There is not enough data on the relation between airway inflammation or remodeling and lung function. There are some publication indicating, for example, the relationship between serum matrix metalloproteinase-9 concentration and airway obstruction (10) or macrophage surface markers in induced sputum and airflow limitation (5). On the other hand, there is growing evidence that airway inflammation and remodeling are related to genetic factors (11-13).
As it is suspected that airway remodeling leads to irreversible airway obstruction or is the main cause of the severe course of the lung obstructive diseases, there is need to search for direct relations between airway remodeling and lung function. Airway remodeling leads to bronchial wall thickening what can be assessed by HRCT (14-16). Therefore, the aim of the present study was to compare radiological features of airway remodeling in asthma and chronic obstructive pulmonary disease (COPD) patients and to assess the correlations between airway wall thickness as well as airway luminal diameter and lung function in asthma and COPD.
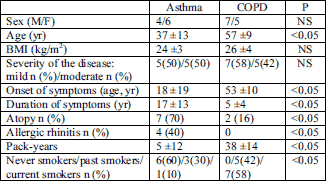
The study was a part of a research project approved by the Bioethics Committee of the Medical University of Warsaw (No. 164/2004). All patients had signed an informed consent form. This prospective study was performed in 10 patients with asthma and 12 patients with COPD between December 2004 and December 2006. The diagnosis of asthma or COPD was based on GINA (17) or GOLD (18) guidelines respectively. The following inclusion criteria were applied: 1) stable disease (no exacerbation during 4 weeks before the study onset); 2) mild to moderate severity of the disease; 3) no anti-inflammatory treatment (inhaled corticosteroids) for at least three months preceding the study onset. The demographic and clinical data of asthma and COPD patients are shown in Table 1.
| Table 1. Demographic characteristics of asthma and COPD patients. |
 |
The patients’ evaluation included: physical examination, chest radiograph, lung function testing, allergy skin prick tests (Alergopharma, Germany), and total serum IgE. Lung function testing was performed in accordance with the European Respiratory Society and American Thoracic Society guidelines (19, 20), and included pre- and postbronchodilator spirometry, metacholine challenge test (Lungtest 1000, MES, Poland), body plethysmography with airway resistance measurement (pre- and postbronchodilator) and diffusion lung capacity for carbon monoxide (DLCO) (Vmax Series 229/V6200, Sensor Medics Corporation, Yorba Linda, USA). Spirometry and plethysmography were performed directly before the chest HRCT, whereas the bronchial challenge was performed afterwards.
Chest HRCT and measurements of bronchial intersections
Inhaled salbutamol (400 µg via a spacer) was administered directly before HRCT scanning to achieve maximal bronchodilation during the procedure. In all patients thorax HRCT was performed with 16-row CT (LightSpeed 16 General Electric, USA), without intravenous contrast administration. The following parameters were used: collimation - 1.25 mm, current – 140 kV, 250 mA, matrix size 512 x 512. The CT image data were reconstructed with a high spatial frequency algorithm and viewed at a window level of – 450 HU and a window width of 1500 HU, which have been estimated as the most accurate parameters to visualize and measure the bronchial diameters. Five selected lung levels were analyzed: 1) superior margin of the aortic arch, 2) tracheal carina, 3) 1 cm below the carina, 4) inferior pulmonary veins, and 5) 2 cm above the diaphragm.
The CT images were enlarged (magnification x 10). Cross-sections of bronchi with external diameter range 1–5 mm were identified and submitted for analysis. Only cross-sections perpendicular to the long airway axis were selected. This was achieved by the comparison of the largest luminal diameter (DL) and the largest luminal diameter perpendicular to DL (DS); only airways with a DL/DS ratio
1. WT - wall thickness. With the assumption that the bronchial wall thickness is constant on the cross section: WT = (D – L)/2.
