The demonstration of the presence of cannabinoid receptor in the brain led to search for endogenous ligand of cannabinoid receptor. In 1992, Dewane et al. (7) have isolated a compound from water-insoluble fraction of the porcine brain, which binds to the brain CB1 receptor. This compound was characterized as arachodonylethanolamide and named “anandamide” based on its chemical nature and a Sanskrit word “ananda”, meaning bliss (7). Anandamide is the first of five endogenous cannabinoid receptor agonists that have been identified up to the present in mammalian tissues. Homo-
Marijuana has been in use for over 4000 years as a recreational and as therapeutic drug (9). It has been administered for the treatment of a wide array of health concerns. The endogenous cannabinoid system is involved in analgesia, cognition, memory, locomotor activity, appetite, vomiting and immune control (1). In clinical studies, marijuana or/and cannabinoids has been reported to reduce an acute (10) and chronic (11) pain. Moreover, treatment with cannabinoids has been shown to have a therapeutic value in the prevention of nausea and vomiting caused by anticancer drugs (12, 13), and in the treatment of anorexia (14). Human trials on the use of cannabinoids in neurological disorders associated with spasticity, ataxia and muscle weakness, as well as epilepsy are not conclusive, because very similar symptoms can be caused by cannabinoids itself (1).
Cannabinoids affect immune response machinery and for this reason may modulate the development of inflammation, infection and tissue damage (1, 15). Experimental studies have shown that treatment with cannabinoids decreases the area of ischemic myocardial necrosis (16), attenuates joint damage in animal models of arthritis (17) and induces neuroprotection (18).
In the gut, administration of cannabinoids inhibits gastric acid secretion (19) and small intestinal secretion (20), delays gastric emptying and decreases gastrointestinal transit (21). Moreover, study performed by Germano et al. (22) has shown that administration of synthetic CB1 receptor agonist, WIN 55,212-2 inhibits the development of stress-induced gastric ulcer in rats. Similar cannabinoids-evoked protection has been found in colitis (23, 24). The opposite effect has been observed in small intestine. Cannabinoids can induce ileitis (25); whereas administration of cannabinoids receptor antagonists inhibits the development of indomethacin-induced intestinal ulcers (26).
The aim of our present study was to check whether the administration of a natural endogenous CB1 receptor agonist, anandamide or CB1 antagonist affects the development of stress-induced gastric ulcers and cerulein-induced acute pancreatitis.
Studies were carried out in two separate series on male Wistar rats weighing 220-250 g (the first series) or 140-160 g (the second series). Animals were housed in cages with wire mesh bottoms, with normal room temperature and a 12-hour light-dark cycle. The experimental protocol was approved by the Committee for Research and Animal Ethics of Jagiellonian University.
The first series of studies was performed to evaluate the effect of cannabinoids on gastric ulcers induced by water immersion and restraint stress (WRS). In the second series, we examined the influence of cannabinoids on the development of cerulein-induced acute pancreatitis. Rats from the first series were fasted for 24 h prior to experiment with unlimited access to water. In the second series, drinking of water and food were available ad libitum.
WRS was induced by rat immobilization in individual cage and immersion in water at 23°C for 3.5 h to the level of xiphoid process as described previously by Takagi et al. (27).
Acute pancreatitis was induced by caerulein (Sigma-Aldrich, Gmbh, Steinheim, Germany) administered intraperitoneally (i.p.) 5 times with 1 h intervals at a dose of 50 µg/kg per injection.
Both series of studies were carried out on the following experimental groups : [1] control rats injected with vehicle i.p.; [2-4] rats treated with endogenous ligand for CB1 receptor, anandamide (CB1 Ki value = 61 nM; CB2 Ki value = 1930 nM; ALEXIS® Biochemicals, San Diego, CA, USA) at the dose of 0.8, 1.5 or 3.0 µmol/kg/dose i.p.; [5] rats treated with synthetic CB1 receptor antagonist, AM 251 (Ki = 7.49 nM; ALEXIS® Biochemicals, San Diego, CA, USA) at the dose of 4 µmol/kg i.p.; [6] vehicle-treated rats exposed to WRS or cerulein-induced pancreatitis; [7-9] rats treated with anandamide at the dose of 0.8, 1.5 or 3.0 µmol/kg/dose i.p. and exposed to WRS or cerulein-induced pancreatitis; [10] rats treated with AM 251 at the dose of 4 µmol/kg i.p. and exposed to WRS or cerulein-induced pancreatitis; [11] rats treated with combination of AM 251 (4 µmol/kg/dose i.p.) plus anandamide (1.5 µmol/kg/dose i.p.) and exposed to WRS or cerulein-induced pancreatitis.
In the first series of studies, anandamide and/or AM 251 were administered once, 30 or 40 min before exposure to WRS, respectively. In the second, anandamide and/or AM 251 were administered twice. The fist dose of anandamide and/or AM 251 was injected 30 min and 40 min before the start of cerulein administration, respectively; the second dose was administered after next 3 h. Eight animals were used in each experimental group, in each series of the study.
