Furthermore, activated platelets release IL-1 and chemokine RANTES that mediates monocyte recruitment through vascular wall (5,6). Platelets amplify also respiratory burst of neutrophils (7,8) and facilitates neutrophil-dependent phagocytosis of bacteria (9). The above pro-inflammatory mechanisms of platelet-monocyte or platelet neutrophil interactions are causally involved in various types of inflammatory processes (10, 7,11, 12).
It is well known that adhesion of platelets to neutrophils under static or flow conditions involves selectin P on platelets and P-selectin glikoprotein ligand (PSGL-1) on neutrophils, as well as GP IIb/IIIa on platelets and CD11b/CD18 on leukocytes (13-15,15,16). Moreover, for platelet-neutrophil adhesion to occur autocrine/paracrine activation of platelets/neutrophils by means of specific lipid mediators such as TXA2, PAF or cysteinyl leukotrienes is required (17).
Eosinophils play a role in allergic disease and host response to parasite, a function that is not shared by other leukocytes. Nevertheless these cells possess rich pro-inflammatory machinery that in some aspects is very similar to that found in other types of leukocytes (18, 19). Also as regards to interactions with platelets, activated platelets via a selectin-P-mediated mechanism adhere to unstimulated eosinophils (20). Still, in contrast to platelet-neutrophil or platelet-monocyte interactions little is known on other mechanisms of platelet-eosinophil interactions as well as on their biological consequences.
Therefore, the aim of the present work was to assess the ability of eosinophils to interact with platelets as compared to neutrophils and to analyze whether the same adhesion molecules (selectin P, GPIIb/IIIa) and lipid mediators (TXA2, PAF, cysteinyl leukotrienes) are involved in platelet-eosinophil and platelet-neutrophil interactions. For that purpose we isolated human platelets, eosinophils, and neutrophils and analysed platelet-eosinophil and platelet-neutrophil adhesion in response to thrombin, LPS and fMLP using the classic method of Jungi known also as "rosettes" formation assay (21). The involvement of adhesion molecules such as selectin P, glycoprotein IIb/IIIa (GPIIb/IIIa) and lipid mediators such as thromboxane A2 (TXA2), platelet activating factor (PAF) and cysteinyl leukotrienes (cysLTs) were studied using monoclonal antibodies or pharmacological inhibitors.
Venous blood was obtained from human volunteers in University Hospital Blood Bank Center. Volunteer donors had not taken any medicines for preceding 2 weeks. Blood was anticoagulated with sodium citrate (3.2%, 1:9 v/v).
Isolation of eosinophils
Eosinophils were isolated using magnetic cell separation method as described previously (20). Briefly, blood were diluted with an equal volume of phosphate-buffered saline (PBS) / 5% FCS and layered above the Percoll solution (1.082 g/ml, Pharmacia, Sweden) and centrifuged at 1.613 x g at 20°C for 30 min. The pellet containing granulocytes and erythrocytes was recovered and erythrocytes were removed by cold hypotonic lysis (ice-cold 0,2 % NaCl). Then anti-CD16 coated magnetic microbeads (Miltenyi Biotech, Germany) were added to the remaining granulocyte mixture (50 µl per 107 cells) and incubated at 4°C for 30 min. The CD16-microbead-bound neutrophils were removed by retention in the column, using a magnetic separator - VarioMACS (Miltenyi Biotech, Germany) (22). Eosinophils were collected, counted and suspended in Ca++ - free and Mg++ - free PBS containing 0.1 % albumin (2 x 106 cells/ml).
Isolation of platelets and neutrophils
To obtain platelet-rich plasma (PRP), blood was centrifuged at 250 x g for 20 min. PPP was obtained by centrifugation of remaining blood for 5 min at 2000 x g. To obtain washed platelets (WP) platelets were washed twice in PGI2 containing PBS according to Radomski et al. (23) and finally suspended (2x108 platelet/ml) in Ca++ free PBS containing 0.1 % albumin. Filtered platelets (FP) were obtained by gel-filtration on Sepharose 2B columns. Contamination of neutrophils in PRP and WP was less then 1/106 and 1/108, respectively, and in FP it was not detectable.