2. BWT - bronchial wall thickness. Defined as the ratio of the wall thickness to the external diameter; BWT = WT/D.
3. AO (total bronchial area) =
4. AL (luminal area) =
5. WA (airway wall area) = AO-AL (Fig. 1).
6. WA% (the percentage wall area) = (WA/Ao) x 100%.
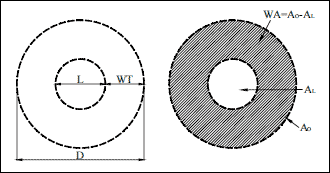 |
Fig. 1. Measurements of the airway cross section: D – airway external diameter, L – airway luminal diameter, WT – wall thickness, AO – airway outer area, AL – airway luminal area, WA –airway wall area. |
Considering that WA and AL may be related to patient’s body surface area (BSA), these variables were calculated additionally as WA/BSA and AL/BSA (21). Two main variables: BWT and WA%, regarded as most objective (6, 22) were taken into account in the comparison of airway wall thickness in both studied groups (6, 22).
Statistical analysis was performed using software Statistica 6.0 (StatSoft Inc. USA). Numerical values were presented as means±SD, for selected variables the value range was presented. To compare the results between the asthma and COPD patients the Mann-Whitney U-test was applied; P<0.05 being regarded statistically significant. For analysis of relations between two quantity variables, Spearman’s r correlation coefficient was used. Spearman’s rank coefficient was used to test potential correlations between two groups of variables.
HRCT airway measurements
The mean number of airways assessed in each patient was 28±5 (25±6 in the asthma group and 29±6 in the COPD group). At each lung level (of the left and right lung) 3±1 airways were measured in each patient. There was no significant difference in the airway wall thickness parameters between the studied groups (Table 2).
| Table 2. Comparison of airway dimensions assessed in HRCT in asthma and COPD patients. |
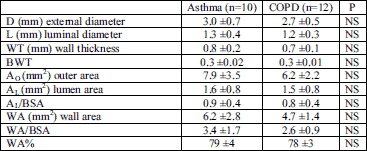 |
| Data are means±SD. See Material and Methods for abbreviations. |
Lung function tests in asthma and COPD patients
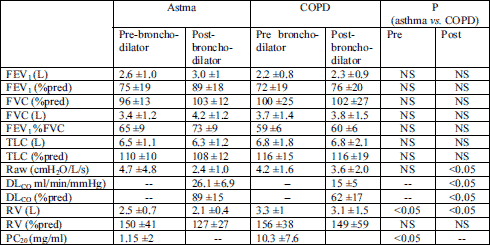
Table 3 presents the comparison of the results of lung function tests in asthma and COPD. Since the chest HRCT was performed after salbutamol inhalation, only the post-bronchodilator lung function parameters were taken into consideration in the analysis of the relations between airway dimensions and lung function.
| Table 3. Comparison of pulmonary function (pre- and post-bronchodiltaor) in asthma and COPD patients. |
 |
The relationship between airway dimensions and lung function in asthma patients
There was no relationship between airway wall thickness (WA% and BWT) and patients’ age or duration of symptoms. There was no correlation between airway dimensions and FEV1% pred or FVC% pred (Table 4). Interestingly, there was a positive correlation noted between WA% and BWT and RV% pred (r=0.72; P<0.05 and r=0.72; P=0.05, respectively). A negative correlation was observed between the lumen area AL (and AL/BSA) and airway resistance (Raw). The most important correlations are presented in Table 4. No other correlations between airway wall thickness and pulmonary function (including the value of PC20) were found in asthma group.
| Table 4. Relations between airway wall thickness or airway lumen dimension and lung function in asthma patients. |
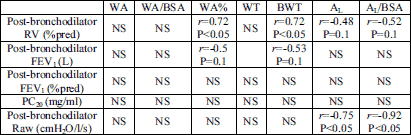 |
| r-Spearman’s correlation coefficient. See Material and Methods for abbreviations. |
The relationship between airway dimensions and lung function in COPD patients
In the COPD group, there was a significant correlation between airway resistance (Raw) and WA%, BWT, AL and AL/BSA. We also noted a significant negative correlation between WA% and DLCO and between BWT and DLCO. A relationship between BWT and WA% and airway hyperresponsiveness (PC20) was found. The most important results of the statistical analysis are presented in Table 5.
| Table 5. Relations between airway wall thickness or airway lumen dimension and lung function in COPD patients. |
 |
| r- Spearman’s correlation coefficient. See Material and Methods for abbreviations. |
There were no significant correlations between BWT or WA% and patients’ age, duration of symptoms, and the number of pack-years. There was no correlation between BWT or WA% and spirometric parameters either. A significant positive correlation was found between WA, WA/BSA, WT and the number of pack-years (Table 5).