Immediately after 3.5 h of WRS or last injection of cerulein, animals were anesthetized with ketamine (50 mg/kg i.p., Bioketan, Vetoquinol Biowet, Gorzów Wielkopolski, Poland). Abdominal cavity was opened and gastric mucosal or pancreatic blood flow was measured by a laser Doppler flowmeter using PeriFlux 4001 Master monitor (Perimed AB, Järfälla, Sweden), as described previously (28). Data were presented as percent of control value obtained in saline treated control rats without exposure to WRS or cerulein.
After the measurement of gastric mucosal or pancreatic blood flow, arterial blood was taken from aorta and serum was collected and frozen at -60°C. In both series of studies, we determined serum concentration of pro-inflammatory interleukin-1ß (IL-1ß). Additionally, in animals from the second series, we measured serum activity of pancreatic enzymes: lipase, amylase and poly-C ribonuclease, as an index of the severity of acute pancreatitis.
In animals from the first series of studies, the number and the area of lesions in the oxyntic mucosa were measured, using computerized planimeter (Morphomat, Carl Zeiss, Berlin, Germany) as described previously (29). The stress-induced lesion was defined as a round or linear mucosal black or red defect of at least 0.1 mm in diameter.
The rate of DNA synthesis, as an index of tissue vitality and ability to the regeneration, was determined in gastric mucosa of animals from the first series of studies, and in pancreatic tissue of animals from the second series. Gastric mucosa scraped from the oxyntic gland area or the portion of minced pancreatic tissue were incubated at 37°C for 45 min in 2 ml of medium containing 8 µCi/ml of [3H]thymidine ([6-3H]thymidine, 20-30 Ci/mmol; Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic), as described in details previously (30). The rate of DNA synthesis was expressed as [3H]thymidine disintegrations per minute per microgram DNA (dpm/µg DNA).
Serum concentration of IL-1b was measured using the commercial BioSource Cytoscreen rat IL-1ß kit (BioSource International, Camarillo, California, USA) based on ELISA. Serum lipase and amylase activity was determined with a Kodak Ectachem DT II System analyzer (Eastman Kodak Company, Rochester, NY, USA) using a commercially available LIPA and AMYL DT Slides (Vitros DT Chemistry System, Johnson & Johnson Clinical Diagnostic, Inc., Rochester, NY, USA). Serum poly-C specific ribonuclease activity was determined using Warsaw and Lee’s procedure (31), employing polycytidylic acid (poly-C) as a ribonuclease substrate, as described previously (32).
Morphological examination of pancreatic tissue, obtained from animals from the second series of study, was performed in hematoxilin and eosin stained slides as describe previously in details (33). Slides were examined by two experienced pathologists without knowledge of the treatment given (four slides per animal). The histological grading of pancreatic edema, leukocyte infiltration, vacuolization of acinar cells, hemorrhages and necrosis was made using a scale ranging from 0, while lack of changes to 3 for maximal expression of alterations. Results have been expressed as a predominant histological grading in each experimental group of animals.
Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using GraphPadPrism (GraphPad Software, San Diego, CA, USA). Results are expressed as mean ± S.E.M. Differences were considered to be statistically significant when P was less than 0.05.
The first series of experiments
Treatment with vehicle, anandamide or AM 251 in rats without exposure to WRS did not induce any gastric mucosal damage (Fig. 1). Exposure to WRS resulted in appearance of multiple erosions of gastric mucosa. The mean lesion area in saline-treated control rats reached 7.8 ± 0.2 mm2. Administration of anandamide alone significantly and dose-dependently reduced the ulcer area; whereas pretreatment with AM 251 significantly increased gastric mucosal damage induced by WRS. Administration of AM 251 before treatment with anandamide significantly reversed protective effect of anandamide against WRS-induced gastric ulcers.
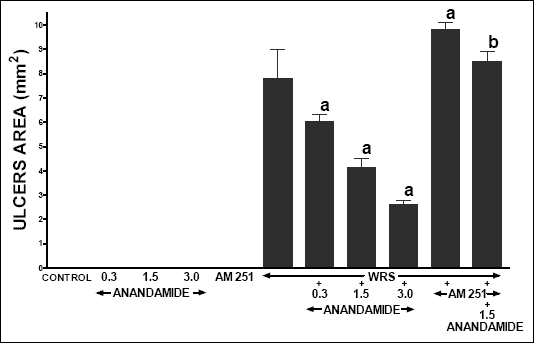 |
| Fig. 1. Effect of administration of anandamide (A) (0.3, 1.5 or 3 µmol/kg) and/or treatment with AM 251 (4 µmol/kg) on the area of water immersion and restrain stress (WRS)-induced gastric lesions. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. WRS alone; bP<0.05 vs. WRS + A 1.5. |
Administration of anandamide or AM 251 was without significant effect on gastric mucosal blood flow in rats without exposure to WRS (Fig. 2). Exposure to WRS reduced gastric mucosal blood flow by 50.3%. Treatment with anandamide alone partly, but significantly reversed this effect. On the other hand, pretreatment with AM 251 prior to WRS increased the WRS-induced reduction in gastric mucosal blood flow, but this result was statistically insignificant. Pretreatment with AM 251 abolished the anandamide-evoked improvement of gastric blood flow in rats exposed to WRS.
