The influence of ghrelin on pancreatic endocrine secretion is unclear. Some studies have reported that ghrelin inhibits insulin secretion in the perfused rat pancreas (16), in anesthetized mice and in isolated mouse islets (17) and in humans (18). Other studies have shown that ghrelin stimulates insulin secretion in isolated rat pancreatic islets (19) and in anesthetized rats (20). Effect of ghrelin on pancreatic exocrine secretion is more established. Study performed by Zhang et al. (21) has shown that ghrelin inhibits the cholecystokinin- and 2-deoxy-D-glucose-stimulated pancreatic exocrine secretion in anesthetized rats, as well as inhibits the potassium-stimulated amylase secretion in incubated pancreatic lobules. Apart from an influence on pancreatic secretion, ghrelin also exhibits a protective effect on the pancreas and stomach. It has been shown that pretreatment with ghrelin reduces pancreatic damage in caerulein-induced pancreatitis (22) and inhibits the development of gastric lesions induced by ethanol (23).
The presence of ghrelin in the pancreas is age-dependent. In the fetal pancreas, ghrelin is expressed in a prominent cell population of pancreatic islets (24). In adult rat and human pancreas, the number of ghrelin-immunoreactive cells is reduced (24) and they have been recognized as the islet alpha cell (19). This observation and findings that ghrelin induces the release of growth hormone (1) and increases food intake (8, 9, 10) taken together suggest that ghrelin may be involved in gut development in young animals.
This study was designed: (a) to determine the influence of ghrelin administration on the pancreatic growth in suckling, weaned and young peripubertal seven week old rats; (b) to investigate the effect of exogenous ghrelin on food intake and the release of growth hormone and insulin-like growth factor-1 (IGF-1) in these young rats.
Animals and treatment
All experiments were performed according to the experimental protocol approved by the Committee for Research and Animal Ethics of the Jagiellonian University. The study was performed on male Wistar rats. Rats were housed in cages with wire mesh bottoms, at normal room temperature (22 ± 1°C) and a 12-h light-dark cycle. It was performed in three series: on suckling, weaned and young peripubertal seven week old rats. Rats from all series were treated with saline or ghrelin (4, 8 or 16 nmol/kg/dose) given intraperitoneally twice a day, last injection was 1 h before the end of the experiment. Rat ghrelin was synthesized in the Yanaihara Institute Inc. by a solid phase methodology with Fmoc-strategy, using peptide synthesizer (Applied Biosystem 9030 Pioneer, Foster, CA, USA).
Suckling rats, from the first study series, were kept with dams and treated with saline or ghrelin for 7 or 14 days starting from the fist postnatal day. The number of pups from each litter was limited to four. Three week old weaned rats and seven week old rats, from the second and third series of the study, were treated with saline or ghrelin for 5 days. Ten animals were used in each experimental group in each series of the study. The dams from the first series of the study and young rats from the second and third series of the study were supplied with standard laboratory chow and water, available ad libitum. Body weight of animals was recorded daily. Also, food intake was recorded in the weaned and young seven week old rats at the end of each day. Food intake in suckling animals was not measured due to technical reasons.
Determination of pancreatic blood flow
At the end of each series of the experiment, animals were anesthetized with ketamine (50 mg/kg i.p., Bioketan, Biowet, Gorzow, Poland), the abdominal cavity was opened and the pancreas was exposed. Pancreatic blood flow was measured by laser Doppler flowmeter using PeriFlux 4001 Master monitor (Perimed AB, Järfälla, Sweden), as described previously (25). Blood flow was measured in five regions of the pancreas and was expressed as percent change from control value.
Determination of serum growth hormone, IGF-1 and glucose concentration
After measurement of pancreatic blood flow, the abdominal aorta was exposed and blood was taken for determination of serum growth hormone, insulin-like growth factor-1 and glucose concentration. Serum growth hormone concentration was determined by radioimmunoassay, using Rat Growth Hormone RIA Kit (LINCO Research, St. Charles, Missouri, USA). Serum IGF-1 concentration was measured by radioimmunoassay, using Mouse/Rat IGF-1 RIA Kit (Diagnostic System Laboratories, Inc., Webster, Texas, USA). Sensitivity of these assays was 0.5 ng/ml. Concentration of serum glucose was determined with a Kodak Ectachem DT II System analyzer (Eastman Kodak Company, Rochester, NY, USA) using Vitros GLU Slides (Vitros DT Chemistry Products, Johnson & Johnson Clinical Diagnostic, Inc., Rochester, NY, USA). Serum glucose concentration was expressed as mmol/l.
