The purpose of the present study was to elucidate the effects of alloxan on the activity of pancreatic lysosomal enzymes in the course of alloxan induced diabetes in rabbits.
It was also important to establish if changes of activity of these enzymes may be a factor leading to diabetic enteropathy.
89 male rabbits, New Zealand breed obtained from Experimental Animals Laboratory, Chorzelow n/Warsaw, Poland, weighing 2.750—3.300 kg were.used in the experiment. The study was fully approved by the University Ethics Commission of the Medical University of Lublin.
Animals were housed one per cage under a 12h/12h light/dark cycle at 21 ± 2°C and 50% relative humidity with standard granulated food (Motycz, Poland) and water available ad libitum. Diabetes mellitus was induced by a single injection of alloxan (Sigma Chemical Company, St. Louis, MO, USA) at a dose of 10 mg/kg into the auricular vein (15).
On day 7 the blood was taken from the marginal auricular vain animals with an empty stomach, and glucose level was measured by a glucometer (Boehringer, Germany) to confirm hyperglycaemia, which is one of the most important signs of diabetes. The onset of diabetes was counted from this point in time. There was one control group (Group 1, n = 18) which did not receive alloxan.
The rabbits with confirmed hyperglycaemia were divided into the following groups: Group 2—21 days hyperglycaemia (n = 18), Group 3—42 days hyperglycaemia (n = 17), group 4—90 days hyperglycaemia (n = 19), group 5—180 days hyperglycaemia (n = 17). After the above-mentioned periods blood samples were taken from the same place and the rabbits were sacrificed by decapitation. The final level of glucose (mmol/l) in the total blood was determined spectrophotometrically by an enzymatic method as described elsewhere (16).
The pancreas was removed and stored at -20°C. The samples were defrosted in 0.9% solution of NaCl at 4°C. 1 g of the tissue of each sample was taken for biochemical investigation. They were placed into a 0.3 M sucrose solution at 4°C in proportions of 1 g of tissue to 5.0 ml of sucrose and homogenised. The obtained homogenate was centrifuged for 10 minutes at 2.200 g at 4°C. The supernatant was decanted and centrifuged for 20 minutes at 35.000 g. The obtained sample containing the free fraction of enzyme was assigned as supernatant 1 (12, 13). The precipitate was placed into 5.0 ml of 0.3 M sucrose containing 0.1% Triton X-100 and stored for 24 hours at 4°C. Triton was used to rupture the lysosomal membrane. Then the precipitate was centrifuged for 20 minutes at 35.000 g. The supernatant, containing the fraction of bound enzyme, was decanted and assigned as supernatant 2.
Total enzyme activity assays were based on the fact that examined enzymes decompose specific substrate releasing free 4-metyloumbeliferol (13). Spectrophotometric evaluation was performed using substrates (Sigma) which form coloured complexes reacting with the proteases (18).
Galactoso-6-sulphatase [EC 2.5.1.5] activity was measured in 44.1 mg of potasium salt of 4-metyloumbeliferol sulphate dissolved in 100 ml of 0.1 M citrate buffer pH 5.0. The amount of 52.7 mg of 4-methyloumberlipherol laurate dissolved in 10 ml of acetone and diluted ten times in 0.1 M acetate buffer pH 5.0 with addition of 0.1% Triton X-100 were used for lipase [EC.3.1.1.3] activity evaluation. Beta-D-galactosidase [EC 3.2.1.23] activity assay was performed in 51 mg of 4-metyloumbeliferol-ß-D-galactopyranoside dissolved in 100 ml 0.1 M citrate buffer pH 5.0. N-acetyl-ß-D-glucosaminidase [EC 3.2.1.30] activity was measured in 57.2 mg of 4-metyloumbeliferol-N-- acetyl-ß-D-glucosaminidine dissolved in 100 ml 0.1 M citrate buffer pH 4.3 with addition of 0.3 M NaCl. Samples of 100 µl of 1st and 2nd supernatant were incubated with 500 µl of each above mentioned substratum for 18 hours at 37°C. The reaction was inhibited by addition of 600 µl alkaline buffer, and after 5 min. extinction was read at the length of wave 360 nm on the spectrophotometer.
