The aim of the present study was to analyze daily variations in hypoxic sensitivity in human subjects, in normal conditions of moderate activity during the day and a short sleep time at night (04:00-6:00). Daily changes in the timing system and in the control of breathing are particularly important in the pathogenesis of nocturnal sleep-related breathing disorders.
The study was approved by a local Ethics Committee and informed consent was obtained from all study participants. Fifty six male subjects, all healthy nonsmokers aged 18-28, were enrolled in the study. All subjects were asked to abstain from food, alcohol, or caffeinated drinks for at least 12 h before the experiment. They were placed for 24 h on a modified constant-routine protocol (10). They were isolated from the external environment, stayed in a room with ambient temperature of 20-22°C, artificial lightning and small meal every 3 h with a total energy content of 1500 kJ. The subjects were permitted to sleep between 04:00 and 06:00. During the day they presented moderate physical activity, could move, read, watch movies.
Each subject was placed on a repetitive 3-h test cycle. Each cycle began with a 10-min seated rest followed by a rebreathing test (9). A computerized experimental setup for ventilatory studies during changes in inspiratory gas mixtures consisting of a closed circuit with a built-in CO2 scrubber (soda lime) to maintain automatically a constant end-tidal PCO2 and with an electromagnetic valve closing every 5 breaths at 100 ms from the beginning of inspiratory effort to measure mouth occlusion pressure (P0.1) was used in the study (MES, Cracow, Poland). The rebreathing test took 16 min. Oxygen arterial blood saturation (SaO2) was monitored by a finger pulse oximeter (Trident, Poland). Total of 8 rebreathing tests were performed during the 24 h period of the constant-routine protocol. Hypoxic ventilatory drive was determined by minute ventilation (MV). Slopes of the regression curves MV/SaO2 and P0.1/SaO2 were analyzed. All data were expressed as means ±SD. Whitney-Mann U test was used for the statistical analysis. P<0.05 was accepted as the level of statistical significance.
Daily changes in the ventilatory response to progressive isocapnic hypoxia, presented as the mean values of the MV/SaO2 and P0.1/SaO2 slopes, are illustrated in Fig. 1, Fig. 2 and Fig. 3. In all cases the maximum level of hypoxic reactivity occurred at 12.00 am. The values at other times of the day are relatively stable, with the possible tendency to show ultradian rhythmicity, particularly in the hypoxic ventilatory response presented as the P0.1/SaO2 slope (Fig. 3).
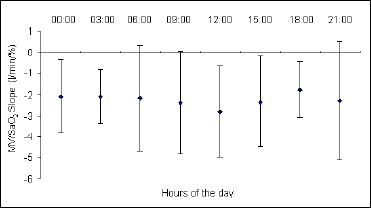 |
Fig. 1. Circadian changes in hypoxic ventilatory responses presented as the mean values of the MV/SaO2 slope. The 12:00 slope was significantly different from those at 0:00, 06:00, 18:00, and 21:00 hours (P<0.05). |
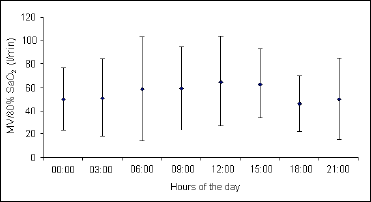 |
Fig. 2. Circadian changes in hypoxic ventilatory response presented as the mean values of minute ventilation (MV) at SaO2 of 80%. Minute ventilation at 12:00 was significantly different from those at 0:00, 18:00, and 21:00 hours (P<0.05). |
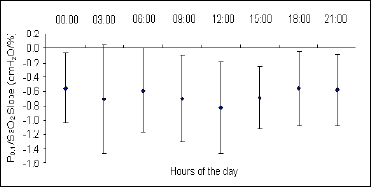 |
Fig. 3. Circadian changes in hypoxic ventilatory response presented as the mean values of the P0.1/SaO2 slope. There were significant differences in mouth occlusion pressure between 12:00 vs. 00:00 and vs. 21:00, and between 15:00 vs. 18:00 (P<0.05). |