HRCT is a valuable tool in airway wall thickness and airway lumen dimension assessment (6, 23). It enables a non-invasive, reproducible evaluation of the airways at the level of bronchioles (14, 21, 24). It was found useful in airway dimension measurements during provocative challenge tests (23, 25) and after bronchodilator inhalation (23-25). A relationship between basement membrane thickness assessed in biopsy specimens and airway wall thickness assessed by HRCT was documented (15).
On the other hand, HRCT has its limitations. It enables the imaging of the total enlargement of the wall area or lumen area, but it does not provide with any information about each layer of the bronchial wall. Exposure of a patient to higher doses of radiation, compared with conventional chest X-ray, is still an important limitation of the method.
Comparison of airway dimensions in asthma and COPD patients
Awadh et al. (6) were one of the first researchers who applied HRCT to assess airway wall dimensions in patients with asthma. Not only did they show that airway walls are thickened in asthma, but they also found a relationship between the severity of the disease and airway wall thickness. Their observations were confirmed by other authors (15, 26-29). Beigelman-Aubry et al. (23) found that lumen area is smaller in asthma patients in comparison with healthy subjects, but there was no difference when measurements were undertaken after salbutamol inhalation.
Salbutamol influences the reversible component of airway obstruction related to airway smooth muscle constriction. Therefore, to assess less reversible or irreversible airway structural changes associated with remodeling we performed all the measurements after bronchodilator inhalation. A similar study procedure was applied previously (15, 26). Okazawa et al. (22) showed that lumen area is diminished, whereas airway walls are thickened in asthma patients compared with healthy subjects. Niimi et al. (27) observed that a greater thickness of the right apical bronchus correlates with a lower FEV1 % predicted, but they did not find significant differences in the lumen area in patients with severe compared with mild and moderate asthma and even with healthy subjects. In the present study, there was no control group of healthy subjects, as the goal of the study was to compare airway dimensions between asthma and COPD patients.
The knowledge about airway wall thickness in COPD comes mainly from indirect measurements performed during autopsy (30) or after pulmonary tissue resection (31). It has been documented that in the course of the disease airway walls thicken and the lumen diameter is diminished (31). This was also noted in HRCT scans (32, 33).
We were unable to find studies directly comparing airway dimensions assessed in HRCT in patients with asthma and COPD. Harmanci et al. (7) evaluated airway remodeling in asthma and COPD patients using HRCT, but they focused on qualitative structural changes. Comparing the results of the studies by Niimi et al. (27) and Nakano et al. (33), it seems that the wall of the right apical bronchus is thicker in asthmatics when compared to COPD patients with the same degree of airway obstruction. The tendency, found in the present study, for WA%, BWT, and other airway thickness parameters to be insignificantly higher in the asthma group is in line with these observations.
The greater thickness of airway wall in asthma can be partially explained by histological findings. It has been shown that the basement membrane thickness (34), smooth muscle layer (30), and the number of blood vessels (35) in patients with asthma are greater than those in patients with COPD. Therefore, it is likely that airway remodeling results in a greater thickening of the airway wall in asthma when compared to COPD. The results of the present study did not directly confirm this hypothesis, but this may be due to a small number of patients in both groups studied.
Niimi et al. (27) found that in asthmatic subjects the cross sectional bronchial wall area was increased; however the increase was not accompanied by a decrease in its lumen when compared with healthy subjects. This finding is in contrast with that of Nakano et al. (33) who analyzed data from patients with COPD; they observed that the airway luminal area is smaller and airway wall thickness is greater in subjects with COPD when compared with healthy persons. In the present study, the airway wall thickness indices (WA and WT) and airway outer area (AO) were greater in the asthmatic subjects when compared with patients with COPD. It is not clear if WA and WT are good parameters for the comparative analysis of airway structural changes in asthma and COPD. However, there are publications in which such comparisons are performed (27). As the values of wall area and airway lumen area (WA and AL) depend on individual anthropometric data, these indices are often presented in relation to the body surface area (21, 27, 36) like it was in our study.