Polymorphonuclear neutrophils (PMNs) were isolated as follows: a sample of erythrocytes and leukocytes was mixed with 3 % solution of dextrane (1:1 v/v) and allowed to sediment for 30 min at 37°C. After sedimentation of erythrocytes the upper fraction which contained PMNs and monocytes was used for Ficoll-gradient isolation of PMNs according to Boyum et al. (24). Residual erythrocytes were removed by means of hypoosmotic lysis with ice-cold 0,2% NaCl solution. Finally, 2x106 leukocytes were suspended in Ca++ free PBS containing 0.1 % albumin. More than 95% of the isolated leukocytes were neutrophils in microscope evaluation. Cells were viable in more than 98 % as evidenced by Trypan blue staining.
Rosettes assay
Rosettes assay was performed according to Jungi (21) (Fig. 1). The mixture of washed platelets (2x108/ml) and isolated eosinophils (2x106/ml) or isolated PMNs (2x106/ml), were added with Ca++ and Mg++ (2 mM each). Then it was incubated for 5 minutes with or without an inhibitor and stimulated with thrombin, fMLP or LPS and incubated at 22°C. Number of rosettes per 100 eosinophils or neutrophils was counted in triplicate with the use of Zeiss Photomicroscope. A leukocyte with 4 or more adherent platelets was considered as a rosette. In control experiments platelet-neutrophil adhesion was assayed using washed and filtered platelets. As there was no difference washed platelets were used for all experiments.
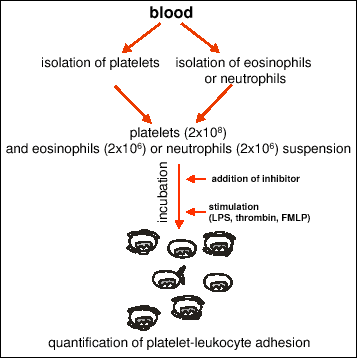 |
Fig. 1. The scheme of the assay for platelet-leukocyte adhesion (rosettes formation). |
Reagents
Endotoxin (LPS, Escherichia coli serotype 0127 : B8), hemotactic peptide - N-formyl-Met-Leu-Phe (fMLP) (25) were purchased from Sigma Chemicals International, thrombin from Polfa-Krakow, Poland. WEB 2170 - a PAF receptor antagonist was from Boehringer Ingelheim, Germany; MK 886 - an inhibitor of 5-lipoxygenase activating protein (FLAP), acetylsalicylic acid were from Biomol Research Lab, Inc., USA. Fucoidin (26) - an non-selective antagonist of selectins was from Sigma, blocking mouse antibodies against human selectin P, selectin E selectin L and CD18 were from Pharmingen, Germany. Abciximab - a monoclonal antibody against platelet glycoprotein IIb/IIIa was a kind gift from Lilly Co, USA (27).
Statistical analysis
All values were expressed as means +/- SEM. Differences between means were evaluated by the unpaired Student's t-test. P value less than 0.05 was considered to be significant.
In the suspension of non-activated platelets (2x108/ml) plus eosinophils (2x106/ml) or non-activated platelets plus neutrophils 10-15% of leukocytes were surrounded by adhering platelets. This spontaneous rosettes formation was higher for eosinophils as compared to neutrophils (Fig. 2). Platelet-eosinophil adhesion was stimulated by thrombin (30 mU/ml), fMLP (1 µM) or LPS (0.01 µg/ml). Each of the stimuli activated platelet-eosinophil adhesion as well as platelet-neutrophil adhesion. However, number of rosettes formed after the stimulation was higher when eosinophils were used. For example, average maximum of platelet-eosinophils adhesion and platelet-neutrophil adhesion induced by thrombin (30 mU/ml) 10 minutes after the stimulation was 39 and 24, respectively (number of rosettes per 100 leukocytes).