The level of protein was determined by the method of Lowry et al (18). Enzyme activities are expressed in nmol/1mg of protein/1 hour of incubation. The total activity was counted as the sum of free and bound fractions.
The statistical analysis was done using the SAS system v. 6.11 (SAS Institute Inc., SAS Campus Drive, Carry, NC 27513, USA). Results are expressed as means ± SD. Differences between groups were analysed by ANOVA. The correlation coefficients (r) between analysed characters (X, Y) were counted. If P < 0.05, differences between the mean values were considered statistically significant.
The initial serum glucose concentration had an average value of 6.36 ± 2.03 mmol/l in the serum. Twenty one days after injection of alloxan, the concentration had increased to 21.79 ± 9.49 mmol/l. It reached its peak level of 32.02 ± 19.11 mmol/l on the 42nd day. The level decreased, falling in all groups to 23.15 ± 10.83 mmol/l on day 180 (Group 5). Serum glucose concentrations are presented in Table 1. The differences between the control group and hyperglycaemic groups were statistically highly significant (p < 0.01).
Activities of lipase, galactoso-6-sulphatase, ß-D-galactosidase, N-acetyl-ß-D-- gluco-saminidase are presented in Table 1.
The mean total activity of lipase (Fig. 1) was 71.48 nmol/mg/h in the control group. It was about 45.3% higher in Group 2, and about 214.7% higher in Group 3. Afterwards it slightly decreased in Group 4, reaching the level of 124.1 nmol/mg/h (about 73.6% higher than in control one). In Group 5, it was 90.31 nmol/mg/h, which means it was the same level as that in the control ones. The activity of the bound fraction was higher than free fraction until day 42. On day 90 free fraction activity exceeded the bound one, and they settled on approximately the same level on day 180.
| Table 1. Activities of lipase, galactoso-6-sulphatase (sulphatase), ß-D-galactosidase (lactase) and N-acetyl-ß-D-glucosaminidase (NAGL) in rabbit pancreas in the course of experimental diabetes (nmol/mg of protein/1 h of incubation, means ± SD) and glucose concentration in blood serum (mmol/l, means ± SD). *As compared with control group, P < 0.05 (ANOVA). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
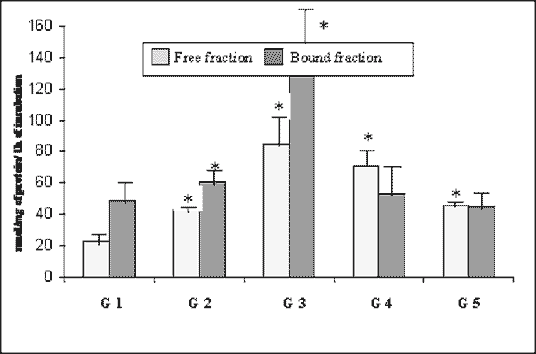 |
| Fig. 1. Activity of lipase in the rabbit pancreas in the course of experimental diabetes (nmol/mg of protein/1 h of incubation, means ± SD). * As compared with control, p < 0.05 (ANOVA). |
The mean total activity of galactoso-6-sulphatase (Fig. 2) in the pancreas was 6.13 nmol/mg/h of that in the control group. By day 21, this value had increased by about 40%. The activity decreased, and then increased to a value
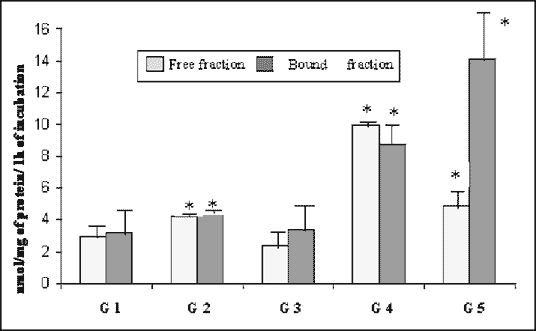 |
| Fig. 2. Activity of galactoso-6-sulphatase (sulphatase) in the rabbit pancreas in the course of experimental diabetes (nmol/mg of protein/1 h of incubation, means ± SD). *As compared with control, p < 0.05 (ANOVA) |
