Parasympathetic nerves
The parasympathetic nerve fibers innervating the pancreas originate mainly from the dorsal vagal nucleus and partly from the nucleus ambigous of the brain stem (1). Most of these fibers run within the posterior vagal truncus (2) near the small curvature of the stomach and the pylorus to the pancreas (3). Some of the fibers run intramurally along the pylorus and the proximal part of the duodenum before joining the pancreas (4). The majority of the vagal preganglionic fibers terminate on the pancreatic ganglia, performing interneuronal synapses.
Sympathetic nerves
The pancreas receives its postganglionic sympathetic innervation mainly from neurons with their cell bodies in the celiac and superior mesenteric ganglia. These fibers are distributed to nerve cell bodies in the pancreatic ganglia and to ducts, blood vessels, and islets. Some sympathetic fibers run within the pancreatic parenchyma, directly terminating on acinar cells. The sympathetic innervation of the exocrine pancreas, however, is modest as compared with the islets and the pancreatic blood vessels (5).
Comparable to the enteric nevous system, an intrapancreatic nervous system exists that enables a degree of independence of the pancreas when cut off from the CNS and the gut. The morphological equivalent is an intrapancreatic ganglionated plexus with terminal axons supplying both the endocrine and exocrine portions of the organ (6, 7). The pancreatic ganglia are the nervous integration centers of the pancreatic exocrine (and endocrine) secretion. They receive input from vagal preganglionic, sympathetic postganglionic, sensory and enteric fibers. Postganglionic nerve fibers surround almost every acinus, forming a periacinar plexus containing cholinergic, noradrenergic, petidergic, and nitrergic fibers which in majority terminate at the acinar cells (6—8). Only a subset of pancreatic neurons appears to project to the islets (7).
There is strong support for the hypothesis that intrapancreatic postganglionic neurons regulate both enzyme and bicarbonate secretion. These neurons are.activated by central input on the pancreatic ganglia during the cephalic phase and by vagovagal reflexes initiated by gastric and intestinal phase stimulation. Acetylcholine released by these neurons acts directly on muscarinic receptors on acinar cells (and presumably duct cells) to elicit secretion (5).
Role of the vagus nerves
Electrical stimulation of the vagal trunks in animals (9, 10) and efferent vagal activation induced by insulin hypoglycemia in humans (11) or by administration of the glucose antagonist 2-deoxyglucose in dogs (12) have been shown to cause strong stimulation of pancreatic enzyme output. In addition, the pancreatic enzyme response to intestinal perfusion with amino acids, peptides and fatty acids, and to a meal is strongly inhibited by atropine (13—17) and vagotomy (11, 14, 17, 18).
Experimental evidence for an enteropancreatic reflex has come from a study on the latency of pancreatic enzyme response, i. e., the time required to measure a significant increase in pancreatic enzyme output after a rapid intraduodenal bolus application of tryptophan or sodium oleate compared with intraportal injection of CCK (14). In this study performed in dogs, even the shortest possible circulation time for a maximal dose of CCK (>30 s) was significantly longer than the observed latency of amylase response to the intestinal nutrients (<20 s). Furthermore, truncal vagotomy and atropine both increased the latency to the intestinal stimulants 10-fold but hat no effect on the latency to intraportal CCK. Thus it was concluded that, at least in dogs, a cholinergic, vagovagal enteropancreatic reflex mediates the early pancreatic enzyme response to intestinal stimulants. Studies on the latency of the pancreatic fluid secretory response suggest that the early pancreatic fluid responses to intestinal tryptophan or sodium oleate are also mediated, at least in part, via a vagovagal, cholinergic enteropancreatic reflex (19).
Follow-up studies performing stepwise extrinsic denervation of the pancreas ruled out possible splanchnic pathways of enteropancreatic reflexes (17). Celiac and superior mesenteric ganglionectomy did not alter the pancreatic protein reponse to intestinal tryptophan. Atropine significantly reduced the pancreatic protein response to low loads of tryptophan before but not after truncal vagotomy. These data suggest that, in dogs, the pancreatic protein response to intraduodenal tryptophan is, at least in part, mediated by long, cholinergic enteropancreatic reflexes with both afferent and efferent limb carried by the vagus nerves (17). Histochemical examinations demonstrated that in the rat there are enteric neurons that project to the pancreatic ganglia, thus providing anatomical evidence for these enteropancreatic reflexes (20, 21).