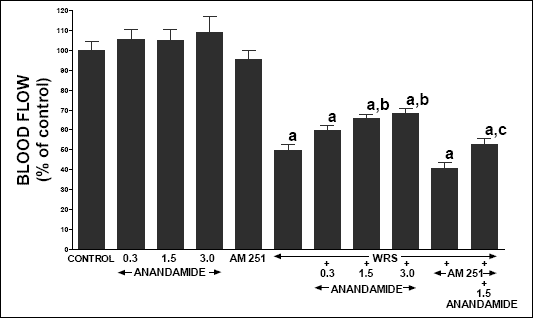 |
| Fig. 2. Effect of water immersion and restrain stress (WRS), administration of anandamide (A) (0.3, 1.5 or 3 µmol/kg) and/or treatment with AM 251 (4 µmol/kg) on gastric mucosal blood flow. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.01 vs. control (C); bP<0.05 vs. WRS alone; cP<0.05 vs. WRS + A 1.5. |
In vehicle-treated control rats without exposure to WRS, serum concentration of pro-inflammatory IL-1b reached a value 67.3 pg/ml (Fig. 3). Administration of anandamide or AM 251 alone was without significant effect on this parameter in these rats. Exposure to WRS led to almost twofold increase in serum concentration of IL-1ß. Treatment with anandamide attenuated the WRS-evoked increase in serum concentration of IL-1ß, and this effect was statistically significant after anandamide used at the dose of 1.5 and 3.0 µmol/kg. Administration of AM 251 significantly increased serum level of IL-1ß in rats exposed to WRS, as well as markedly reversed the anandamide-evoked decrease in IL-1ß in these rats.
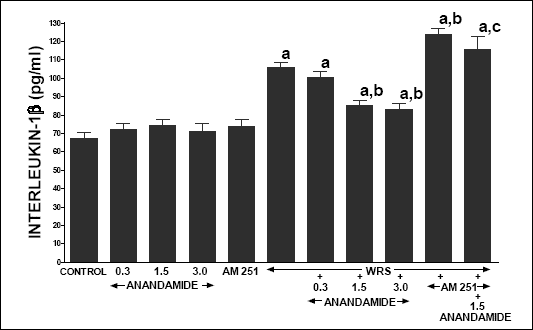 |
| Fig. 3. Effect of water immersion and restrain stress (WRS), administration of anandamide (A) (0.3, 1.5 or 3 µmol/kg) and/or treatment with AM 251 (4 µmol/kg) on serum interleukin-1ß concentration. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. WRS alone; cP<0.05 vs. WRS + A 1.5 |
In vehicle-treated control rats without exposure to WRS, gastric mucosal DNA synthesis reached a value of 43.7 ± 2.0 dpm/µg DNA (Fig. 4). Treatment with anandamide or AM 251 was without effect on gastric mucosal DNA synthesis in rats without exposure to WRS. Exposure to WRS reduced gastric mucosal DNA synthesis by 43%. Treatment with anandamide at the dose of 1.5 and 3.0 µmol/kg partly, but significantly reversed the WRS-induced decrease in gastric mucosal DNA synthesis. In rats exposed to WRS, administration of AM 251 tended to reduce gastric mucosal DNA synthesis, but this effect was statistically insignificant. On the other hand, pretreatment with AM 251 abolished the anandamide-evoked increase in gastric DNA synthesis in rat exposed to WRS.
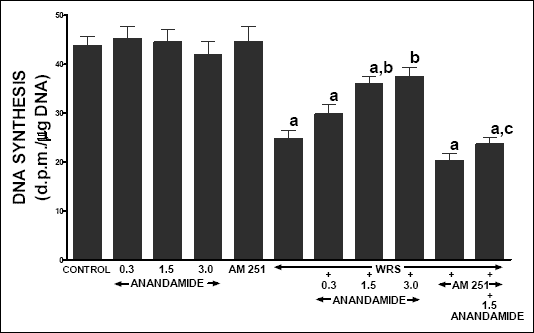 |
| Fig. 4. Effect of water immersion and restrain stress (WRS), administration of anandamide (A) (0.3, 1.5 or 3 µmol/kg) and/or treatment with AM 251 (4 µmol/kg) on gastric mucosal DNA synthesis. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. WRS alone; cP<0.01 vs. WRS + A 1.5 |
The second series of experiments
In vehicle treated control rats, pancreases did not show macroscopically and at light microscopic level any tissue alteration (Table 1). Also, administration of AM 251 did not affect morphology of pancreatic tissue in rats without induction of pancreatitis. In some animals treated with anandamide alone, we observed minimal pancreatic edema without any additional histological alteration. In the rest of rats treated with anandamide without induction of acute pancreatitis, morphological features were as in control group. Administration of cerulein caused acute edematous pancreatitis in all rat tested. The pancreas was grossly swollen and enlarged with a visible collection of edematous fluid. At light microscopic level, prominent interlobular and moderate intralobular edema (grade 3 and 2) was accompanied with scarce perivascular inflammatory leukocyte infiltration. Vacuolization was observed in more than 50% of acinar cells. No necrosis or hemorrhages was observed. Treatment with anandamide, before and during administration of cerulein, increased the pancreatitis-induced pancreatic edema and inflammatory leukocyte infiltration; whereas administration of AM 251 reduced the cerulein-evoked pancreatic edema and vacuolization of pancreatic acinar cell. Additionally pretreatment with AM 251 inhibited the anandamide-evoked aggravation of pancreatic tissue damage in rats with acute pancreatitis.