Mills et al (10) proposed protocol of "constant routine". It is a protocol aiming to explore daily patterns in constant behavioral and environmental conditions. This approach has been employed to the studies of daily rhythms of respiratory function in humans (6, 11, 12). The imposed prolonged wakefulness of the "constant routine" protocol is a small disadvantage compared to the interpretative problems which would arise by changes in the state of arousal, different caloric intake, and the level of activity (13). Raschke (7) and Raschke and Moller (8) demonstrated daily rhythmicity in O2 sensitivity, applying a protocol that allowed subjects to sleep. Stephenson et al (6) showed daily variations in the CO2 threshold (but not in the CO2 sensitivity) in subjects who did not sleep. Short sleep loss has been shown to have minimal effects on human or canine respiratory chemoreflex (14). We studied the subjects applying a little modification of "constant routine" protocol, as our modified protocol allowed subjects to sleep between 04:00 and 06:00 (one sleep cycle) and to be moderately active during the day. In our study, the hypoxic ventilatory response remained relatively stable in the course of 24 hours, showing a maximum at mid-day (12:00). A tendency to ultradian rhythmicity is similar to another ultradian biological rhythms observed in humans (15-17). We conclude that the respiratory response to hypoxia in the condition of moderate activity remains relatively stable during 24 hours, except for a noon peak. The stability of hypoxic responsiveness may be influenced by changes in arousal during normal activity, as opposed to the "constant routine protocol". We presume that change from wakefulness to night sleep of normal duration is accompanied by depression of respiratory responsiveness, and increased propensity for respiratory instability at night may potentially have to do with the circadian timing system.
Acknowledgments: This study was supported by KBN grant 2P05B02626.
- Ralph MR, Foster RG, Davies FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990; 247: 975-978.
- Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med 2002; 165, 8: 1041-1047.
- Modarreshadeh M, Bruce EN. Ventilatory variability induced by spontaneous variations of PaCO2 in humans. J Appl Physiol 1994; 76: 2765-2775.
- Khoo MCK, Marmarelis VZ. Estimation of peripheral chemoreflex gain from spntaneous sigh responses. Ann Biomed Engl 1989; 17: 557-570.
- Jensen JI, Mosekilde E, Ward DS. A system dynamics model of human respiratory regulation. In BJ Whipp, DM Wiberg (eds). Modelling And Control Of Breathing. New York, Elsevier. 1983, pp. 316-321.
- Stephenson R, Mohan RM, Duffin J, Jarsky TM. Circadian rhythms in the chemoreflex control of breathing. Am J Physiol Regul Integr Comp Physiol 2000; 278: R282-R286.
- Raschke F. Various components of respiratory control during sleep, rest and strain. In JH Peter, T Podszus, P von Wichert (eds). Sleep Disorders And Internal Diseases. Berlin, Verlag. 1987, pp. 83-88.
- Raschke F, Moller KH. Untersuchungen zur Tagesrhythmik der Chemosensitivitat und beitrag zu nachtlichen Atmungsregulationsstorungen. Pneumologie 1989; 43: 568-571.
- Read DJC, Leigh J. Blood-brain PCO2 raltionship and ventilation during rebreathing. J Appl Physiol 1967; 23:53-70.
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts on the oscillator(s) controlling human circadian rhythms. J Physiol London 1978; 285: 455-470.
- Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 2000; 161: 124-1128.
- Spengler CM. Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. AM J Respir Crit Care Med 2000; 162: 1038-1046.
- Vargas M, Jimenez D, Leon-velarde F, Osorio J, Mortola JP. Circadian patterns in men acclimatized to intermittent hypoxia. Respir Physiol 2001; 126: 233-243.
- Espinoza H, Thornton AT, Sharp D, Antic R, McEvoy RD. Sleep fragmentation and ventilatory responsiveness to hypercapnia. Am Rev Resp Dis 1991; 144: 1121-1124.
- Stupfel M, Pletan Y. Respiratory ultradian rhythms of mean and low frequencies: A comparative physiological approach. Chronobiologia 1983; 10: 283-292.
- Machleidt W. Ultradiane Rhythmen in EEG. Z EEG-EMG 1980; 11: 148-154.
- Klein R, Armitage R. Rhythms in humans performance: 11/2 - hour oscillations in cognitive style. Science 1979; 204:1326-1328.