Studies on the effect of truncal vagotomy or atropine on the pancreatic secretory response to different loads of intestinal nutrients (15, 17) have shown.that vagal cholinergic mechanisms are the major mediators of the pancreatic secretory response to low loads of intestinal stimulants, whereas hormones mediate the secretory response particularly to high loads of intestinal stimuli (18, 22).
Role of M1 receptors
Since at least three pharmacologically distinct subtypes (M1, M2, M3) of the muscarinic receptor do exist, the question emerged, which subtype is responsible for the vagal cholinergic control of pancreatic exocrine secretion. Based upon studies in which the M3 receptors were pharmacologically blocked, the M3 receptor was considered as the typical muscarinic receptor of the acinar cell (23—26). In-vivo studies in humans and dogs, however, revealed a physiological role of M1 receptors in the control of pancreatic exocrine secretion (27—29). A broad series of studies comparing the effects of atropine and of the M1 receptor antagonist telenzepine on human pancreatic secretion led to the conclusion, that the non-selective inhibition by atropine even is predominantly achieved via M1 receptors (29). More recently, analysis of the rat acinar cell muscarinic receptor by polymerase chain reaction revealed expression of both M1 and M3 subtypes (30). In addition, pharmacological blockade of M1 receptors caused a significantly greater inhibition of amylase secretion in isolated pancratic acinar cells than blockade of the M3 receptors (30).
Since at least three pharmacologically distinct subtypes (M1, M2, M3) of the muscarinic receptor do exist, the question emerged, which subtype is responsible for the vagal cholinergic control of pancreatic exocrine secretion. Based upon studies in which the M3 receptors were pharmacologically blocked, the M3 receptor was considered as the typical muscarinic receptor of the acinar cell (23—26). In-vivo studies in humans and dogs, however, revealed a physiological role of M1 receptors in the control of pancreatic exocrine secretion (27—29). A broad series of studies comparing the effects of atropine and of the M1 receptor antagonist telenzepine on human pancreatic secretion led to the conclusion, that the non-selective inhibition by atropine even is predominantly achieved via M1 receptors (29). More recently, analysis of the rat acinar cell muscarinic receptor by polymerase chain reaction revealed expression of both M1 and M3 subtypes (30). In addition, pharmacological blockade of M1 receptors caused a significantly greater inhibition of amylase secretion in isolated pancratic acinar cells than blockade of the M3 receptors (30).
M1 receptors and pancreatic bicarbonate secretion
The stimulation of pancreatic bicarbonate secretion by intraduodenal amino acids such as tryptophan has been well established (13, 15, 35). However, tryptophan does not increase plasma secretin concentrations over basal levels (35), indicating the existence of other pathways that stimulate pancreatic bicarbonate secretion. Telenzepine was found to significantly decrease the pancreatic bicarbonate response to intraduodenal tryptophan in conscious dogs up to total abolition (18, 22, 28) (Fig. 1). Thus, intraduodenal amino acids stimulate bicarbonate secretion in part via M1 receptors. This mechanism
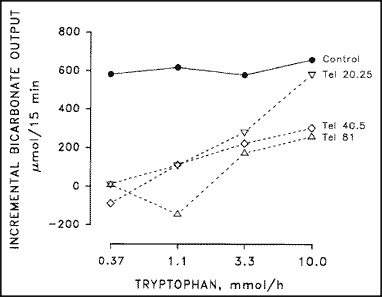 |
Fig. 1. Effect of different doses of telenzepine
(Tel; nmol/kg/h iv.) on the incremental pancreatic bicarbonate response
to graded loads of intraduodenal tryptophan. Results are means of 6
dogs. Data are adapted from Niebergall-- Roth et al. (22).
|
seems to play a major role in the mediation of the bicarbonate response to low loads of intraduodenal amino acids, since the inhibitory effect of telenzepine was greater when low loads of tryptophan were given (Fig. 1).
M1 receptors and pancreatic enzyme secretion
During secretin infusion (in order to stimulate pancreatic fluid secretion), telenzepine significantly decreased canine pancreatic enzyme output by up to 85% (18, 36) (Fig. 2). Since secretin does not stimulate pancreatic enzyme secretion in dogs (18, 28, 36), this finding suggests that M1 receptors are important mediators of basal pancreatic enzyme secretion. The observation that telenzepine still depresses basal enzyme output after truncal vagotomy (18) (Fig. 2) suggests that the basal cholinergic tone of the pancreas does not significantly depend on the integrity of the vagus nerves but can be mainly ascribed to the intrinsic cholinergic nerves.