One should remember that features of remodeling can be changed by anti-inflammatory treatment like inhaled glicocorticosteroids (iGCS) (14), therefore patients in this study had not been receiving iGCS during at least 3 months preceding the study. Airway wall thickness observed in HRCT is related to some of the histological features of remodeling assessed in biopsy specimens (15). However, the impact of airway structural changes and their dimensions on lung function needs to be elucidated.
The relation between airway dimensions and lung function in asthma
Saglani et al. (16) and Little et al. (26) did not find any relation between airway wall thickness and airway obstruction, expressed as FEV1 % predicted in asthmatics. On the other hand, the results of some other studies suggest that there is a correlation between airway wall thickening and disease severity (6, 27).
The present study results did not confirm the correlation between the airway wall thickness and FEV1 % predicted. It should be noted, however, that airway wall remodeling is only one of the factors contributing to airflow limitation in asthma. Mucous membrane edema, inflammatory cell infiltration, increased mucus secretion and smooth muscle constriction may influence airflow limitation. On the other hand, many genetic and environmental factors can influence spirometric results and airflow limitation (37-39). The impact of smooth muscle constriction was minimized by salbutamol inhalation prior to the HRCT procedure; however, the other aforementioned components of airway obstruction could have influenced our results.
Boulet et al. (40) observed that airway hyperresponsiveness and wall thickening were related to each other in a group of asthma patients with persistent obstruction, but they did not find such a correlation in the group without post-bronchodilator airway obstruction. In the present study, we did not note any relation between bronchial hyperresponsiveness and airway wall thickness assessed by HRCT. Our results are similar to those obtained in other studies (26, 41).
The correlation between residual volume and airway wall thickness as well as lumen area seems to be a very interesting finding. This may suggest that airway wall thickening results in air trapping in patients with asthma. This is consistent with results of some other studies (8, 28).
The relation between airway dimensions and lung function in COPD
In the present study, we found no relationship between airway wall thickening and spirometric parameters in COPD patients. However, we observed that patients with a thicker airway wall (higher BWT and WA%) had a lower PC20 value and higher airway resistance.
Nakano et al. (42) evaluated the dimensions of the right apical bronchus in a group of 114 smokers and found a correlation between WA% and FEV1 % pred, FVC % pred, and the RV/TLC ratio. This suggests a relation between airway wall thickness and airway obstruction. In another study, the same authors showed that WA% in the HRCT of the proximal airways correlates with WA% assessed in biopsy specimens. Similarly, Tiddens et al. (43) observed that proximal airway wall thickening correlates with obstruction and inflammatory features found in the distal bronchi. The authors suggest that in the course of COPD, a similar inflammatory process and a similar degree of airway remodeling are present along the whole bronchial tree, regardless of the airway caliber. We may, therefore, assume that airway wall thickening of larger airways observed in HRCT indicates a similar process in small airways.
The results of the present study suggest that in COPD, greater WA% and BWT values are associated with a lower lung diffusion capacity. We might speculate that airway remodeling in COPD is accompanied by structural changes in the lung parenchyma, which impaires DLCO. Nakano et al. (33) defined two groups of COPD patients according to the type of remodeling (airway or lung tissue remodeling) predominating in HRCT. Lung tissue remodeling (emphysema) was defined as the proportion of low attenuation areas in the lungs (LAA%). The authors found that WA% and LAA% correlated with FEV1% predicted and FVC% predicted, but only LAA% was related to DLCO (33). In the present study, we did not perform such an analysis, as we focused on the airway rather than lung parenchyma evaluation.
One of the most significant limitations of our study was a relatively small number of patients enrolled. This had two reasons: the inclusion criteria (the patients had to be steroid-naive) and lack of consent for chest HRCT which is associated with a high dose of radiation. Therefore, our results should be treated with caution. Another limitation was the lack of a second radiologist evaluating the airway dimensions. However, it should be noted that the values of airway wall dimensions regarded as most objective (WA% and BWT) were similar to those obtained in other studies, where one (27), or two radiologists (6, 15, 33) participated.