| Table 1. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) or treatment with AM 251 (4 µmol/kg/dose) on morphological signs of pancreatic damage. |
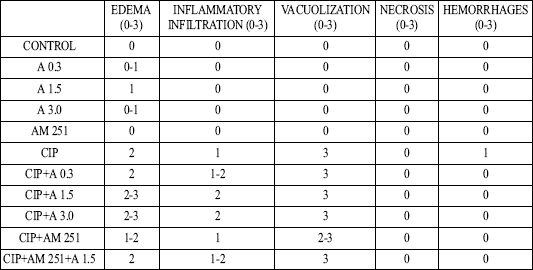 |
| Numbers represent the predominant histological grading in each experimental group. |
In control vehicle-infused rats, serum activity of lipase (Fig. 5), amylase (Fig. 6) and poly-C ribonuclease (Fig. 7) reached 59.2 ± 3.9, 3594 ± 167 and 167.3 ± 18.9 U/l, respectively. In rats without induction of pancreatitis, administration of anandamide or AM 251 did not affect serum activity of these pancreatic enzymes. Cerulein administered for 5 h induced acute pancreatitis and significantly increased serum activity of lipase, amylase and poly-C ribonuclease. Pretreatment with anandamide additionally and markedly increased serum activity of these pancreatic enzymes in rats with cerulein-induced pancreatitis; whereas administration of AM 251 significantly reduced serum activity of lipase, amylase and poly-C ribonuclease in rats with acute pancreatitis. Additionally, pretreatment with AM 251 partly reversed the anandamide-evoked increase in serum activity of theses enzymes in rats with acute pancreatitis.
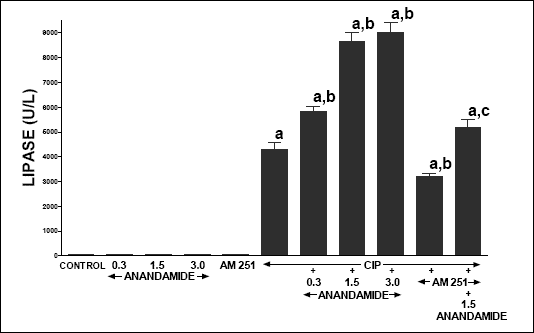 |
| Fig. 5. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on serum activity of lipase. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. CIP alone; cP<0.05 vs. CIP + A 1.5. |
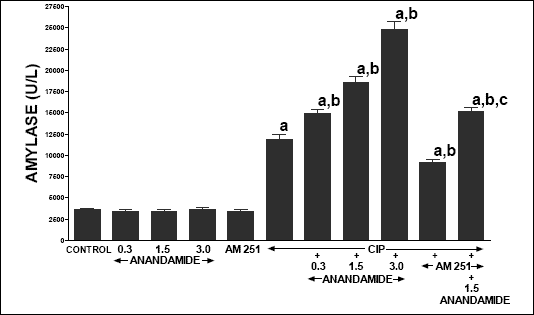 |
| Fig. 6. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on serum activity of amylase. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. CIP alone; cP<0.05 vs. CIP + A 1.5. |
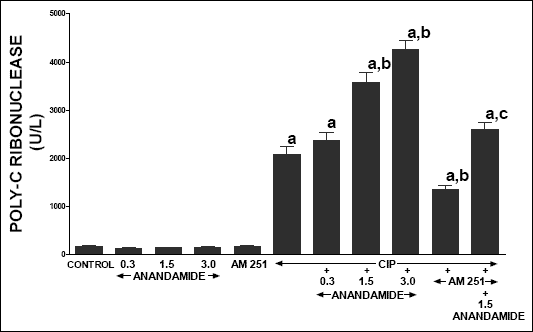 |
| Fig. 7. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on serum activity of poly-C ribonuclease. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. CIP alone; cP<0.05 vs. CIP + A 1.5. |
Treatment with any doses of anandamide or AM 251 was without any significant effect on pancreatic blood flow in rats without acute pancreatitis (Fig. 8). Administration of cerulein reduced pancreatic blood flow by 51.2% when compared to vehicle-infused control rats. Treatment with anandamide, AM 251 or combination of AM 251 plus anandamide did not significantly affect pancreatic blood flow in animals with cerulein induced pancreatitis.
