It is generally accepted that potassium ion channel activation prevents the development of action potentials in smooth muscles. According to Thorneloe and Nelson (1) several K+ channels, with heterogeneous molecular basis, contribute to regulatory basal K+ conductance in nerves or smooth muscle cells: Kv (voltage-gated); BK+Ca (large conductance calcium-activated); SK+Ca (small conductance calcium-activated); Kir (inward rectifier); stretch-dependent and K+ATP (ATP-sensitive). There is increased evidence of BK+Ca and K+ATP ion channels role in ASM reactivity, concerning both afferent and efferent airway sensory nerve activity.
K+ATP ion channels link membrane excitability to metabolism. Several endogenous agonists (e.g., intracellular nucleotides and calcitonin-gene related peptide; CGRP) activate K+ATP resulting in hyperpolarization and relaxation, a response that is similar to the effect of the K+ATP openers pinacidil or mogusteine (2). Nielsen-Kudsk (3) showed that pinacidil relaxes isolated trachea of the guinea pig. The ASM relaxation produced by pinacidil was selectively blocked by the antidiabetic glibenclamide. Furthermore, Fabiani et al (4) suggested that K+ATP agonists inhibit acetylcholine release from parasympathetic nerves of the airways. Morita and Kamei (5) demonstrated a suppression of capsaicin-induced cough in guinea pigs, which was prevented by glibenclamide.
BK+Ca have been shown to play a role in ASM in a number of species and may be important regulators of the membrane potential and intrinsic tone of smooth muscle (6). Fox et al (7) demonstrated that a selective opener of BK+Ca, NS1619, inhibits the activity of myelinated and unmyelinated sensory nerves in guinea pigs airways in vitro and citric acid-induced cough. BK+Ca probably represent a common mechanism, which mediates the inhibition of airway reflexes evoked by a number of sensory nerve receptors (e.g., µ-opioid or 5-HT1). According to Patel et al (8) NS1619 also inhibits cholinergic contractile responses by prejunctional interaction, but this effect is not coupled with the autoinhibitory function on muscarinic receptors.
Therefore, in this study we investigated whether openers of potassium channels, K+ATP - pinacidil and BK+Ca - NS1619, modulate cough reflex and airway smooth muscle (ASM) reactivity in in vivo and in vitro conditions in guinea pigs
Animals
The study protocol was approved by a local Ethics Committee of the Jessenius Faculty of Medicine in Martin, Slovakia, in accordance with the revised Declaration of Helsinki from 1983. Adult, male TRIK strain guinea pigs, weighing 150-350 g, were used in the study. The animals were obtained from the Department of Experimental Pharmacology, Slovak Academy of Sciences, Dobra Voda, Slovakia, and were housed in the approved animal holding facility. The experiments were carried out one week of animals' adaptation period.
The function of both ATP or calcium-sensitive potassium ion channels was tested in two groups of guinea pigs: group A - "Agonist" group (n= 8) - received the K+ATP opener pinacidil in a dose of 1 mg/kg subcutaneously (sc) or were exposed to an aerosol of BK+Ca agonist NS1619 (200 µM) for 10 min; and group B - "Antagonist/agonist" group - received a combined medication consisting of pretreatment with potassium ion channels antagonist glibenclamide (K+ATP) in a dose 3 mg/kg intraperitoneally (ip) or tetraethylammonium - TEA (BK+Ca) in a dose 8 mg/kg, ip, followed by the agonist described for group A after 20 min.
-
In these experiments we tested:
- Influence of the above mentioned agents on citric acid-induced cough in conscious guinea pigs;
- Specific airway resistance in vivo
- Response of ASM to cumulative doses of contractile mediators in vitro.
Chemicals
Modulators of potassium ion channels activity, citric acid, and the contractile mediators acetylcholine and histamine were obtained from Sigma-Aldrich (Lambda Life, Slovakia). Codeine was purchased from Lachema (Czech Republic). Pinacidil, glibenclamide and NS1619 were dissolved in 10% DMSO, TEA, codeine was dissolved in water for injection, and all other drugs in 0.9 % saline.
Method of chemically induced cough
Awaken guinea pigs were individually placed in a body plethysmograph box (HSE type 855, Hugo Sachs Elektronik, Germany) and were exposed to citric acid aerosol in a concentration 0.3 M for 3 min. The citric acid aerosol was generated by a jet nebulizer (PARI jet nebulizer, Paul Ritzau, Pari-Werk, Germany, output 5 l/s, particles mass median diameter 1.2 µm) and delivered to the head chamber of the plethysmograph.