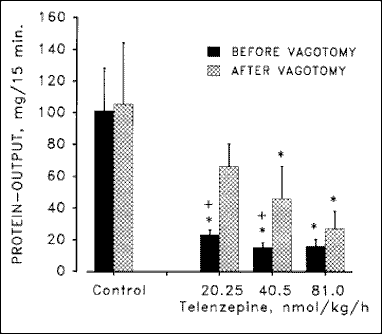 |
Fig. 2. Effect of different doses of telenzepine
on pancreatic protein output during intravenous infusion of secretin
(20.5 pmol/kg/h) before and after truncal vagotomy. Results are means
± SEM of 6 dogs. *p <0.05 vs. control. +p
< 0.05 before vs. after vagotomy. Data are adapted from Niebergall-Roth
et al. (18).
|
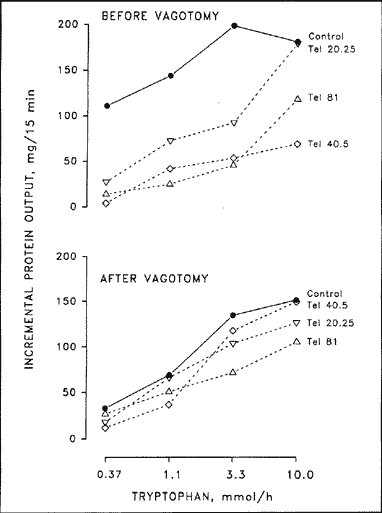 |
Fig. 3. Effect of different doses of telenzepine
(Tel; nmol/kg/h iv.) on the incremental pancreatic protein response
to graded loads of intraduodenal tryptophan before and after truncal
vagotomy. Results are means of six dogs. Data are adapted from Niebergall-
Roth et al. (18).
|
The involvement of M1 receptors was confirmed by studying the effect of telenzepine on the latency of pancreatic amylase secretion after a rapid intraduodenal bolus application of tryptophan or sodium oleate (37). In this study, telenzepine increased the latency of amylase response to tryptophan and oleate by more than 10-fold (Table 1), indicating that enteropancreatic reflexes stimulate pancreatic enzyme secretion via activation of M1 receptors.
| Table 1. Effect of telenzepine and L-364,718 on latency (in sec., unless otherwise stated) of pancreatic amylase response to intravenous (iv.) and intraduodenal (id.) stimulants. | ||||||||||||||||
|
||||||||||||||||
| Data are means ± SEM of 6 dogs. *p < 0.05 vs. control; #p < 0.05 vs. caerulein. Data are adapted from Niebergall-Roth et al. (37). |
Besides cholinergic nerves, the hormone CCK is considered as the most important mediator of postprandial pancreatic exocrine secretion, particularly pancreatic enzyme output. To investigate possible interactions between cholinergic M1 receptors and CCK, we have studied the effects of telenzepine in dogs during blockade of CCK-A-receptors by L-364,718 (MK-329, devazepide). L-364,718 is an extremely potent competitive CCK antagonist with no intrinsic activity and a high specifity for peripheral CCK-A-receptors (38).
Exogenous stimulation of the pancreatic protein output with the CCK analogue caerulein is not affected by telenzepine (18, 36) (Fig. 4). This is in accordance with earlier findings that in dogs neither atropine nor vagotomy had any significant effect on pancretic secretory response to exogenous CCK or caerulein (15, 39, 40). In addition, L-364,718 depressed the canine pancreatic protein response to tryptophan irrespective of the integrity of the vagus nerves (18) (Fig. 5). Thus, endogenously released CCK controls canine pancreatic enzyme output at least in part by a pathway that does not involve M1 receptors, probably as a classical humoral factor acting at acinar CCK receptors.
 |
Fig. 4. Effect of different doses of telenzepine
(Tel; nmol/kg/h iv.) on the incremental pancreatic protein response
to graded doses of intravenous caerulein. Results are means of 6 dogs.
Data are adapted from Niebergall-Roth et al. (36).
|
Obviously, this mechanism seems to represent a species particularity. In humans, the stimulatory effect of physiological doses of exogenous CCK or caerulein was almost completely inhibited by atropine (41—43), suggesting that exogenous CCK at physiologic concentrations stimulates pancreatic secretion by interaction with the cholinergic system. In rats, truncal vagotomy, atropine, hexamethonium and the sensory neurotoxin capsaicin completely abolished the pancreatic protein response to exogenous CCK given in a dose that produced postprandial plasma concentrations (44). Thus, in rats the stimulatory action of exogenous CCK in a physiologic dose on pancreatic secretion is mainly mediated via capsaicin-sensitive vagal sensory afferents originating from the gastroduodenal mucosa (44).