We conclude that chest HRCT seems a valuable tool in evaluating small airway dimensions in patients with asthma and COPD. The results of our study suggest that in asthma the diminished area of the airway lumen and airway wall thickening are reflected by an increase in the indices of air trapping. In COPD patients, reduction in the airway lumen area and airway wall thickening may result in higher airway resistance and responsiveness, and it is likely that airway wall thickening is related to the degree of exposure to tobacco smoke. The lack of correlations between spirometric parameters and airway dimensions assessed in HRCT indicates that the airway wall thickness and lumen diameter are not the only factors which influence airflow limitation during forced expiration.
Conflict of interests: None declared.
- Gorska K, Krenke R, Korczynski P, Kosciuch J, Domagala-Kulawik J, Chazan R. Eosinophilic airway inflammation in chronic obstructive pulmonary disease and asthma. J Physiol Pharmacol 2008; 59(Suppl 6): 261-270.
- Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008; 59(Suppl 6): 19-34.
- Gorska K, Krenke R, Domagala-Kulawik J, et al. Comparison of cellular and biochemical markers of airway inflammation in patients with mild-to-moderate asthma and chronic obstructive pulmonary disease: an induced sputum and bronchoalveolar lavage fluid study. J Physiol Pharmacol 2008; 59(Suppl 6): 271-283.
- Kosciuch J, Przybylowski T, Gorska K, et al. Relationship between airway basement membrane thickness and lung function tests in patients with astma. Pneumonol Alergol Pol 2009; 77: 256-263.
- Domagala-Kulawik J, Maskey-Warzechowska M, Hermanowicz-Salamon J, Chazan R. Expression of macrophage surface markers in induced sputum of patients with chronic obstructive pulmonary disease. J Physiol Pharmacol 2006; 57(Suppl 4): 75-84.
- Awadh N, Muller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax 1998; 53: 248-253.
- Harmanci E, Kebapci M, Metintas M, Ozkan R. High-resolution computed tomography findings are correlated with disease severity in asthma. Respiration 2002; 69: 420-426.
- Laurent F, Latrabe V, Raherison C, Marthan R, Tunon-de Lara J. Functional significance of air trapping detected in moderate asthma. Eur Radiol 2000; 10: 1404-1410.
- Kauczor HU, Hast J, Heussel P. Focal air-trapping at expiratory high-resolution CT: comparision with pulmonary function tests. Eur Radiol 2000; 10: 1539-1546.
- Brajer B, Batura-Gabryel H, Nowicka A, Kuznar-Kaminska B, Szczepanik A. Concentration of matrix metalloproteinase-9 in serum of patients with chronic obstructive pulmonary disease and a degree of airway obstruction and disease progression. J Physiol Pharmacol 2008; 59(Suppl 6): 145-152.
- Mroz RM, Holownia A, Chyczewska E, et al. Cytoplasm-nuclear trafficking of CREB and CREB phosphorylation at Ser133 during therapy of chronic obstructive pulmonary disease. J Physiol Pharmacol 2007; 58(Suppl 5): 437-444.
- Mroz RM, Noparlik J, Chyczewska E, Braszko JJ, Holownia A. Molecular basis of chronic inflammation in lung diseases: new therapeutic approach. J Physiol Pharmacol 2007; 58(Suppl 5): 453-460.
- Mroz RM, Holownia A, Chyczewska E, Braszko JJ. Chronic obstructive pulmonary disease: an update on nuclear signaling related to inflammation and anti-inflammatory treatment. J Physiol Pharmacol 2008; 59(Suppl 6): 35-42.
- Capraz F, Kunter E, Cermik H, Ilvan A, Pocan S. The effect of inhaled budesonide and formoterol on bronchial remodeling and HRCT features in young asthmatics. Lung 2007; 185: 89-96.
- Kasahara K, Shiba K, Okazawa T, Okuda K, Adachi M. Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax 2002; 57: 242-246.