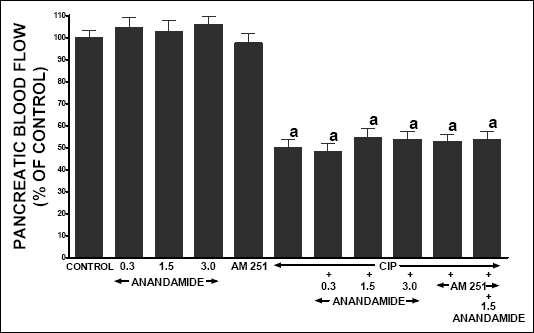 |
| Fig. 8. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on pancreatic blood flow. Mean ± S.E.M. N=8 rats in each experimental group.aP<0.05 vs. control (C). |
In control rats infused with vehicle, serum IL-1ß concentration was 65.3 ± 3.2 pg/ml (Fig. 9). Treatment with anandamide or AM 251 was without a significant effect on serum level of IL-1ß in rats without induction of acute pancreatitis. Cerulein caused an increase in serum IL-1ß concentration to a value of 186.5 + 7.8 pg/ml and this increase was enhanced by all doses of anandamide. Administration of AM 251 led to a significant reduction in the caerulein-induced increase in serum IL-1ß concentration, as well as partly reduced the anandamide-evoked increase in serum IL-1ß in rats with acute pancreatitis.
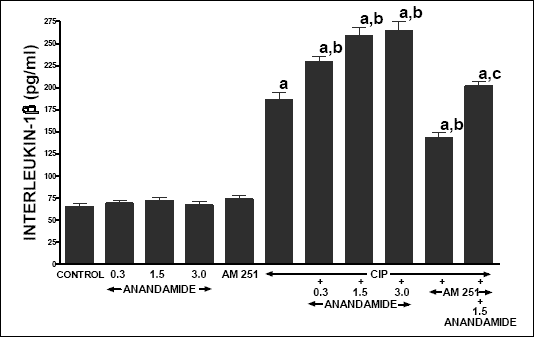 |
| Fig. 9. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on serum concentration of pro-inflammatory interleukin-1ß. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. CIP alone; cP<0.05 vs. CIP + A 1.5. |
In vehicle-treated control rats, pancreatic DNA synthesis reached 67.5 ± 2.4 dpm/µg DNA (Fig. 10). Treatment with any dose of anandamide or AM 251 did not significantly affect pancreatic DNA synthesis in animals treated with vehicle. In rats with caerulein-induced pancreatitis, pancreatic DNA synthesis was reduced by 37%. In this group of animals, treatment with anandamide partly reversed the caerulein-induced fall in pancreatic DNA synthesis. Administration of AM 251 alone was without effect on pancreatic DNA synthesis in rats with pancreatitis, but AM 251 administered in combination with anandamide significantly inhibited the anandamide-evoked increase in incorporation of [3H]thymidine to pancreatic DNA.
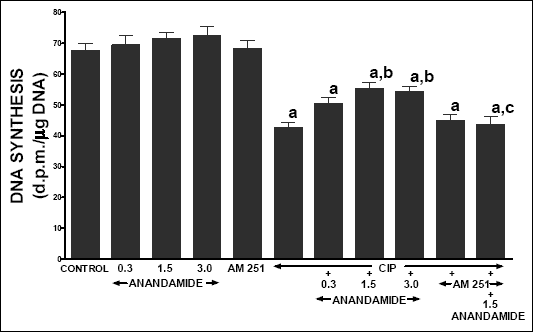 |
| Fig. 10. Effect of cerulein-induced pancreatitis (CIP), anandamide (A) administration (0.3, 1.5 or 3 µmol/kg/dose) and/or treatment with AM 251 (4 µmol/kg/dose) on pancreatic DNA synthesis. Mean ± S.E.M. N=8 rats in each experimental group. aP<0.05 vs. control (C); bP<0.05 vs. CIP alone; cP<0.05 vs. CIP + A 1.5. |
Previous study has shown that pretreatment with WIN 55,212,-2, a synthetic agonist of CB1 receptor inhibits the development of stress-induced gastric ulcer in rats (22). Our present study confirms and extends this observation. Anandamide is a natural agonist for both CB receptors, but its affinity for CB1 receptors (Ki = 61 nM) is markedly higher than for CB2 receptors (Ki = 1.93 µM) (8). In our study, we have found that pretreatment with anandamide protects gastric mucosa against lesions evoked by WRS. This gastroprotective effect has been associated with the preservation of gastric mucosal blood flow and gastric mucosal DNA synthesis. Gastric blood flow plays an important role in the protection and healing of gastric mucosa (34). Blood flow supplies the mucosa with oxygen and bicarbonates, and removes hydrogen ions, carbon dioxide and toxic agents diffusing from the gastric lumen. Mucosal ischemia contributes to gastric ulceration in various clinical and experimental conditions; whereas hyperemia protects gastric mucosa against damaging agents (34, 35). In our present study, exposure to WRS reduced gastric mucosal blood flow; whereas treatment with anandamide markedly reversed this effect of WRS. This observation indicates that gastroprotective effect of anandamide involves the preservation of gastric mucosal blood flow. However, it is not clear whether the anandamide-evoked preservation of gastric mucosal blood flow is a mechanism or result of mucosal protection. The relationship between gastric mucosal blood flow and gastric mucosal damage seems to be bidirectional. Improvement of gastric mucosal blood flow reduces gastric mucosal damage, but also the reduction of gastric lesions leads to improvement of gastric mucosal circulation.