Determination of pancreatic weight, DNA content and DNA synthesis
After blood withdrawal, the pancreas was carefully dissected out from the body and weighed. The rate of DNA synthesis in the pancreas was determined by incorporation of [3H]thymidine into DNA as described previously (26). The weighed portion of minced pancreatic tissue was incubated at 37°C for 45 min in 2 ml of medium containing 8 µCi/ml of [3H]thymidine ([6-3H]-thymidine, 20-30 Ci/mmol, Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic). The reaction was stopped, using 0.4 N perchloric acid. DNA content of the samples was determined by Giles and Myers procedure (27). The incorporation of [3H]thymidine into DNA was determined by counting 0.5 ml DNA-containing supernatant in a liquid scintillation system. DNA synthesis was expressed as disintegrations per minute [3H]thymidine per microgram DNA (dpm/ µg DNA). DNA content in the pancreas was expressed as mg per pancreas.
Determination of amylase activity in the pancreas
The weighed portion of pancreatic tissue was placed in 2 ml of saline containing 0.01% (wt/vol) soybean trypsin inhibitor (SIGMA Co. Saint Louis, MO, USA). The tissue was mechanically and ultrasonically homogenized, and centrifuged at 30,000 g for 10 min. An aliquot from the supernatant was taken for measurement of amylase activity. Activity of amylase was determined by an enzymatic method (Amylase reagent set (kinetic), Alpha Diagnostic sp. z o.o., Warszawa, Poland). Pancreatic activity of amylase in tissue samples was recalculated per total weight of the pancreas.
Statistical analysis
Results were expressed as mean ± S.E.M. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using GraphPadPrism. A difference with P value less than 0.05 was considered statistically significant.
The first series of studies with suckling rats
The body weight of suckling rats treated with ghrelin for 7 or 14 days at the dose 4, 8 or 16 nmol/kg/dose was not significantly different in comparison with control rats treated with saline (Table 1). Also, pancreatic blood flow and serum concentration of glucose were not statistically affected by administration of ghrelin in suckling rats; whereas pancreatic DNA synthesis and pancreatic DNA content were significantly reduced by treatment with ghrelin for 7 or 14 days at the highest dose, 16 nmol/kg/dose (Table 1). Pancreatic weight (Table 1) and pancreatic amylase activity (Fig. 1) were significantly lowered by all used doses of ghrelin.
| Table 1. Effect of ghrelin administration for 7 or 14 days on pancreatic growth in suckling rats |
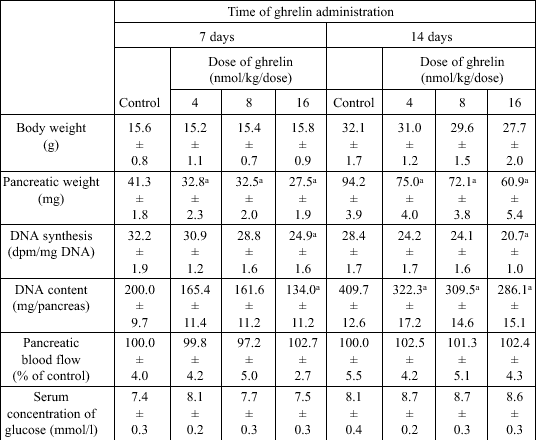 |
| Mean ± S.E.M.