- Saglani S, Papaioannou G, Khoo L, et al. Can HRCT be used as a marker of airway remodeling in children with difficult asthma? Respir Res 2006; 7: 46-51.
- GINA Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention. NHLBI/WHO Workshop Report. National Heart, Lung and Blood Institute, National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Washington, 2006.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructve Pulmonary Disease. NHLBI/WHO Workshop Report. Bethesda, National Heart, Lung and Blood Institute, April 2001. 2003 update. GOLD website www.goldcopd.com
- Standarized lung function testing. Official statement of the European Respiratory Society. Eur Respir J 1993; 16(Suppl): 1-100.
- Crapo RO, Casaburi R., Coates AL, et al. Guidelines for methacholine and exercise challenge testing -1999. Am J Respir Crit Care Med 2000; 161: 309-329.
- King GG, Muller NL, Pare PD. Evaluation of airways in obstructive pulmonary disease using high-resolution computed tomography. Am J Respir Crit Care Med 1999; 159: 992-1004.
- Okazawa M, Muller N, McNamara AE, Child S, Verburgt L, Pare PD. Human airway narrowing measured using high resolution computed tomography. Am J Respir Crit Care Med 1996; 154: 1557-1562.
- Beigelman-Aubry C, Capderou A, Grenier PA, et al. Mild intermittent asthma: CT assessment of bronchial cross-sectional area and lung attenuation at controlled lung volume. Radiology 2002; 223: 181-187.
- Seneterre E, Paganin F, Bruel JM, Michel FB, Bousquet J. Measurement of the internal size of bronchi using high resolution computed tomography (HRCT). Eur Respir J 1994; 7: 596-600.
- Paganin F, Vignola AM, Seneterre E, Bruel JM, Chanez P, Bousquet J. Heterogeneity of airways obstruction in asthmatic patients using high resolution computed tomography. Chest 1995; 105(Suppl): 145s-146s.
- Little SA, Sproule MW, Cowan MD, et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax 2002; 57: 247-253.
- Niimi A, Matsumoto H, Amitani R. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med 2000; 162: 1518-1523.
- Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J 2003; 22: 965-971.
- Ketai L, Coutsias C, Williamson S, Coutsias V. Thin-section CT evidence of bronchial thickening in children with stable asthma: bronchoconstriction or airway remodeling? Acad Radiol 2001; 8: 257-264.
- Dunnill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax 1969; 24: 176-179.
- Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airways dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990; 142: 563-570.
- Daveci F, Murat A, Turgut T, Altuntas E, Hamdi Muz M. Airway wall thickness in patients with COPD and healthy current smokers and healthy non-smokers: assessment with high resolution computed tomographic scanning. Respiration 2004; 71: 602-610.
- Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162: 1102-1108.
- Milanese M, Crimi E, Scordamaglia A. On the functional consequences of bronchial membrane thickening. J Appl Physiol 2001; 91: 1035-1040.
- Tanaka H, Yamada G, Saikai T, et al. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am J Respir Crit Care Med 2003; 168: 1495-1499.
- Matsumoto H, Niimi A, Tabuena RP, et al. Airway wall thickening in patients with cough variant asthma and nonasthmatic chronic cough. Chest 2007; 131: 1042-1049.
- Ostrowski S, Barud W. Factors influencing lung function: are the predicted values for spirometry reliable enough? J Physiol Pharmacol 2006; 57(Suppl 4): 263-271.
- Ziora D, Madaj A, Wieckowka E, Ziora K, Kozielski K. Correlation of spirometric parameters taken at a single examination with the quality of life in children with stable asthma. J Physiol Pharmacol 2007; 58(Suppl 5): 801-809.
- Nosal S, Durdik P, Sutovska M, et al. Changes of airway obstruction parameters in healthy children caused by mother’s smoking during pregnancy. J Physiol Pharmacol 2008; 59(Suppl 6): 523-529.
- Boulet L, Belanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med 1995; 152: 865-871.
- Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med 2003; 168: 983-988.
- Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005; 171: 142-146.
- Tiddens HA, Pare PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med 1995; 152: 260-266.