The rate of DNA synthesis is an index of tissue vitality and ability to regeneration. Several reports demonstrated that after exposure to stress the gastric mucosa exhibits a decrease in cell proliferation and DNA synthesis (36, 37). The same effect has been observed in our present study. Additionally, we have found that pretreatment with anandamide partly reversed the WRS-induced fall in gastric mucosal DNA synthesis. This finding is an additional evidence that anandamide exhibits gastroprotective effect against stress ulcers.
Interleukin-1ß is a well-known mediator of acute inflammation and plays a crucial role in the release of other members of pro-inflammatory cytokine cascade (38). Interleukin-1ß stimulates the synthesis and release of inflammatory mediators such as TNF-alpha, PAF, prostaglandins and pro-inflammatory interleukins (38, 39). The essential role of interleukin-1ß in inflammatory process is evidenced by the observation that administration of interleukin-1ß receptor antagonist prevents the release of pro-inflammatory mediators and reduces the severity of inflammation and tissue damage (39). These data are in agreement with our present observation that exposure to WRS leading to gastric mucosa damage increases the serum level of interleukin-1ß. Additionally we have found that protective effect of anandamide against stress-induced gastric lesions involves inhibition of the release of interleukin-1ß.
The integrity of the gastric mucosa depends upon the balance between damaging and protective factors. It is of interest that pretreatment with AM 251, a selective CB1 receptor antagonist, significantly increased the area of gastric lesions and serum concentration of interleukin-1ß in rats exposed to WRS. Additionally, administration of AM 251 before treatment with anandamide markedly increased gastric mucosal damage evoked by WRS, as well as increased the serum level of pro-inflammatory interleukin-1ß and decreased the gastric mucosal blood flow and gastric DNA synthesis. These observations indicate that endogenous cannabinoids are involved in the protection of gastric mucosa against damage evoked by WRS and gastroprotective effect of anandamide is strictly dependent on the stimulation of CB1 receptors.
The next interesting finding of our present study is observation that in contrast to results obtained in the stomach, pretreatment with anandamide increases the severity of cerulein-induced pancreatitis. Treatment with anandamide prior to administration of cerulein increased pancreatic tissue damage and increased serum concentration of pro-inflammatory interleukin-1ß and serum activity of pancreatic enzymes. Opposite effect was observed after administration of AM 251, a selective CB1 receptor antagonist. Pretreatment with AM 251 inhibited the development of cerulein-induced acute pancreatitis, as well as reversed the injurious effect of anandamide in this model of acute pancreatitis. Our observation that stimulation of CB1 receptors during induction of acute pancreatitis enhances the severity of this disease is in agreement with study performed by Matsuda et al. (40). They have found that administration of CB1 receptor antagonist prolongs the survival of rats with severe necrotizing acute pancreatitis evoked by injection of sodium taurocholate into the bilio-pancreatic duct. Also clinical case report indicates that cannabis may induce acute pancreatitis (41).
Our present study has indicated that pretreatment with anandamide protects gastric mucosa; whereas in the pancreas increases the severity of acute pancreatitis. The mechanism of different effects of CB1 receptor activation in these both organs remains unknown and needs further studies to elucidate. We can speculate that deleterious effect anandamide in the pancreas may depend on the influence of cannabinoids on capsaicin-sensitive sensory nerves. These nerves co-express CB1 and capsaicin-sensitive vanilloid (TRPV1) receptors to a very high degree (42), and cannabinoids act on both these receptors (8). CB1 receptors are negatively coupled to calcium channels; whereas vanilloid receptors open cation channels. Anandamide was found to be considerably more potent in inhibiting calcium mobilization than in activating vanilloid receptors (8). Stimulation of CB1 receptors abolishes the capsaicin-induced increase in intracellular calcium in sensory nerves (43), as well as cannabinoids may directly inhibit capsaicin-sensitive nerves by dephosphorylating and desensitizing vanilloid receptor 1 (TRPV1) via calcium calcineurin mechanism (44). Capsaicin-sensitive sensory nerves are involved in the maintenance of tissue integrity. In the pancreas, a stimulation of these nerves (45, 46) or administration of its main neurotransmitter, CGRP (46, 47), prior to induction of acute pancreatitis, attenuates the pancreatic damage; whereas deactivation of sensory nerves increases the severity of pancreatitis (46, 48). For this reason, it is most likely that anandamide-evoked inhibition of capsaicin-sensitive nerves is responsible for the aggravation of pancreatic damage in cerulein-induced acute pancreatitis.