 |
Fig. 2. Comparison of platelet-eosinophil and platelet-neutrophil adhesion induced by fMLP thrombin, and LPS. Basal denotes spontaneous platelet-neutrophil or platelet-eosinophil adhesion. Data are presented as mean ± SEM from at least 6 experiments. * p < 0.05; ** p<0.001 in comparison to basal. |
Regardless whether thrombin, fMLP or LPS was used to stimulate platelet-eosinophil adhesion, inhibition of P-selectin by fucoidin (100 µM) or by anti-CD62P antibody (1 µm/ml) profoundly attenuated platelet-eosinophil adhesion (Fig. 3A). Anti-L selectin antibody (1 µm/ml) had an modest inhibitory effect, while anti-E selectin antibody (1 µm/ml) was not effective. Similar results were obtained for platelet-neutrophil adhesion (Fig. 3B). In the presence of blocking antibody against GPIIb/IIIa (abciximab 3 µm/ml), platelet-eosinophil as well as platelet-neutrophil adhesion were strongly attenuated (Fig. 3A,B).
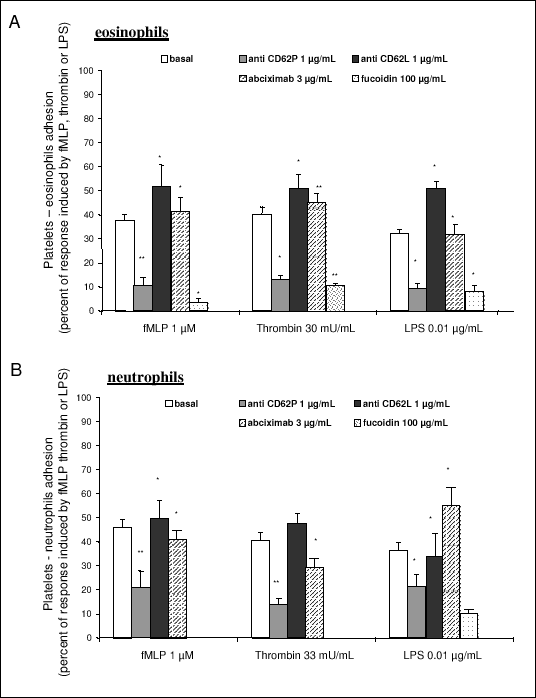 |
| Fig. 3. Involvement of adhesion molecules in platelets-eosinophil (A) and platelet-neutrophils (B) adhesion. Monoclonal antibodies (anti-GPIIb/IIIa - abciximab, CD62P and anti CD62L) were used or non-selective selectin inhibitor fucoidin. Data are expressed as percent of response to fMLP (1 µM), thrombin (30 mU/ml) or LPS (0.01 µg/ml), respectively. Basal denotes spontaneous platelet-neutrophil or platelet-eosinophil adhesion. Results are presented as mean ± SEM from at least 3 experiments.* p < 0.05; ** p<0.001 in comparison to the response induced by fMLP, Thrombin or LPS. |
As shown in Fig. 4A, inhibition of cyclooxygenase with aspirin (300 µM) resulted in a profound inhibition of platelet-eosinophil adhesion, while effects of FLAP-inhibitor (MK 886, 10 µM) or PAF receptor antagonist (WEB 2170, 100 µM) were only noticeable when LPS was used as a stimulus (Fig. 4A). Similarly to platelet-eosinophils adhesion aspirin was a potent inhibitor of platelet-neutrophil adhesion (Fig. 4B). However, in contrast to platelet-eosinophils adhesion, the inhibitory effects of MK 886 (10 µM) and WEB 2170 (100 µM) on platelet-neutrophil adhesion was more pronounced though the magnitude of this effect depended on the stimulus that was used (Fig. 4B).