-
The following two methods were used for detection of cough and to distinguish cough efforts from sneezing and movements:
- changes in the expiratory airflow interrupting the basic respiratory pattern during cough, as measured by pneumotachograph connected to the head chamber of the plethysmograph.
- typical cough movements and sounds recognized by a trained observer.
A minimum time difference between any two measurements of cough response, to prevent cough receptor adaptation, was 2 h. Measurements were realized taking into account the metabolism time of the drugs used (48 h in case of codeine and K+ATP modulators and 24 h in case of BK+Ca modulators).
Evaluation of ASM reactivity in vitro
Changes in ASM reactivity in response to cumulative doses of acetylcholine and histamine after administration of the above mentioned agents were tested by a tissue bath method. The animals were sacrificed by transversal interruption of the neck spinal cord 30 min after administration of pinacidil and glibenclamide/pinacidil or after inhalation of NS1619 and combined therapy of TEA/NS1619. Four strips (two of tracheal and two of pulmonary smooth muscle) obtained from each animal were placed in 30 ml organ bath chambers filled with Krebs-Henseleit´s buffer of the following composition (mM): NaCl 112.9, KCl 4.7, CaCl2 2.8, MgSO4 0.5, NaHCO3 24.9, glucose 11.1, saturated with 95% O2 and 5% CO2 at 36.0 ±0.5°C, pH 7.5 ±0.1. Single strips were fixed onto the sliding arm and the other end was bound with a thin thread to a hook of a transducer (TSR 10 G, Vyvoj, Slovakia) and amplifier (M1101, SUPR, Microtechna, Slovakia). The tension was transmitted through an amplifier to a linear recorder (LINE RECORDER TZ 4620, Laboratorni pristroje, Czech Republic) to monitore the intensity of contractile responses. Tissues strips were initially set to 4 g tension (30 min loading phase). After this period, the tension in each tissue segment was readjusted to a baseline of 2 g (30 min adaptation phase). During this period, tissue strips were washed at 10 min intervals. The amplitude of isometric contraction (mN) of tracheal and pulmonary smooth muscles in response to cumulative doses of acetylcholine and histamine at concentrations 10-8-10-3 mol/l were used for reactivity evaluation.
The evaluation of ASM reactivity in vivo conditions
ASM reactivity was expressed as specific airway resistance (RV) values calculated by Pennock (9). This non-invasive plethysmograph technique is commonly used for evaluation of bronchoactive substances effect (10). Conscious adult male TRIK strain guinea pigs were individually placed in a double chambers body pletysmograph box for laboratory animals (HSE type 855, Hugo Sachs Elektronik, Germany) consisting of head and body chambers. Nasal airflow was registered in the head chamber and the thoracic airflow in the body chamber. The value of specific airway resistance, i.e., the extent of bronchoconstriction, is proportional to the phase difference between the nasal and thoracic respiratory airflows.
Baseline (N) specific airway resistance measurement took 1 min and was followed by citric acid exposure and cough response registration at 0.5, 1, 2 and 5 h.
Data analysis
All data were expressed as means ±SE. Significance of differences was evaluated using a paired t-test. P<0.05 was considered significant.
Modulators of potassium ion channels and citric acid-induced cough
Both potassium ion channel agonists reduced the number of citric acid-induced cough efforts (Fig. 1). Pinacidil, a K+ATP opener, led to a significant decline in the number of citric acid-induced coughs at all time points measured in comparison with the baseline number of cough efforts. The character and intensity of the decline was comparable to then effect of codeine. Pretreatment with glibenclamide partially reduced the pinacidil influence on coughs.
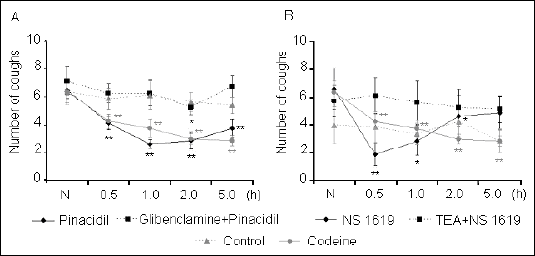 |
| Fig. 1. Influence of potassium channels openers: pinacidil (A) and NS1619 (B), given alone and after pretreatment with their respective selective blockers: glibenclamide and TEA on the number of citric acid-induced coughs (see text for details). The values labeled as N represent baseline levels of cough before application of any agents. *P<0.05; **P<0.01 vs. N. |
NS1619, an agonist of BK+Ca, significantly decreased the number of coughs at 0.5 h and 2 h. This effect was completely abolished by pretreatment with the selective BK+Ca inhibitor tetraethylammonium.