Capsaicin-sensitive sensory nerves are also implicated in gastric mucosa protection. Stimulation of these nerves by low doses of capsaicin reduces the formation of gastric lesion induced by acid-dependent and acid-independent ulcerogens (49) and capsaicin-sensitive nerves are involved in gastric adaptation to repeated stress (50) or aspirin (51) insults. Inhibition of sensory nerves by cannabinoids should increase gastric mucosal damage. However, in our present study we have observed that administration of anandamide reduces the area of gastric lesions evoked by WRS. It is most likely that deleterious effect of sensory nerves inhibition is balanced and reversed by the influence of cannabinoids on gastric acid secretion. Gastric acid plays an essential role in the development of gastric ulcers and inhibition of gastric acid secretion is a main mechanism of gastroprotection evoked by proton pump inhibitors (52, 53) and blockers of H2 receptors (54), as well as a reduction in gastric acid secretion is involved in gastroprotective effects of prostaglandins (55) or EGF (56). On the other hand, numerous studies have shown that cannabinoids inhibit gastric acid secretion (19, 57, 58) and for this reason gastroprotective effect of anandamide seems to be dependent on inhibiting gastric acid secretion.
Finally, our present study demonstrates that anandamide through CB1 receptor activation exhibits opposite effects on the maintenance of tissue integrity in the stomach and pancreas. In the stomach anandamide exhibits protective effect against stress-induced gastric mucosal lesions; whereas in the pancreas, increases the severity of cerulein-induced pancreatitis.
- Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anesthesia 2001; 56: 1059-1068.
- Mechoulam R, Gaoni Y. A total synthesis of dl-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc 1965; 87: 3273-3275.
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 1997; 74: 129-180.
- Devane WA, Dymarz FA 3rd, Johnson MR, Melin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988; 34: 605-13.
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346: 561-564.
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365: 61-65.
- Dewane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946-1949.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002; 54: 161-202.
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol 1998; 38: 179-200.
- Jain AK, Ryan JR, McMahon FG, Smith G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol 1981; 21(8-9 Suppl): 320S-326S.
- Noyes R Jr., Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther 1975; 18: 84-89.
- Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br J Cancer 1983; 48: 657-663
- Tramer MR, Carroll D, Campbell FA, Reynolds DJM, Moor RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001; 323: 16-21.
- Beal JE, Olson R, Lefkowitz L, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage 1997; 14: 7-14.
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 2005; 5: 400-411.
- Ugdyzhekowa DS., Krylatow AV, Bernatskaya NA, Maslow LN, Mechoulam R, Pertwee RG. Activation of cannabinoid receptors decreases the area of ischemic myocardial necrosis. Bull Exp Biol Med 2002;133:125-126.
- Malfait AM, Gallily R, Sumariwalla PF et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA 2000; 97: 9561-9566.
- Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Targets CNS Neurol Disord 2005; 4: 677-684.
- Coruzzi G, Adami M, Coppelli G, Frati P, Soldani G. Inhibitory effect of the cannabinoid receptor agonist WIN 55,212-2 on pentagastrin-induced gastric acid secretion in the anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol 1999; 360: 715-718.
- Tyler K, Hillard CJ, Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. Eur J Pharmacol 2000; 409: 207-211.
- Izzo AA, Mascolo N, Capasso R, Germano MP, De Pasquale R, Capasso F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedebergs Arch Pharmacol 1999; 360: 221-223.
- Germano MP, D’Angelo V, Mondell MR, et al. Cannabinoid CB1-mediated inhibition of stress-induced gastric ulcers in rats. Naunyn Schmiedebergs Arch Pharmacol 2001; 363: 241-243.
- Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004; 113: 1202-1209.
- Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Physiol 2006; 291: G364-G371.
- McVey DC, Schmid PC, Schmid HH, Vigna SR. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1). J Pharmacol Exp Ther 2003; 304: 713-22.
- Croci T, Landi M, Galzin A-M, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-a in the gut and systemic anti-inflammatory activity of SR 141716 (Rimonabant) in rodents. Br J Pharmacol 2003; 140: 115-122.
- Takagi K, Kasuya Y, Watanabe K. Studies on the drugs for peptic ulcer. a reliable method for producing stress ulcer in rats. Chem Pharm Bull (Tokyo) 1964; 12: 465-472.
- Konturek SJ, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol 1994; 15: 19-28.
- Konturek SJ, Brzozowski T, Dembinski A, Warzecha Z, Konturek PK, Yanaihara N. Interaction of growth hormone-releasing factor and somatostatin on ulcer healing and mucosal growth in rats: role of gastrin and epidermal growth factor. Digestion 1988; 41: 121-128.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Immunohistochemical expression of FGF-2, PDGF-A, VEGF and TGF beta RII in the pancreas in the course of ischemia/reperfusion-induced acute pancreatitis. J Physiol Pharmacol 2004; 55: 791-810.