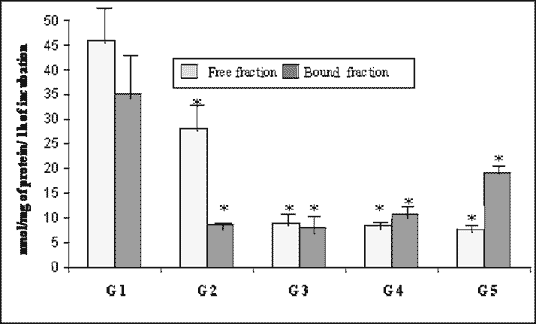 |
| Fig. 3. Activity of ß-D-galactosidase (lactase) in the rabbit pancreas in the course of experimental diabetes (nmol/mg of protein/1 h of incubation, means ± SD). *As compared with control, p < 0.05 (ANOVA). |
of 18.73 nmol/mg/h on day 90 and remained so until day 180. It was 205% higher than in the control. Mean bound fraction activities were slightly higher than free fraction until day 90, when free fraction was more active. On day 180 bound fraction activity was 209% higher than that of the free one
Consequently, during the course of experiment, the mean total activity of ß-galactosidase was decreasing from the peak value 80.98 nmol/mg/h in the control group (Fig. 3), reaching its bottom 16.87 nmol/mg/h (20% of control) on day 42. After this interval it slightly began to recover to the value of 26.9 nmol/mg/h, which was about 30% of initial activity on day 180. Free fraction was more active than the bound one until day 90, when the latter revealed higher activity that remained until day 180.
The mean total activity of N-acetyl-ß-D-glucosaminidase was at its highest level of 77.21 nmol/mg/h in the control group, significantly dropping by 71% to the level of 22.56 nmol/mg/h on day 42. Despite the slight increase to 30.13 nmol/mg/h on day 90, it tended to decline reaching the bottom level of 14.09 nmol/mg/h (18.2% of control) on day 180. The activity of free fraction was higher than the bound one throughout the experimental period, except day 90 when the bound enzymes were more active than the free ones (Fig. 4).
The mean level of glucose in serum, and the mean total activities of lipase, galactoso-6-sulphatase, ß-D-galactosidase and N-acetyl-ß-D-glucosaminidase in pancreatic gland are presented in Figure 5.
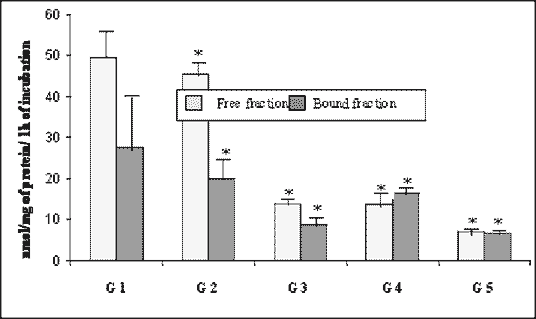 |
| Fig. 4. Activity of N-acetyl-ß-D-glucosaminidase (NAGL) in the rabbit pancreas in the course of experimental diabetes (nmol/mg of protein/1 h of incubation, means ± SD). * As compared with control, p < 0.05 (ANOVA) |
| Fig. 5. Changes of glucose concentration in serum (mmol/l) and total activity of lipase, galactoso-6-sulphatase (sulphatase), ß-D-galactosidase (lactase) and N-acetyl-ß-D-glucosaminidase (NAGL) (nmol/mg of protein/1 h of incubation, means) in rabbit pancreas in the course of experimental diabetes. |
Statistical analysis of correlation coefficients between analysed characters showed a negative correlation between glucose concentration and mean total activ ity of ß-D-galactosidase (r = -0.978, p < 0.01), and N-acetyl-ß-D- -glucosaminidase (r = -0.877, p = 0.05). There were no correlations between glucose concentration and mean total activity of galactoso-6-sulphatase (r = 0.378) and lipase (r = 0.723).