Modulators of potassium ion channels and ASM reactivity in vivo
Our experiments demonstrated that both potassium ion channels agonists influenced specific airway resistance in conscious guinea pigs (Fig. 2). Pinacidil administration caused a decline in specific airway resistance (RV) at all time points of the measurement. NS1619 significantly reduced the value of RV at 0.5 h and 1 h in comparison with the baseline value. The effects of both K+ ion channel openers were completely antagonized by pretreatment with the selective inhibitors of these channels activity.
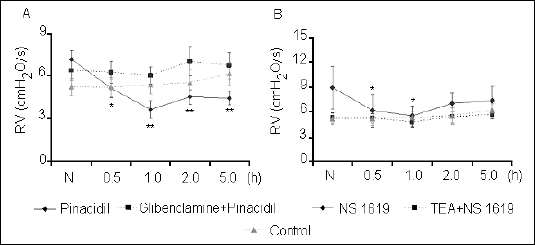 |
| Fig. 2. Changes in specific airway resistance (RV) after administration of potassium channels openers: pinacidil (A) and NS1619 (B) alone, and after pre-treatment with their respective blockers: glibenclamide and TEA. The values labeled as N represent baseline measurements of RV before application of any agents. *P<0.05; **P<0.01 vs. N. |
Modulators of potassium ion channels and ASM reactivity in vitro
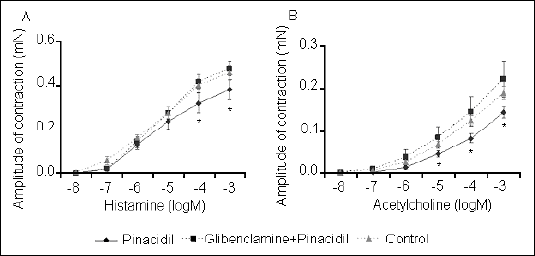
We found that administration of the K+ATP agonist pinacidil resulted in a significant decrease in the tracheal smooth muscle response to cumulative doses of the contractile mediators histamine and acetylcholine (Fig. 3). Likewise, the contractile response of tracheal smooth muscle to these mediators was significantly attenuated by the BK+Ca opener NS1619 (Fig. 4). Observed influence of potassium ion channel agonists was completely inhibited by pretreatment with antagonists.
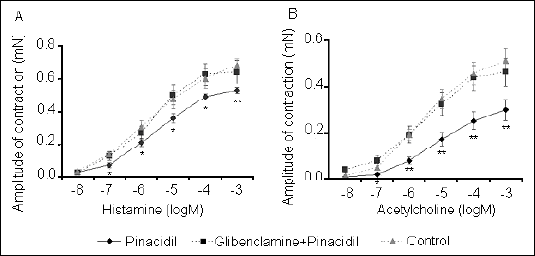 |
| Fig. 3. Pinacidil-induced changes in tracheal smooth muscle contractile response, expressed as the amplitude of contraction, to cumulative doses of histamine (A) and acetylcholine (B). The influence of pretreatment with glibenclamide on the pinacidil effect is also shown.*P<0.05; **P<0.01 vs. control values. |
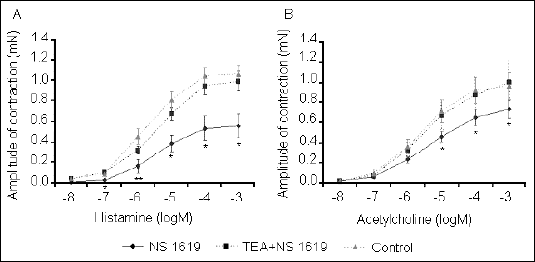 |
| Fig. 4. NS1619-induced changes in tracheal smooth muscle contractile response, expressed as the amplitude of contraction, to cumulative doses of histamine (A) and acetylcholine (B). The influence of pretreatment with TEA on the NS1619 effect is also shown.*P<0.05; **P<0.01 vs. control values. |
Reactivity of pulmonary smooth muscle to histamine and acetylcholine was reduced mildly after prior administration of pinacidil and significant changes were registered only at higher concentrations of both contractile mediators (Fig. 5). Furthermore, the BK+Ca agonist NS1619 did not changed the pulmonary smooth muscle contractile response. In this case, the recorded values of reactivity were comparable with those in the negative control group (Fig. 6).