- Warshaw AL, Lee KH. Serum ribonuclease elevations and pancreatic necrosis in acute pancreatitis. Surgery 1979: 86: 227-234.
- Dembinski A, Warzecha Z, Konturek SJ, et al. Extract of grapefruit-seed reduces acute pancreatitis induced by ischemia/reperfusion in rats; Possible implication of tissue antioxidants. J Physiol Pharmacol 2004; 55: 799-809.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur J Pharmacol 2004; 486: 107-119.
- Sřrbye H, Svanes K. The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis 1994; 12: 305-317.
- Dembinski A, Warzecha Z, Ceranowicz P, et al. Role of capsaicin-sensitive nerves and histamine H1, H2 and H3 receptors in the gastriprotective effect of histamine against stress ulcers in rats. Eur J Pharmacol 2005; 506: 211-221.
- Flynn R, Stuart RC, Gorey TF, et al. Stress ulceration and gastric mucosal cell kinetics: the influence of prophylaxis against acute stress ulceration. J Surg Res 1993; 55: 188-192.
- Warzecha Z, Dembinski A, Brzozowski T, et al. Histamine in stress ulcer prophylaxis in rats. J Physiol Pharmacol 2001; 52: 407-421.
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood 1991; 77: 1627-1652.
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N England J Med 1993; 328: 106-113.
- Matsuda K, Mikami Y, Takeda K, et al. The cannabinoid 1 receptor antagonist, AM 251, prolongs the survival of rats with severe acute pancreatitis. Tohoku J Exp Med 2005; 207, 99-107.
- Grant P, Gandhi P. A case of cannabis-induced pancreatitis. JOP 2004; 5: 41-43.
- Ahluwalia J, Urban L, Capogna M, Bevans S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000; 100: 685-688.
- Millns PJ, Chapman V, Kendall DA. Cannabinoid inhibition of the capsaicin-induced calcium response in rat dorsal root ganglion neurons. Br J Pharmacol 2001; 132: 969-971.
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci USA 2006; 103: 11393-11398.
- Dembinski A, Warzecha Z, Konturek PJ, Ceranowicz P, Konturek SJ. Influence of capsaicin-sensitive afferent neurons and nitric oxide (NO) on cerulein-induced pancreatitis in rats. Int J Pancreatol 1996; 19: 179-189.
- Warzecha Z, Dembinski A, Ceranowicz P, Stachura J, Tomaszewska R, Konturek SJ. Effect of sensory nerves and CGRP on the development of caerulein-induced pancreatitis and pancreatic recovery. J Physiol Pharmacol 2001; 52: 679-704.
- Warzecha Z, Dembinski A, Ceranowicz P, et al. Protective effect of calcitonin gene-related peptide against caerulein-induced pancreatitis in rats. J Physiol Pharmacol 1997; 48: 775-787.
- Warzecha Z, Dembinski A, Jaworek J, et al. Role of sensory nerves in pancreatic secretion and caerulein-induced pancreatitis. J Physiol Pharmacol 1997; 48: 43-58.
- Brzozowski T , Konturek SJ, Sliwowski Z, Pytko-Polonczyk J, Szlachcic A, Drozdowicz D. Role of capsaicin-sensitive nerves in gastroprotection against acid–independent and acid-dependent ulcerogens. Digestion 1996; 57: 424-432.
- Brzozowski-T, Konturek SJ, Pytko-Polonczyk J, Warzecha Z. Gastric adaptation to stress: role of sensory nerves, salivary glands, and adrenal glands. Scand J Gastroenterol 1995; 30: 6-16.
- Konturek SJ, Brzozowski T, Stachura J, Majka J. Role of neutrophils and mucosal blood flow in gastric adaptation to aspirin. Eur J Pharmacol 1994; 253: 107-114.
- Bergmann JF, Chassany O, Simoneau G, Lemaire M, Segrestaa JM, Caulin C. Protection against aspirin-induced gastric lesions by lansoprazole: simultaneous evaluation of functional and morphologic responses. Clin Pharmacol Ther 1992; 52: 413-416.
- Hata M, Shiono M, Sekino H, et al. Prospective randomized trial for optimal prophylactic treatment of the upper gastrointestinal complications after open heart surgery. Circ J 2005; 69: 331-334.
- Burgess P, Larson GM, Davidson P, Brown J, Metz CA. Effect of ranitidine on intragastric pH and stress-related upper gastrointestinal bleeding in patients with severe head injury. Dig Dis Sci 1995; 40: 645-650.
- Konturek SJ, Radecki T, Brzozowski T, Piastucki I, Zmuda A, Dembinska-Kiec A. Aspirin-induced gastric ulcers in cats. Prevention by prostacyclin. Dig Dis Sci 1981; 26: 1003-1012.
- Skov Olsen P. Role of epidermal growth factor in gastroduodenal mucosal protection. J Clin Gastroenterol 1988; 10(Suppl 1): S146-S151.
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48: 859-867.
- Adami M, Frati P, Bertini S. Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br J Pharmacol 2002; 135: 1598-1606.