Alloxan induced diabetes was characterised by the state of hypoinsulinemia and hyperglycaemia. In diabetic animals increased levels of serum triglycerides and cholesterol was observed (19). Due to defective beta-cell production of insulin, blood levels of insulin did not increase in response to elevated blood glucose levels. Hyperglycaemia in diabetes is caused by increased hepatic production of glucose combined with diminished peripheral utilisation. In the cells, where glucose entry is not insulin-dependant, on elevated blood glucose level results in increased intracellular glucose and its metabolites concentration..Elevated intracellular glucose concentrations and adequate supply of NADPH cause a significant increase of sorbitol, which accumulates in these cells. Diabetes mellitus leads to a broad spectrum of symptoms and manifestations in the field of gastroenterology. Motility disorders, infectious complications, secondary disease of the digestive tract are considered and discussed (20). As a result of impaired exocrine pancreatic function, individuals with diabetes may be particularly susceptible to digestion and motility disorders related symptoms such as abdominal pain or discomfort, bloating, early satiety, nausea and vomitting (21).
Heartburn, constipation and nocturne urge to defecate were significantly more frequent in these patients. Furthermore, a feeling of incomplete defecation, a need to strain at defecation, and urgency were more common (22). Enck and co-workers (23) observed increased prevalance of diarrhoea and postprandial fullness in their diabetic patients. In other study, Hess et al. (24) revealed that presence of diabetes mellitus increased the risk of developing fatal acute pancreatitis in dogs. All the above reports suggest a strong correlation between both the endocrine and exocrine part of pancreas in the course of diabetic patients.
Further hyperglycaemia may promote the condensation of glucose or its metabolites with cellular proteins. These glycated proteins may mediate some of the early microvascular changes in diabetes. The long lasting elevation of blood glucose level is widely believed to cause the chronic complications of diabetes-premature atherosclerosis, retinopathy, nephropathy and neuropathy. Burlin et all (11) suggest a direct connection between the lysosomal apparatus and insulin-controlled metabolic pathways, and a potential role for lysosomal enzymes as indicators of the metabolic pathways compensation in diabetes.
The present results are corresponding with those obtained by our former studies of altered lysosomal enzymes activities in the course of experimental diabetes in rabbits (16, 25). Mean total activities of lipase and sulphatase increased in 21 day diabetes group, which was also true for both free and bound fractions. A highly significant increase in activity was noted in free fractions of both lipase and sulphatase as the value was almost twice as high as in the control, and significant activity increase in the bound fractions. At the same time ß-D-galactosidase was highly significant and NAGL significantly decreased in their free and bound fraction enzyme activities. These results were previously suggested in the works of other authors (26, 27, 28).
An increase in activities of both fractions of lipase as well as decrease in both lactase and NAGL fractions in comparison to control seem to be the most specific statistically for 42 day group. Above results regarding NAGL activity are concordant to those already obtained by Borelli et al. (29). The highly significant increase of both lipase fractions was noted, reaching their peak values in the course of experiment. A decrease in both fractions of lactase and.NAGL was also highly significant, whilst lactase reached its bottom value. However, surprisingly decreased activities of all total, free, and bound fraction of sulphatase seem to be more of a misevaluation rather than a result reflecting the progress of pathological changes.
In 90 days diabetes activities of lactase and NAGL were highly significantly lower than their initial values, as can be seen in other reports (26, 29).
In homogenates of 180 day diabetes rabbits free fraction of lipasde, and both fractions of sulphatase were highly significantly higher, whilst as also observed by Lundquist (30), lipase bound fraction was lower and all lactase and NAGL fractions highly significantly lower than the controls. It was also noted in 180 days group, that the mean total activity of NAGL was the lowest throughout the examination period. Above observations are similar to those reported by Skoglund et al. (31). Chua (32) observed an increase in proteolysis in the diabetic hearts which was also associated with decreased total activity and latency of NAGL, and an increased proportion of dense lysosomes in the particular fraction. Bhimji and co-workers (19) reported, that in their study NAGL activity was significantly increased in hearts of diabetic rabbits. They concluded that altered activities of lysosomal enzymes may be responsible for some aspects of diabetic cardiomyopathy.