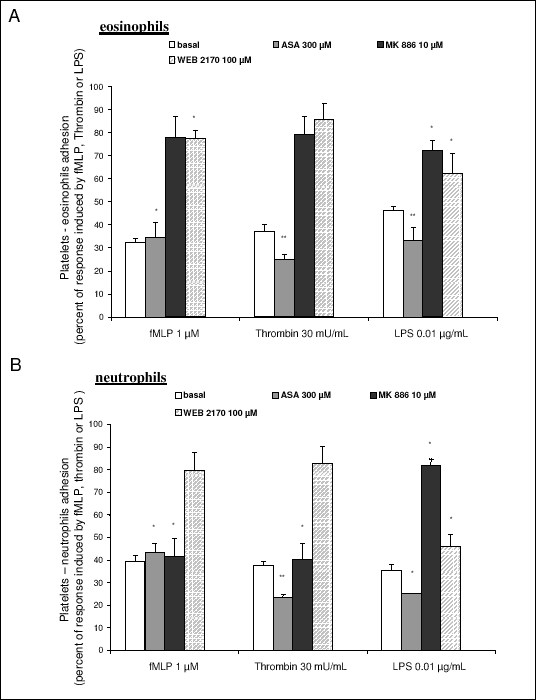 |
| Fig. 4. Involvement of lipid mediators in platelets-eosinophil (A) and platelet-neutrophils (B) adhesion. ASA- aspirin; WEB 2170 (PAF receptor antagonist); MK 886 (inhibitor of FLAP). Data are expressed as percent of response to fMLP (1 µM), thrombin (30 mU/ml) or LPS (0.01 µg/ml), respectively. Basal denotes spontaneous platelet-neutrophil or platelet-eosinophil adhesion. Results are presented as mean ± SEM from at least 3 experiments. p < 0.05; ** p<0.001 in comparison to to the response induced by fMLP, Thrombin or LPS. |
In the present study we demonstrated that similarly to platelet-neutrophil interaction adhesion of platelets to eosinophils involved not only adhesion molecules (selectin P, glycoprotein IIb/IIIa), but also lipid mediators among which TXA2 was the major mediator while PAF and cysteinyl leukotrienes played a minor role. In contrast to numerous studies on the mechanism and biochemical consequences of platelet-neutrophil interactions there are limited number of studies related to interactions of eosinophils with platelets (28, 29). This might be due to the methodological difficulties to obtain homogenous population of human eosinophils. Here we took an advantage of the novel immunomagnetic method of eosinophils isolation based on negative selection and anti-CD16-coated immunomagnetic beads (22). Using this technique to isolate eosinophils and classic method to isolate neutrophils we compared the ability to platelet to adhere to eosinophils and to neutrophils as well as studied mechanisms involved in both types of interactions.
We found that platelets adhered avidly to eosinophils both after activation of platelets by thrombin, eosinophils by fMLP (25) or simultaneous activation of platelets (30) and eosinophils by LPS (31). In that respect eosinophils behaved similarly to neutrophils (17,28,32). Mechanisms of adhesion of platelets to neutrophils were studied extensively in static and dynamic conditions (15). Here we confirmed that mechanism of platelet-neutrophil adhesion involved adhesion molecules such as selectin P and glycoprotein IIb/IIIa on platelets and their counterparts on leukocyte as well as lipid mediators such as TXA2, PAF and cysteinyl leukotrienes. Similarly to platelet-neutrophil interactions when platelets adhered to eosinophils, selectin P and glycoprotein IIb/IIIa were engaged. Obviously, eosinophils similarly to neutrophils and monocytes express PSGL-1 (33) and CD11b/CD18 (34) to bind their counterparts on platelets as neutrophils do (14-16,35). Our results show that when activated platelet encounter neutrophil or eosinophil similar type of adhesive event take part. It is however worth to add, that in our experimental conditions relative number of neutrophils and eosinophils was similar that obviously do not occur in vivo in physiological conditions.