N=10 in each group of animals. aP<0.05 compared with control at the same time of observation. |
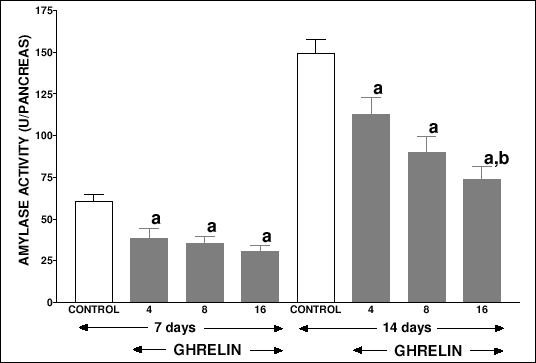 |
| Fig.1. Pancreatic amylase activity in suckling rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 7 or 14 days. Mean ± S.E.M. N = 10. aP<0.05 compared with control at the same time of observation; bP<0.05 compared with ghrelin at the dose 4 nmol/kg/dose at the same time of observation. |
Serum growth hormone level in suckling control rats treated with saline for 7 or 14 days reached 27.2 ± 1.6 or 24.1 ± 1.7 ng/ml, respectively (Fig. 2 and 3). Treatment with ghrelin, at any dose used, caused a significant increase in serum concentration of growth hormone. There was no difference in serum insulin-like growth factor-1 level between suckling control rats treated with saline for 7 or 14 days and suckling rats treated with any used dose of ghrelin (Fig. 2 and 3).
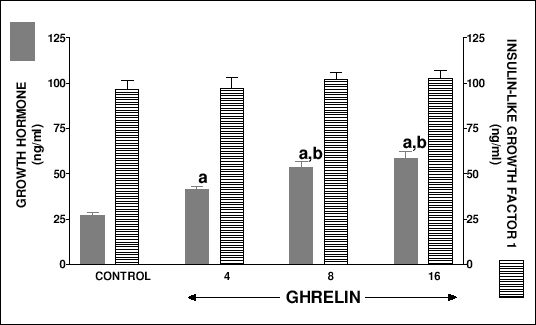 |
| Fig.2. Serum concentration of growth hormone and insulin-like growth factor-1 in suckling rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 7 days. Mean ± S.E.M. N = 10 in each group of animals. aP<0.01 compared with control; bP<0.05 compared with ghrelin at the dose 4 nmol/kg/dose. |
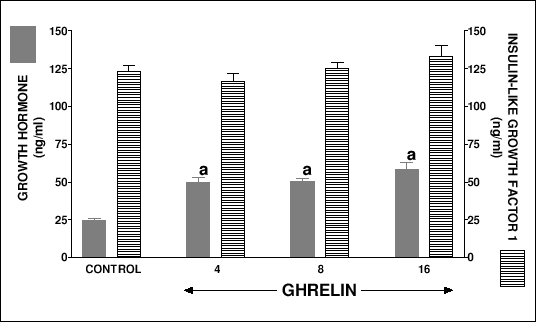 |
| Fig.3. Serum concentration of growth hormone and insulin-like growth factor-1 in suckling rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 14 days. Mean ± S.E.M. N = 10 in each group of animals. aP<0.001 compared with control. |
The second series of studies with weaned rats
In control weaned rats treated with saline for 5 days, the body weight reached 83.9 ± 2.3 g and treatment with ghrelin at any dose used failed to affect this parameter (Table 2). Administration of ghrelin tended to reduce the daily food intake, however this effect was statistically significant only after ghrelin administered at the highest dose, 16 nmol/kg. In contrast to this effect, pancreatic weight, DNA content (Table 2) and amylase activity (Fig. 4) were significantly increased by all used doses of ghrelin. Pancreatic DNA synthesis was significantly increased only by ghrelin at the highest dose, 16 nmol/kg (Table 2). There was no difference in pancreatic blood flow or serum concentration of glucose between control weaned rats and weaned rats treated with ghrelin (Table 2).
| Table 2. Effect of ghrelin administration for 5 days on pancreatic growth in weaned rats |
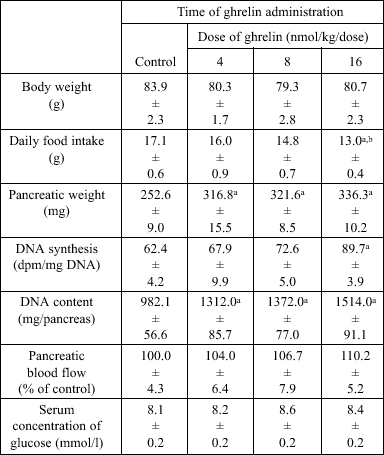 |
| Mean ± S.E.M. N=10 in each group of animals. aP<0.05 compared with control. |
 |
Fig. 4. Pancreatic amylase activity in weaned rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 5 days. Mean ± S.E.M. N = 10. aP<0.01 compared with control; bP<0.001 compared with ghrelin at the dose 4 nmol/kg/dose; cP<0.05 compared with ghrelin at the dose 8 nmol/kg/dose. |
Ghrelin administered for 5 days in weaned rats increased serum concentration of growth hormone (Fig. 5). The increase in serum level of growth hormone after ghrelin at the dose 8 or 16 nmol/kg/dose was significantly higher than that after ghrelin used at the dose 4 nmol/kg/dose. Serum concentration of insulin-like growth factor-1 was significantly higher in weaned rats treated with any dose of ghrelin than in control rats (Fig. 5).