The alterations in activity of lysosomal pancreatic enzymes of alloxan induced diabetes, presented in this study, may be responsible for some aspects of previously reported diabetic enteropathy and chronic complications, or may provide a mechanism for the pancreatic beta-cells to moderate their content of insulin (33, 34).
We conclude that in the course of alloxan-induced diabetes activities of lysosomal pancreatic lipase and sulphatase were increasing following the levels of glucose, whilst activities of ß-D-galactosidase and N-acetyl-ß-D-- glucosaminidase were declining, being inversely correlated to the level of glucose and activities of the first two mentioned enzymes.
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183—1197.
- Aughsteen AA. Morphometric studies on the juxta-insular and tele-insular acinar cells of the pancreas in normal and streptozotocin-induced diabetic rats. J Electron Microsc (Tokyo) 1993; 42: 79—87.
- Bendayan M. Contacts between endocrine and exocrine cells in the pancreas. Cell Tiss Res 1982; 222: 227—230.
- Korc M, Owerbach D, Quinto C, Rutter WJ. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels are determined by insulin. Science 1981; 213: 351—353.
- Groger G, Layer P. Exocrine pancreatic function in diabetes mellitus. Eur J Gastroenterol Hepatol 1995; 7: 740—746.
- Hirano T, Manabe T, Printz H, Saluja A, Steer M. Secretion of lysosomal and digestive enzymes into pancreatic juice under physiological and pathological conditions in rabbits. Nippon Geka Hokkan 1992; 61: 103—124.
- Kuijpers GAJ, Van Nooy IGP, De Pont JJ, Bonting SL. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta 1984; 778: 324—331.
- Kuijpers GAJ, Van Nooy IGP, De Pont JJ, Bonting SL. Anion secretion by the isolated rabbit pancreas. Biochim Biophys Acta 1984; 774: 269—276.
- Konturek SJ, Krzyzek E, Bilski J: The importance of gastric secretion in the feedback control of intterdigestive nd postprandial pancreatic secretion in rats. Regul Pept 1991; 36: 85—97.
- Bilski J, Konturek PK, Krzyzek E, Konturek SJ: Feedback control of pancreatic secretion in rats. Role of gastric acid secretion. J Physiol Pharmacol 1992; 43: 237—257.
- Burlina AB, Goi G, Fabi A, Lombardo A, Gaburro D, Tetmanti G. Behaviour of some lysosomal enzymes in the plasma of insulin dependent diabetic patients during artificial pancreas treatment. Clin Biochem 1987; 20: 423—427.
- Novikoff AB. Lysosomes in the physiology and pathology of cells. Contributions of staining methods. In: de Reuck AVS, Churchill JA. (eds), Ciba Foundation Symposium on Lysosomes. Boston: Little Brown; 1963.
- Barrett AJ. Lysosomal enzymes. In: Dingle JT. (ed) Lysosomes. Amsterdam: North-Holland Publishing Co; 1972.
- Burdan F, Siezieniewska Z, Maciejewski R, Burski K, Wójtowicz Z. Temporary elevation of pancreatic lysosomal enzymes, as a result of omeprazole-induced peripancreatic inflammation in male Wistar rats. J Physiol Pharmacol 2000; 51: 462—470.
- Kuscher B, Lazar M, Furman M, Lieberman TW, Leopold JH. Resistance of rabbits and guinea pigs to the diabetogenic effects of streptozotocin. Diabetes 1969; 18: 542—544.
- Maciejewski R, Hermanowicz-Dryka T, Wójtowicz Z, Dryka T, Burski K, Moghal N. et al. Changes in the activity of salivary gland cathepsins in the course of alloxan-induced diabetes mellitus in rabbits. Med Sci Res 1998; 26: 673—678.