 |
| Fig. 5. Pinacidil-induced changes in pulmonary smooth muscle contractile response, expressed as the amplitude of contraction, to cumulative doses of histamine (A) and acetylcholine (B). The influence of pretreatment with glibenclamide on the pinacidil effect is also shown. *P<0.05; **P<0.01 vs. control values. |
 |
| Fig. 6. NS1619-induced changes in of pulmonary smooth muscle contractile response, expressed as the amplitude of contraction, to cumulative doses of histamine (A) and acetylcholine (B). Lack of the influence of pretreatment with TEA on the NS1619 effect is also shown. |
In the present study, we found that subcutaneous administration of the K+ATP agonist pinacidil and inhalation of the BK+Ca opener NS1619 produced a marked decline of citric acid-induced cough efforts in the guinea pig. The antitussive activity of these K+ ion channels agonists was partially (pinacidil) or completely (NS1619) antagonized by pretreatment with the selective blockers glibenclamide and TEA, reespectively. These results strongly imply that the antitussive effects of pinacidil and NS1619 are mediated via the activation of K+ATP and BK+Ca ion channels. The study confirmed the findings of Morita et al (11) findings who demonstrated that K+ATP openers significantly and dose-dependently suppress the capsaicin-induced cough. According to our results, a significant suppressing effect of a single dose of pinacidil (1 mg/kg) lasted for 5 h and the strength of cough suppression is close to the effects of centrally acting codeine. Our results also are in line with those of Fox et al (7) who found an inhibitory effect of NS1619 on citric acid-induced cough. Moreover, we demonstrated that the antitussive effect of a single dose of NS1619 (200 µM) given by inhalation was sustained for 2 h.
Although cough and bronchoconstriction are believed to have separate afferent neural pathways, they often occur simultaneously and have been considered to be closely related (12). Several literature data indicate that promoting K+ efflux through potassium ion channels inhibits bronchoconstriction (3, 13, 14). Our experiments showed that both K+ATP and BK+Ca ion channels are involved in ASM reactivity. Therefore, it is possible that their antitussive effect is, at least in part, a consequence of bronchodilation.
Although pinacidil significantly inhibited ASM reactivity both in vivo and in vitro conditions, it appears that mechanisms other than bronchodilatory activity might be involved in the antitussive effect of the K+ATP agonist. While the influence of pinacidil on ASM reactivity was completely antagonized by pretreatment with glibenclamide, its antitussive effect was abolished only partially. Recently, Poggioli et al (15) also reported that pinacidil has antitussive effects in doses that have no influence on the stimuli-induced bronchospasms.
In contrast, the BK+Ca agonist NS1619 produced rather mild attenuation of ASM reactivity in both in vivo and in vitro conditions, but it caused an impressive suppression of citric acid-induced cough. In this regard, Fox et al (7) reported that increased activity of BK+Ca channels results in decreased firing of A
Our experiments in vitro showed that ASM reactivity, both tracheal and pulmonary, to cumulative doses of acetylcholine was reduced more after pinacidil than after histamine. Aside from K+ATP opening, other mechanism may participate in bronchodilatory properties of pinacidil. There is increasing evidence that K+ATP activity plays an important role in NO-mediated, cGMP-independent, response to acetylcholine (4, 17). This evidence also concerns different types of smooth muscles, such as those in blood vessels (18, 19).
In the presented study, the agonist of BK+Ca NS1619 prevented histamine-induced contractility of tracheal smooth muscle in vitro. Buchheit et al (20) obtained similar results in the experiments with another selective BK+Ca agonist - SCA 40. Unlike K+ATP, BK+Ca channel function in ASM reactivity in response to acetylcholine seems to be variable and depends on a number of ill-defined factors, such as the method or the sort of experimental animals used. The response of guinea pig tracheal smooth muscle to cumulative doses of acetylcholine was less significantly attenuated after NS1619 in our study in comparison with the same effect of pinacidil. Thus, our results point to a more important role of K+ATP channels in NO-mediated response to acetylcholine and confirm those of Corompt et al (21) who demonstrated that BK+Ca did not appear to functionally participate in the relaxation to an NO-donor in human bronchi ana guinea pig trachea precontracted with acetylcholine. On the contrary, Janssen et al (22) showed a key role of BK+Ca channels in isolated human bronchi or trachea strips of dogs. We found that pulmonary tissue strips reactivity was not influenced by NS1619, which fits well with the results of Snetkov and Ward (23). These authors argue that small bronchioles differ in the expression of BK+Ca channels compared with large airways, with possible implications for the channel function and, consequently, ASM changes.
In summary, both potassium ion channels tested play an important role in cough and other defense reflexes of the airways, which are coupled with reactivity of airway smooth muscles. Our experiments may provide a basis for future studies on these ion channel openers as potential antitussive agents in therapy of inflammatory airway diseases, such as asthma.