Besides the action of CCK as a classical humoral factor, in dogs also experimental evidence exists for an interaction between the cholinergic system and the hormone CCK. The inhibition of the pancreatic protein response to intraduodenal tryptophan caused by combination of telenzepine and L-364,718 was significantly greater than the sum of the inhibitory effects of each antagonist when given alone. In particular, the combination of the two antagonists in very low doses that had no significant effects on protein output when given separately, totally abolished the protein response when given together (18) (Fig. 6). This finding indicates that in dogs — in addition to directs effects of CCK on the pancreatic acinar cell — a mechanism of potentiative interaction exists between cholinergic nerves ending on M1 receptors and CCK. In addition, L-364,718 was shown to interrupt the enteropancreatic reflex mediating the early amylase response to intraduodenal bolus application of tryptophan and oleate (37), indicating that the activation of (possibly vagal) CCK receptors is essential to run this reflex.
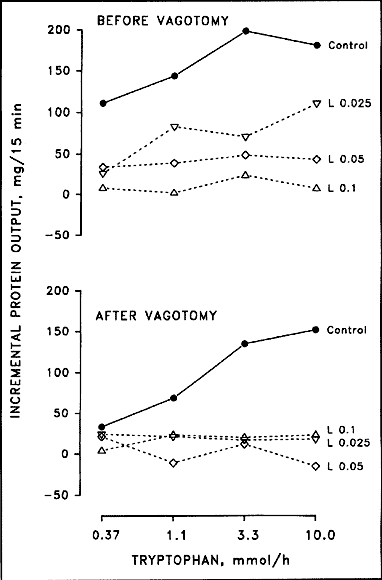 |
Fig. 5. Effect of different doses of L-364,718
(L; mg/kg/h iv.) on the incremental pancreatic protein response to graded
loads of intraduodenal trypotphan before and after truncal vagotomy.
Results are means of 6 dogs. Data are adapted from Niebergall- Roth
et al. (18).
|
The question, where the interaction between the cholinergic system and CCK in the dog occurs, needs still to be answered. Firstly, CCK and cholinergic nerves may interact at the level of the acinar cell in as much a threshold of cholinergic input conditions the pancreatic secretory response of the acinar cell to hormonal acting CCK. However, this hypothesis is incompatible with the observation, that in dogs neither vagotomy, nor atropine, nor telenzepine alters the pancreatic protein response to exogenous CCK or caerulein in dogs (13, 18, 45). Secondly, CCK may activate cholinergic vagal neurons and thus modulate the intrapancreatic neurons. An anatomical basis is provided by the discovery of type A receptors for CCK on afferent vagal fibers (46).
An intriguing hypothesis that integrates both — humoral and vagally mediated — pathways of CCK in the dog was provided by Grossman (cited in
 Fig. 6. Effect of different doses of telenzepine (Tel; nmol/kg/h iv.), L-364,718 (L; mg/kg/h iv.), or both on the incremental pancreatic protein response to graded loads of intraduodenal tryptophan. Results are means of 6 dogs. Data are adapted from Niebergall-Roth et al. (18). |
(47)). Intraduodenal infusion of nutrients would release CCK from endocrine cells of the duodenum. When low loads of nutrients were infused, only small amounts of CCK would be released, that would not be discharged into the circulating blood but would diffuse across the intracellular space to the nervous CCK receptor and initiate a vago-vagal reflex. When high doses of nutrients were given intraduodenally, greater amounts of CCK would be released from the intestinal endocrine cells and discharged into the bloodstream and could thus stimulate pancreatic protein output by acting at the acinar CCK receptor. The higher treshold for hormonal than for nervous stimulation by CCK would explain the observation that in dogs L-364,718 inhibited the protein reponse to low and high amounts of intraduodenal nutrients, whereas telenzenzepine was mainly effective for inhibiting the response to low amounts of nutrients (18, 28, 36) (Figs. 3 & 5). Furthermore, this mechanism would account for the finding that L-364,718 inhibits the pancreatic protein response even to low loads of intestinal amiono acids, that do not cause measurable elevation of plasma CCK (18).
