined subjects. This increase in leptin release in Hp-infected children and adults were, however, not paralleled by a decrease in plasma ghrelin concentration. The question remains what is the possible mechanism of the alterations of ghrelin release by Hp infection. Hp may act directly or through its cytotoxins on the secretory activity of the Gr cells. The fact that prolong Hp infection and accompanying atrophic gastritis results in the decrease in the number of the Gr cells that is paralleled by the decrease in plasma levels of pepsinogen I, which is considered as biomarker of the integrity of the oxyntic mucosa, suggests that Hp may indeed interfere directly with the Gr-cells and ghrelin release but this requires further studies (59). Hp infection seems to enhance the release of leptin, which originates predominantly from the adipocytes and less from the gastric glands, and it is not clear how the Hp in the stomach could affect the release of this peptide. Although we found that Hp infection alters serum levels of ghrelin and leptin, further studies are needed to determine whether the alterations in serum ghrelin and leptin in Hp infection play any important role in appetite control energy homeostasis in Hp infected children and adults.
- Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: Evidence from a prospective investigation. BMJ 1991; 302: 1302-1305.
- Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology 1989; 96: 615-625.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330: 1267-1271.
- Sipponen P. Natural course of Helicobacter pylori gastric infection. Ital J Gastroenterol Hepatol 1998; 30 (Suppl 3) S264-269.
- Konturek PC, Bielanski W, Konturek SJ, Hahn EG. Helicobacter pylori associated gastric pathology. J Physiol Pharmacol 1999; 50: 695-710.
- Megraud F. Transmission of Helicobacter pylori; faecal-oral versus oral-oral route. Aliment Pharmacol Ther 1995; 9 (suppl 2): 85-91.
- Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: Inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol 1996; 143: 257-262.
- Vaira D, D’Anastasio C, Holton J, et al. Campylobacter pylori in abattoir workers: Is it a zoonosis? Lancet 1988; 2: 725-726.
- Husson MO, Vincent P, Grabiaud MH, Furon D, Leclerc H. Anti-Helicobacter pylori IgG levels in abattoir workers. Gastroenterol Clin Biol 1991; 15: 723-726.
- Morris A, Nicholson G, Lloyd G, Haines D, Rogers A, Taylor D. Seroepidemiology of Campylobacter pyloridis. NZ Med J 1986; 99: 657-659.
- Fox JG. Non-human reservoirs of Helicobacter pylori. Aliment Pharmacol Ther 1995; 9 (suppl 2): 93-100.
- El-Zaatari FA, Woo JS, Badr A, et al. Failure to isolate Helicobacter pylori from stray cats indicates that H. pylori in cats may be an anthroponosis – an animal infection with a human pathogen. J Med Microbiol 1997; 46: 372-376.
- Thomson MA, Storey P, Greer R, Cleghorn GJ. Canine-human transmission of Gastrospirillum hominis. Lancet 1994; 344: 1037-1038.
- Goodman KJ, Correa P, Tengana Aux HJ, et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol 1996; 144: 290-299.
- Dore MP, Bilotta M, Vaira D, et al. High prevalence of Helicobacter pylori infection in shepherds. Dig Dis Sci 1999; 44: 1161-1164.
- Dore MP, Malaty HM, Gaham DY, Fanciulli G, Delitala G, Realdi G. Risk factors associated with Helicobacter pylori infection among children in a defined geographic area. Clin Infect Dis 2002; 15 (2): 91-95.
- Bielanski W, Konturek SJ. New approach to 13C-urea breath test: capsule-based modification with low-dose of 13C-urea in the diagnostic of Helicobacter pylori infection. J Physiol Pharmacol 1996; 47: 545-553.
- Papiez D, Konturek PC, Bielanski W, et al. Prevalence of Helicobacter pylori infection in Polish shepherds and their families. Dig Dis Sci 2003; 35: 10-15.
- Kojima M , Kanagawa K. Ghrelin; Structure and Function. Physiol Rev 2005; 85: 495-522.
- Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 2004; 55: 137-154.
- Konturek SJ, Czesnikiewicz-Guzik M. Brain-gut axis and ford intake control. Pediatria Wspolczesna. Gastroenterologia, Hepatologia i Zywienie Dziecka 2004; 6: 351-359.
- Konturek SJ, Bielanski W, Konturek PC, Hahn EC. Studies on the relationship between gastric ghrelin and Helicobacter pylori (Hp) infection in children and adults. Helicobacter 2004; 9: 551.
- Falley NJ, Stranghellini V, Heading RC, Koch KI, Malagelada JR, Tytgat GNJ. Functional gastroduodenal disorders. Gut 1999; 45 (Suppl II): 1137-1142.
- Bielanski W. Epidemiological study on Helicobacter pylori infection and extragastroduodenal disorders in Polish population. J Physiol Pharmacol 1999; 50: 723-733.
- Konturek PC, Konturek SJ, Starzynska T, Marlicz K, Bielanski W. Helicobacter pylori – gastrin link in MALT lymphoma. Aliment Pharmacol Ther 2000; 14: 1311-1318.
- Konturek PC, Brzozowski T, Pajdo R, et al. Ghrelin a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol 2004; 55: 325-336.
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone releasing acylated peptide is synthesized in a distinct endocrine cell type in gastrointestinal tract of rats and Humans. Endocrinology 2000; 141: 4255-4261.
- Konturek JW, Konturek SJ, Kwiecien S, et al. Leptin in the control of gastric secretion and gut hormones in humans infected with Helicobacter pylori. Scand J Gastroenterol 2001; 36: 1148-1154.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone-releasing acetylated peptide from the stomach. Nature 1999; 402: 1492-502.