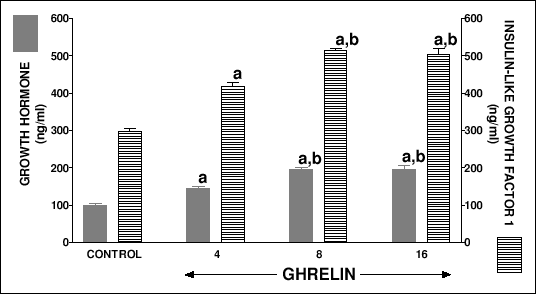 |
| Fig.5. Serum concentration of growth hormone and insulin-like growth factor-1 in weaned rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 5 days. Mean ± S.E.M. N = 10 in each group of animals. aP<0.001 compared with control; bP<0.001 compared with ghrelin at the dose 4 nmol/kg/dose. |
The third series of studies with peripubertal seven week old rats
In peripubertal seven week old rats, the body weight, daily food intake, pancreatic weight, DNA synthesis and DNA content, (Table 3) and pancreatic activity of amylase (Fig. 6) were significantly increased by all used doses of ghrelin. Maximal stimulatory effect appeared after treatment with ghrelin at the dose, 8 nmol/kg/dose. Pancreatic blood flow and serum concentration of glucose were not affected by ghrelin administration in peripubertal seven week old rats (Table 3).
| Table 3. Effect of ghrelin administration for 5 days on pancreatic growth in peripubertal seven week old rats |
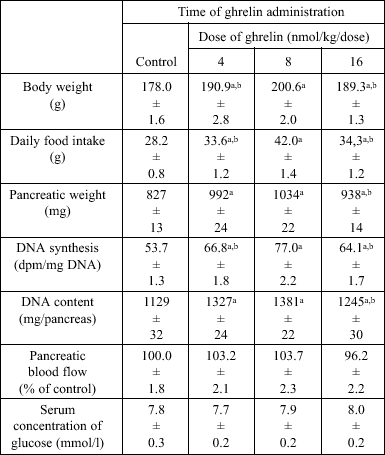 |
| Mean ± S.E.M.; N=10 in each group of animals; aP<0.05 compared to control; bP<0.05 compared to ghrelin at the dose 8 nmol/kg/dose. |
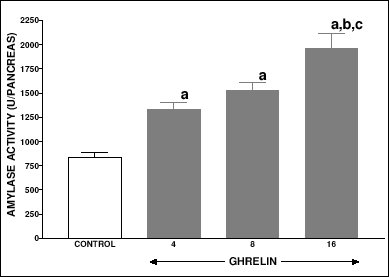 |
Fig. 6. Pancreatic amylase activity in peripubertal seven week old rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 5 days. Mean ± S.E.M. N = 10. aP<0.001 compared with control; bP<0.01 compared with ghrelin at the dose 8 nmol/kg/dose. |
Serum concentration of growth hormone in peripubertal seven week old control saline-treated rats reached a value 148.1 ± 8.7 ng/ml (Fig. 7). All used doses of ghrelin significantly increased serum concentration of growth hormone in peripubertal rats. Also, administration of ghrelin significantly increased serum concentration of insulin-like growth factor-1 (Fig. 7). Maximal increase in serum concentration of insulin-like growth factor was observed after ghrelin at the dose 8 nmol/kg.