Although several neurotransmitters found in pancreatic nerve cell bodies or fibers can stimulate or inhibit pancreatic exocrine secretion under distinct experimental conditions (Table 2), the physiological significance of most of them in the neural control of the exocrine pancreas needs to be further evaluated. The most likely candidates are nitric oxide (NO), serotonin (5-HT) and the neuropeptides vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase activating polypeptide (PACAP) and gastrin releasing polypeptide (GRP) (48)..Experimental evidence exists that nitrergic, serotonergic and GRP-containing nerves modulate the cholinergic control of pancreatic exocrine secretion.
| Table 2. Non-adrenergic non-cholinerigig neurotransmitters present in the pancreas and their effect on pancreatic exocrine secretion. | ||||||||||||||
|
Nitric oxide (NO)
The gas NO fulfills several characteristics of a neurotransmitter in that it is released from nerves and affects the function of effector cells. However it is not preformed or stored in presynaptic cells like other transmitters. The significance of NO for the regulation of pancreatic exocrine secretion has been established by several studies in humans and animals (for review see (49)). In conscious dogs, the inhibitory effect of the NO synthase inhibitor NG-nitro-L-arginine (L-NNA) was most pronounced after vagal stimulation of the pancreas by sham feeding (50) or 2-deoxy-D-glucose induced neuroglycopenia (49, 51). These findings suggest that the regulatory effect of NO on pancreatic secretion is related to a vagal pathway.
Serotonin (5-HT)
The serotonergic nerve fibers found in the pancreas are probably of enteric origin (20). The axons of the serotonergic enteropancreatic neurons appear to form inhibitory axo-axonic synapses in the pancreas (52). 5-HT inhibits veratridine-mediated amylase secretion; thus the targets of the serotonergic enteropancreatic innervation are likely to include the cholinergic axons that innervate acinar cells. This effect of 5-HT was blocked by the 5-HT1P receptor antagonist, N-acetyl-5-hydroxytryptophyl-5-hydroxytryptophanamide (5-HTP-DP). This observation suggest that serotonergic nerves inhibit pancreatic enzyme secretion via presynaptic 5-HT1P receptors on cholinergic neurons (52).
Gastrin releasing polypeptide (GRP)
The amphibian peptide bombesin, its mammalian structural counterpart GRP, and its C-terminal decapeptide neuromedin C, respectively, strongly stimulate.pancreatic fluid, bicarbonate and enzyme secretion in humans, pigs, dogs, and rats (53—57). Electrical field stimulation evoked release of GRP in isolated perfused pig and rat pancreas (54, 58). In the isolated perfused porcine pancreas, atropine inhibited the enzyme response to exogenous GRP, suggesting that acetylcholine somehow sensitizes the acinar cell to the effects of GRP. Another possiblity is that, in addition to acting directly at the acinar bombesin/GRP receptor, GRP-containing nerves modulate pancreatic exocrine secretion through stimulation of acetycholine release from postganglionic cholinergic fibers. This hypothesis is supported by the findings that tetrodotoxin inhibited GRP stimulated amylase release in rat pancreatic lobules (57) and that bombesin had no effect on enzyme output from dog pancreatic acini (59).
Despite an increasing knowledge about the function of pancreatic innervation several unanswered questions still remain.
Regarding the cholinergic component in the control of pancreatic exocrine secretion, the study of the effects of M3 receptor antagonists in vivo could clarify the physiological role of acinar M3 receptors. The relative contribution of M1 and M3 receptors to the cholinergic control of pancreatic exocrine secretion is another intriguing question and could be answered by the comparison of the effects of M1 and M3 receptor antagonists under distinct experimental conditions.
The function of the enteropancreatic innervation still remains hypothetical. Further studies revealing the nature of the enteric stimuli that selectively activate either the excitatory or the inhibitory components of the enteropancreatic innervation will provide the needed insight into the function of this system.
Now that neurochemical investigations have revealed a number of peptides and other neuroactive substances in the nerve cell populations of the pancreas, subsequent studies should investigate the physiological significance of these substances as neurotransmitters of these cells. In addition, further investigation of interaction pathways between these neurotransmitters could contribute to the understanding of the integration and/or summation of vagal, sympathetic, enteric and intrinsic pancreatic signals.
Finally, attention should be paid to the role of the pancreatic innervation in pathophysiological processes. Future studies are necessary to establish whether and how pancreatic neurotransmission is altered or disrupted unter pathophysiological conditions. A more comprehensive perspective of the neural mechanisms associated with such processes will lead to a better understanding of pancreatic disease.