- Popovic V, Miljic D, Pekic S, et al. Low plasma ghrelin level in gastrectomized patients is accompanied with enhanced sensitivity to the ghrelin-induced growth hormone release. J Clin Endocrinol Met 2005; 11: 1888-1905.
- Rindi G, Necchi V, Savio A et al. Characterization of gastric ghrelin cells in man and other mammals: Studies in adult and fetal tissues. Histochem Cell Biol 2002; 117: 511-519.
- Cummings DE, Purnell JQ, Frayo RS, Schimidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714-1719.
- Takaya K, Ariyasu H, Kanamoto N et al. Ghrelin strongly stimulates growth hormone (GH) release in humans. J Clin Endocrinol Metab 2000; 85: 4908-4911.
- Schmidt DA, Held K, Ising M, Uhr M, Weikel JC, Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH and cortisol, but does not affect leptin in normal controls. Europsychopharmacology 2005; 30: 187-192.
- Kamegai J, Tamura H, Schimizu T, Ishii S, Sugihara H, Wakabayashi. Chronic central infusions of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001; 50: 2438-2443.
- Riediger T, Traebert M, Schmidt HA, Scheel C, Lutz TA, Scharrer E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci Lett 2003; 341: 151-155.
- Wren AM, Seal LJ, Cohen MA et al. Ghrelin enhances appetite and increases food intake in humans. J Endocrinol Metab 2001; 86: 5992-5995.
- Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader-Willi syndrome. Nat Med 2002; 8(7): 643-644.
- DelParigi A, Tschop M, Heiman ML, et al. High circulating ghrelin: A potential cause for hyperphagia and obesity in Prader-Willi syndrome. J Clin Endocrinol Metab 2002; 87: 5461-5464.
- Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin and ghrelin before and after weight loss induced by gastric bypass surgery in morbidity obese subjects. J Clin Endocrinol Metab 2003; 88: 1594-1602.
- Hanusch-Enserer U, Brabant G, Rodenn M. Ghrelin concentration in morbidity obese patients after adjustable gastric bending. N Engl J Med 2003; 384: 2159-2160.
- Brzozowski T, Konturek PC, Konturek SJ, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Reg Pept 2004; 120: 39-51.
- Konturek PC, Brzozowski T, Burnat G, et al. Ghrelin – a new gastroprotective factor for gastric mucosa. J Physiol Pharmacol 2004; 55: 325-336.
- Edholm T, Levin F, Hellestrom PM, Schmidt PT. Ghrelin stimulates motility in small intestine through instrinsic cholinergic neurons. Reg Pept 2004; 121: 25-30.
- Konturek PC, Konturek SJ, Ochmanski W. Endocrinology of gastric H+ and duodenal HCO3 secretion the role of grain-gut axis. Eur J Pharmacol 2004; 499: 15-27.
- Konturek SJ, Pepera J, Zabielski, et al. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol 2003; 54: 293-317.
- Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neurohormones control food intake; basic mechanisms and clinical implications. J Physiol Pharmacol 2005; 56: suppl 6; 5-25.
- Kirchgessner AL. Orexins in the brain-gut axis. Endocrinol Rev 2002; 23: 1-15.
- Sakurain T, Amemiya A, Ischi M, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92: 573-585.
- Burdyga G, Lal S, Spiller D, et al. Localization of orexins-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology 2003; 124: 129-139.
- Komaki G, Matsumoto Y, Nishikarata H, et al. Orexin-A and leptin change inversely in fasting non-obese subjects. Eur J Endocrinol 2001; 144: 645-651.
- Adam JA, Menheere PP, van Dielen FM, Soeters PB, Buurman WA, Greve JW. Decreased plasma orexin-A levels in obese individuals. Int J Obes Relat Metab Disord 2002; 26: 274-276.
- Nalund E, Schmidt PT, Hellstrom PM. Gut peptide hormones: Importance for food intake. Scand J Gastroenterol 2005; 40: 250-258.
- Popovic V, Miljic D, Pekic S et al. Low plasma ghrelin vele in gastrectomized patients is accompanied by enhanced sensiiitiivity to the ghrelin-induced growth hormone release. J Clin Endocrinol Met 2005; 11: 1888-1905.
- Nwokolo CU, Freshwater DA, O,Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut 2003; 52: 637-640.
- Gokcel A, Gumurdulu Y, Kayaselcuk F, et al. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol 2003; 148: 423-426.
- Isomoto H, Ueno H, Nishi Y, Wen Ch-Y, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol 2005; 11: 1644-1648.
- Osawa H. Nakazato M, Date Y, et al. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Met 2005; 90: 10-16.
- Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut 2004; 53: 187-194.
- Plonka M, Konturek PC, Bielanski W, Pawlik T, Brzozowski T, Konturek SJ. Relationship between ghrelin and Helicobacter pylori infection in Polish adult shepherds and their children. Aliment Pharmacol Therap (Symp ser) 2006; 2: 1:160-168.
- Tatsuguchi A, Miyake K,Gudis K, et al. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol 2004; 99: 2121-2126.
- Masaoka T, Suzuki H, Imaeda H, et al. Long-term strict monitoring of plasma ghrelin and other serological markers of gastric disease after Helicobacter pylori eradication. Hepatogastroenterol 2005; 52: 1-4.
- Nishi Y, Isomoto H, Uotani S, et al. Enhanced production of leptin in gastric fundic mucosa with Helicobacter pylori infection. World J Gastroenterol 2005; 11: 695-699.