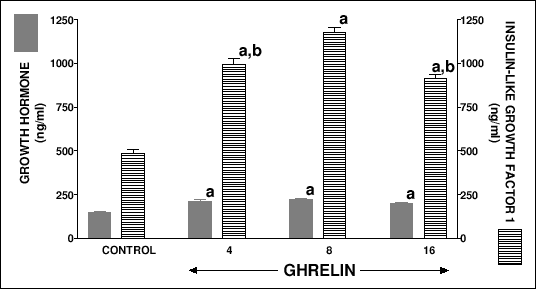 |
| Fig.7. Serum concentration of growth hormone and insulin-like growth factor in peripubertal seven week old rats treated with saline (control) or ghrelin (at the dose 4, 8 or 16 nmol/kg/dose) twice a day for 5 days. Mean ± S.E.M. N = 10 in each group of animals. aP<0.001 compared with control; bP<0.001 compared with ghrelin at the dose 8 nmol/kg/dose. |
Our present study has brought several important findings: (a) effect of ghrelin administration on pancreatic growth in young rats is age-dependent; in sucking rats ghrelin inhibits pancreatic growth, whereas in weaned and in peripubertal seven week old rats, treatment with ghrelin stimulates pancreatic growth; (b) treatment with ghrelin reduces food intake in weaned rats, while ghrelin administered in peripubertal seven week old rats increases appetite; (c) ghrelin administration enhances the serum concentration of growth hormone in suckling, weaned and peripubertal rats and this effect increases with animal age; (d) administration of ghrelin does not affect serum concentration of insulin-like growth factor-1 in suckling rats, in contrast in weaned and peripubertal rats, ghrelin increases serum level of insulin-like growth factor-1.
Previous studies have shown that ghrelin administration stimulates food intake and body weight gain in adult rats (8, 9) and humans (10). These data are in agreement with our present study with peripubertal seven week old rats. In contrast to this observation, our experiments performed on prepubertal animals have shown that treatment with ghrelin does not affect the body weight gain in suckling and weaned rats. Also, the highest dose of ghrelin, 16 nmol/kg, given for 5 days caused a significant reduction in food consumption in weaned rats. These observations taken together indicate that influence of ghrelin administration on appetite and body weight gain depends on the age of rats. Inhibitory effect is probably related to immaturity of the hypothalamus in weaned young animals. According to the classic concept, eating behavior remains under the control of two hypothalamic centers with opposite effects on food intake, a lateral hypothalamic area named "feeding or appetite center" and ventromedial hypothalamic area known as "satiety center" (28). These centers regulate energy intake by integrating the information concerning starvation or satiation with the status of the environment through a variety of neural and blood derived signals (28). Gastric ghrelin enters the brain across the blood-brain barrier (29) and acts on growth hormone secretagogue receptors present in the pituitary and hypothalamus. Previous studies have shown that orexigenic effect of ghrelin is related to activity of the neuropeptide Y-, agouti-related protein- and orexine-ergic neurons present in the hypothalamus (30, 31). It is believed that release of neuropeptide Y plays the most important role in this process (30). In childhood before puberty, hypothalamic neuropeptide Y gene expression and level of this peptide is high (32). In this period of life, hypothalamic excess of neuropeptide Y exhibits inhibitory action on the hormonal gonadotropic (32, 33) and somatotropic axis (34, 35). It is most likely that high basal hypothalamic level of neuropeptide Y blocks the ghrelin-induced release of this peptide in the hypothalamus in prepubertal animals and this mechanism is probably responsible for the lack of orexigenic effect of ghrelin in weaned rats in our present study.
Enteral feeding affects body weight gain and plays the most important role in maintaining pancreatic weight, structure and enzyme composition in animals and humans (36, 37). Contrary, fasting of rats for more than 48 h causes a marked decrease in pancreatic weight and total content of DNA, protein and amylase in pancreatic tissue (37, 38). Our results obtained in peripubertal rats are consistent with these observations. In these rats, administration of ghrelin increased daily food intake and animal body weight, and this effect was associated with the significant increase pancreatic weight, DNA synthesis, DNA content and pancreatic amylase activity. This finding indicates that ghrelin administered in peripubertal seven week old animals stimulates proliferation of pancreatic cells and increases content of digestive enzymes in the pancreas.
In contrast to results obtained in peripubertal rats, treatment with ghrelin reduced daily food intake in weaned rats and this effect was statistically significant after ghrelin administered at the dose 16 nmol/kg. On the other hand, pancreatic weight, pancreatic DNA synthesis and DNA content, and content of amylase in the pancreas were significantly increased by ghrelin administration in weaned rats. This discrepancy between reduction of daily food intake and stimulation of pancreatic growth and enzymatic activity seems to be related to stimulatory effect of ghrelin on the release of growth hormone and IGF-1. Both hormones, especially IGF-1 exhibit anabolic activity (39). This mechanism is probably involved in the promotion of pancreatic growth and in pancreatic amylase activity in weaned rats after treatment with ghrelin.