- Weglicki WB, Ruth RC, Gottwik MG, McNamara DB, Owens K. Lysosomes of cardiac and skeletal muscle: resolution by zonal centrifugation. Rec Adv Stud Card Struct Metab 1975; 8: 503—517.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265—275.
- Bhimji S, Godin DV, McNeill JH. Biochemical and functional changes in hearts from rabbits with diabetes. Diabetologia 1985; 28: 452—457.
- Vogt M, Adamek HE, Arnold JC, Schilling D, Schleiffer T, Riemann JF. Gastrointestinale Komplikationen des Diabetes mellitus. Med. Klin 1999; 94: 329—337.
- Ricci JA, Sidique R, Stewart WF, Sandler RS, Sloan S, Farup CE. Upper gastrointestinal symptoms in a U.S. national sample of adults with diabetes. Scand J Gastroentero 2000; 35: 152—159.
- Spangeus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalance of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroentero 1999; 34: 196—202.
- Enck P, Dubois D, Marquis P. Quality of life in patients with upper gastrointestinal symptoms: results from the Domestic/International Gastroenterology Surviallance Study (DIGEST). Scand J Gastroentero (Suppl) 1999; 48—54.
- Hess RS, Kass PH, Shofer FS, Van Winkle TJ, Washabau RJ. Evaluation of risk factors for fatal acute pancreatitis in dogs. J Am Vet Med. Assoc 1999; 214: 46—51.
- Maciejewski R, Kaus M, Wójtowicz Z, Burdan F, Burski K. Changes in the activities of pancreatic cathepsins D and L and acid phosphatase in the course of alloxan-induced diabetes mellitus in rabbits. Med Sci Res 2000; 28: 113—116.
- Beattie GM, Levine F, Mally MI, Otonkoski T, O’Brien JS, Salmon DR, Hayek A. Acid beta-galactosidase: a developmentally regulated marker of endocrine cell precursors in the human fetal pancreas. J Clin Endocrinol Metab 1994; 78: 1232—1240.
- Garcia-Pascual JJ; Villar E, Corrales JJ, Garcia-Sastre A, Garcia-Diez LC, Corral J, Cabezas JA, Miralles JM. Enzymatic glycosidase activities in experimental obesity. Horm Metab Res 1992; 24: 412—415.
- Strawser LD, Touster O. The cellular processing of lysosomal enzymes and related proteins. Rev Physiol Biochem Pharmacol 1980; 87: 169—210.
- Borelli Mi, Alvarez V, de-Gagliariano E, Couso R, Gagliardiano JJ. Rapidly induced modulation of beta-N-acetylglucosaminidase activity in pancreatic islets. Arch Int Physiol Biochim Biophys 1994; 102: 9—12.
- Lundquist I, Panagiotidis G. The relationship of islet amyloglucosidase activity and glucose-induced insulin secretion. Pancreas 1992; 7: 352—357.
- Skoglund G, Ahren B, Lundquist I. Biochemical determination of islet lysosomal enzyme activities following crinophagy-stimulating treatment with diazoxide in mice. Diabetes Res, 1987; 6: 81—84.
- Chua BH, Long WM, Lautensack N, Lins JA, Morgan HE. Effects of diabetes on cardiac lysosomes and protein degradation. Am J Physiol 1983; 245: 91—100.
- Maciejewski R, Hermanowicz-Dryka T, Czerny K, Wójtowicz Z, Dryka T. Changes of the ATPase, acid phosphatase and alkaline phosphatase reaction intensity in the parotid and submandibular glands of rabbits in experimental diabetes. Folia Hitochem Cytobiol 1999; 37: 99—100.
- Maciejewski R, Burdan F, Hermanowicz-Dryka T, Wójcik K, Wójtowicz Z. Changes in the activity of some lysosomal enzymes and in the fine structure of submandibular gland due to experimental diabetes. Acta Physiol Hung 1999; 86: 127—137.