Acknowledgements: The study was supported by Grant of Agency for Science (VEGA) No. 1/3375/06 and Grant of Ministry of Health No. 2005/13-MFN-05.
- Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 2005; 83: 215-242.
- Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des 2005; 11: 1915-1940.
- Nielsen-Kudsk JE. Potassium channel modulation: a new drug principle for regulation of smooth muscle contractility. Studies on isolated airways and arteries. Dan Med Bull 1996; 43: 429-447.
- Fabiani ME, Vlahos R, Story DF. Epithelium-dependent inhibition of cholinergic transmission in rat isolated trachea by potassium channel openers. Pharmacol Res 1996; 33: 261-272.
- Morita K, Kamei J. Involvement of ATP-sensitive K+ channels in the anti-tussive effect of moguisteine. Eur J Pharmacol 2000; 395: 161-164.
- Zhu FX, Zhang XY, Olszewski MA, Robinson NE. Mechanism of capsaicin-induced relaxation in equine tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 1997; 273: 997-1001.
- Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. Activation of large conductance potassium channels inhibits the afferent and efferent function of airway sensory nerves in the guinea pig. J Clin Invest 1997; 99: 513-519.
- Patel HJ, Giemgycz MA, Keeling JE, Barnes PJ, Belvisi MG. Inhibition of cholinergic neurotransmission in guinea pig trachea by NS1619, a putative activator of large-conductance, calcium-activated potassium channels. J Pharmacol Exp Ther 1998; 286: 952-958.
- Pennock BE, Cox CP, Rogers RM, Cain WA, Wells JH. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol 1979; 46: 399-406.
- Tohda Y, Muraki M, Ywanaga T, Haraguchi R, Fukuoka M, Nakajima S. Dual-phase response model for bronchial asthma. Allergy Asthma Proc 2000; 21: 79-84.
- Morita K, Onodera K, Kamei J. Inhaled pinacidil, an ATP-sensitive K+ channel opener, and moguisteine have potent antitussive effects in guinea pigs. Jpn J Pharmacol 2002; 89: 171-175.
- Karlsson JA, Sant' Ambrogio G, Widdicombe J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol 1988; 65: 1007-1023.
- Stretton D, Miura M, Belvisi MG, Barnes PJ. Calcium-activated potassium channels mediate prejunctional inhibition of peripheral sensory nerves. Proc Natl Acad Sci USA 1992; 89: 1325-1329.
- Fozard JR, Manley PW. Potassium channel openers: Agents for the treatment of airway hyperreactivity. In New Drugs for Asthma, Allergy and COPD, TT Hansel, PJ Barnes (eds). Basel, Prog Respir Res, Karger, 2001, 31: 77-80.
- Poggioli R, Benneli A, Arletti R, Cavazzuti E, Bertolini A. Antitussive effect of K+ channel openers. Eur J Pharmacol 1999; 371: 39-42.
- Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A Discov Mol Cell Evol Biol 2003; 270: 2-10.
- Song P, Rocchi D, Lazzarotti M, Crimi E, Rehder K, Brusasco V. Postjunctional effect of pinacidil on contractility of isolated bovine trachealis. Eur Respir J 1996; 9: 2057-2063.
- Ferrer M, Marin J, Encabo A, Alonso MJ, Balfagon G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen Pharmacol 1999; 33: 35-41.
- Garry A, Sigaudo-Roussel D, Merzeau S, Dumont O, Saumet JL, Fromy B. Cellular mechanisms underlying cutaneous pressure-induced vasodilation: in vivo involvement of potassium channels. Am J Physiol Heart Circ Physiol 2005; 289: H174-H180.
- Buchheit KH, Hofmann A, Pfannkuche HJ. in vitro and in vivo effects of SCA 40 on guinea pig airways. Naunyn Schmiedebergs Arch Pharmacol 1997; 355: 217-223.
- Corompt E, Bessard G, Lantuejoul S, Naline E, Advenier C, Devillier P. Inhibitory effects of large Ca2+-activated K+ channel blockers on beta-adrenergic- and NO-donor-mediated relaxations of human and guinea-pig airway smooth muscles. Naunyn Schmiedebergs Arch Pharmacol 1998; 357: 77-86.
- Janssen LJ, Premji M, Lu-Chao H, Cox G, Keshayjee S. NO+ but not NO radical relaxes airway smooth muscle via cGMP-independent release of internal Ca2+. Am J Physiol Lung Cell Mol Physiol 2000; 278: L899-905.
- Snetkov VA, Ward JP. Ion currents in smooth muscle cells from human small bronchioles: presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp Physiol 1999; 84: 835-846.