In our present study we have found that administration of ghrelin inhibits pancreatic growth in suckling rats. Pancreatic weight, DNA synthesis and DNA content, as well as pancreatic activity of amylase were reduced when compared with control saline-treated rats. This inhibitory effect of ghrelin on pancreatic growth was accompanied by a slight increase in serum growth hormone concentration. However, it must be pointed out that growth hormone concentration in suckling saline-treated control rats was extremely low and administration of ghrelin was without effect on serum level of insulin-like growth factor-1. The major biologic effect of the growth hormone is growth promotion. However, the release of growth hormone is strongly stimulated by starvation, hypoglycemia, stress, infection or exercise (40-43). Direct metabolic action of growth hormone includes stimulation of lipolysis, and reduction in the rate of glucose utilization, what elevates serum glucose concentration (44, 45). Additionally, growth hormone stimulates release of free fatty acids and by this way may directly activate degradation of amino acids in the process of gluconeogenesis (46). It is well established that growth hormone antagonizes insulin action in vivo and that over-physiological concentration of growth hormone frequently results in insulin resistance and glucose intolerance (45, 47). Growth hormone can directly increase the rate of protein synthesis in cells of the body, but its anabolic effect is mainly mediated through insulin-like growth factor-1, which is principally produced in the liver (39, 44, 45). Data mentioned above taken together with our observations that serum level of growth hormone in suckling rats is low, and ghrelin administration does not affect serum insulin-like growth factor-1, can explain why ghrelin administration inhibits pancreatic growth in suckling rats.
A recent study performed by Saito et al. (48) has shown that intracerebroventricular administration of ghrelin inhibits food intake in neonatal chicks. Saito et al have suggested that this effect is caused by activation of the endogenous corticotropin-releasing factor system. In our present study we have observed a reduction in food intake in weaned rats and a reduction in pancreatic growth in suckling rats; however these results are probably not related to activation of the hypothalamo-pituitary-adrenal axis. This thesis is supported by two findings. First of all, previous studies have reported that ghrelin stimulates a release of glucocorticoids in humans (49) and rats (50), but this effect has been associated with stimulation of food intake. On the other hand, action of glucocorticoids leads to increase in serum level of glucose. However in our present study, serum level of glucose was not affected by any dose of ghrelin in any group of animals. This observation indirectly indicates that inhibitory effect of ghrelin administration on pancreatic growth in suckling rats does not depend on glucocorticoids release.
In conclusion, our study has shown that the effect of ghrelin on pancreatic growth in young rats depends on the age of animals. Administration of ghrelin reduces pancreatic growth in suckling rats; whereas in weaned and young seven week old animals, treatment with ghrelin increases pancreatic growth. In weaned and peripubertal rats, pancreatic growth-promoting effect of ghrelin seems to be related to stimulation of the release of anabolic IGF-1.
Acknowledgements: This study was supported by the Polish Committee for Scientific Research (Solicited Project PBZ-KBN-093/P06/2003) and by grant 501/P/108/L from Collegium Medicum of Jagiellonian University.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature 1999; 402: 656-660.
- Date Y, Kojima M, Hosoda H et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255-4261.
- Ariyasu H, Takaya K, Tagami, T et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001; 86: 4753-4758.
- Gnanapavan S, Kola B, Bustin SA et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002; 87: 2988-2991.
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 2001: 12: 118-122.
- Bowers CY, Momany F, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 1984; 114: 1537-1545.
- Smith RG, Pong SS, Hickey G et al. Modulation of pulsatile GH release through a novel receptor in hypothalamus and pituitary gland. Recent Prog Horm Res 1996; 51: 261-285.
- Wren AM, Small CJ, Abbott CR et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540-2547.
- Shimbara T, Mondal MS, Kawagoe T et al. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett 2004; 369: 75-79.
- Wren AM, Seal LJ, Cohen MA et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992-5995.
- Shiiya, T., Nakazato, M., Mizuta et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002; 87: 240-244.
- Shimizu Y, Nagaya N, Isobe T et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res 2003; 9: 774-8.
- Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707-709.
- Bellone S, Castellino N, Broglio F et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab 2004; 89: 1662-1665.
- Saito ES, Kaiya H, Takagi T et al. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. Eur J Pharmacol 2002; 453: 75-79.
- Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 2002; 146: 241-244.
- Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003; 144: 916-921.
- Broglio F, Benso A, Castiglioni C et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab 2003; 88: 1537-1542.
- Date Y, Nakazato M, Hashiguchi S et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 2002; 51: 124-129.
- Lee HM, Wang G, Englander EW, Kojima M, Greeley GH Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002; 143: 185-190.
- Zhang W, Chen M, Chen X, Segura BJ, Mulholland MW. Inhibition of pancreatic protein secretion by ghrelin in the rat. J Physiol 2001; 537: 231-236.
- Dembiñski A, Warzecha Z, Ceranowicz P et al Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol 2003; 54: 561-573.
- Konturek PC, Brzozowski T, Pajdo R et al. Ghrelin - a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol 2004; 55: 325-336.
- Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002; 107: 63-69.
- Konturek SJ, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol 1994; 15: 19-28.
- Warzecha Z, Dembinski A, Ceranowicz P et al. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J Physiol Pharmacol 2003; 54: 575-590.
- Giles KW, Myers A. An improvement diphenylamine method for the estimation of deoxyribonucleic acid. Nature 1965; 206: 93.
- Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 2004; 55: 137-154.
- Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 2002; 302: 822-827.
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes 2001; 50: 2438-2443.
- Toshinai K, Date Y, Murakami N et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003; 144: 1506-1512.
- Plant TM, Shahab M. Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav 2002; 77: 717-722.
- Blogowska A, Rzepka-Gorska I, Krzyzanowska-Swiniarska B. Is neuropeptide Y responsible for constitutional delay of puberty in girls? A preliminary report. Gynecol Endocrinol 2004; 19: 22-25.
- McDonald JK, Lumpkin MD, Samson WK, McCann SM. Neuropeptide Y affects secretion of luteinizing hormone and growth hormone in ovariectomized rats. Proc Natl Acad Sci USA 1985; 82: 561-564.
- Korbonits M, Little JA, Forsling ML et al. The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol 1999; 11: 521-528.
- Fölsch UR. Regulation of pancreatic growth. Clin Gastroenterol 1984; 13: 679-699.
- Konturek SJ, Dembinski A, Warzecha Z et al. Antagonism of receptors for bombesin, gastrin and cholecystokinin in pancreatic secretion and growth. Digestion 1991; 48: 89-97.
- Webster PD, Singh M, Tucker PC, Black O. Effects of fasting and feeding on the pancreas. Gastroenterology 1972; 62: 600-605.
- Zapf J, Froesch ER. Insulin-like growth factors/somatomedins: structure, secretion, biological actions and physiological role. Horm Res 1986; 24: 121-130.
- Roth J, Glick SM, Yalow RS, Berson SA. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 1963; 140: 987-988.
- Roth J, Glick SM, Yalow RS, Berson SA. Secretion of human growth hormone: physiologic and experimental modification. Metabolism 1963; 12: 577-579.
- Greenwood FC, Landon J. Growth hormone secretion in response to stress in man. Nature 1966; 210: 540-541.
- Schalch DS. The influence of physical stress and exercise on growth hormone and insulin secretion in man. J Lab Clin Med 1967; 69: 256-269.
- Ho KK, O'Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J 1996; 43 Suppl: S57-S63.
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med 2003; 37: 100-105.
- Fanelli C, Calderone S, Epifano L et al. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 1993; 92: 1617-1622.
- Glenn KC, Rose KS, Krivi GG. Somatotropin antagonism of insulin-stimulated glucose utilization in 3T3-L1 adipocytes. J Cell Biochem 1988; 37: 371-383.
- Saito ES, Kaiya H, Tachibana T et al. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 2005; 125: 201-208.
- Schmid DA, Held K, Ising M, Uhr M, Weikel JC, Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology 2005; 30: 1187-1192.
- Wren AM, Small CJ, Fribbens CV et al. The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology 2002; 76: 316-324.