As regards the involvement of lipid mediators we analyzed here the involvement of PAF, TXA2 and cysteinyl leukotrienes in the activation of intercellular interactions between platelet- neutrophil and platelet-eosinophil. It is known that in the circulation leukocytes and platelets represent the major source of PAF and TXA2, respectively, while biosynthesis of cysteinyl leukotrienes ensue by the transcellular biosynthesis (36). Autocrine activation of neutrophils by PAF (37) or platelets by TXA2 (38) is an important mechanism by which activation of these cells is amplified. Here we look on their role in the regulation of heterotypic intercellular interactions that may also favour transcellular biosynthesis of cysLTs.
We found that platelet-eosinophil and platelet-neutrophil adhesion stimulated by thrombin, fMLP or LPS were abrogated by aspirin. Accordingly, COX-1- derived product of arachidonic acid, most likely TXA2, plays a key role in platelet-leukocyte adhesion. Indeed, aspirin is relatively selective inhibitor of COX-1-derived TXA2 in platelets, while human neutrophils in contrast to platelets do not possess TXA2 synthase (39). Also there is no evidence that eosinophils do synthesize TXA2 (40). It is unlikely that aspirin effect was related to the inhibition of other COX-1-derived products such as PGE2 or PGD2, as these metabolites inhibit but not amplify activation of platelets and leukocytes (41). Recently, we have shown that TXA2 - mediated platelet-dependent augmentation of respiratory burst of neutrophils (8). Altogether, it appears that platelet-derived TXA2 is a major regulator of platelet-neutrophil as well as platelet-eosinophil interactions.
In the light of the above, it is tempting to suggest that aspirin when administered in a low anti-platelet dose may blunt platelet-leukocyte interactions and in that way may afford part of its anti-inflammatory action. It would be interesting to test whether similar effect of aspirin pertains to eosinophils isolated from atopic individuals or patients with aspirin-sensitive asthma (ASA). ASA-induced bronchospasm is believed to be associated with augmented release of cysteinyl leukotrienes that is platelet-independent (42,43). Involvement of TXA2 in platelet-eosinophil interactions in this condition remains to be tested.
We found that in contrast to platelet-neutrophil interaction pharmacological elimination of PAF or cysteinyl leukotrienes by WEB 2170 or MK 886 had only a minor effect on platelet-eosinophil adhesion. Apparently, PAF and cysteinyl leukotrienes are of more importance in the adherence of activated platelet to neutrophil then to eosinophils. It may well be that transcellullar metabolism of neutrophil -derived leukotriene A4 to leukotriene C4 in platelets (36,44) cannot be mimicked by eosinophils. Indeed, in contrast to neutrophils, eosinophils alone are able to synthethize considerable amount of LTC4 and the intercellular transfer of LTA4 leading to transcellular biosynthesis of LTC4 seems to be directed towards epithelial cells (41) not towards platelets. On the other hand, both intercellular signalling of PAF in neutrophils and an enhanced release of PAF by neutrophils in presence of platelets (17,28) may not occur to the same extent with eosinophils. It remains to be established whether the differential role of PAF and cysLTs in the autocrine/paracrine activation of platelet-neutrophil and platelet-eosinophil interactions have an important relevance to function of these interactions in pathophysiological settings.
In summary, it seems clear that occurrence of platelet-eosinophil and platelet-neutrophil interactions is associated with distinct pathological entities. Still, both types of interactions are governed by similar types of mechanisms involving adhesion molecules (selectin P, glycoprotein IIb/IIIa), and lipid mediators among which platelet-derived TXA2 seems to be the major player, while PAF and cysteinyl leukotrienes play adjunctive role, that is more pronounced in platelet-neutrophil then in platelet-eosinophil interactions. It is becoming increasingly clear how platelet-neutrophil interactions amplify inflammatory response (1,45). It remains to be determined how platelet-eosinophil interactions contribute to atopic diseases or host defence against parasites.
Acknowledgments: We would like to thank Mrs Jolanta Reyman for her excellent technical assistance. This work was supported by the Polish Ministry of Science and Information Society Technologies (MNiI) (grants No 2 P05A 084 26 and P05A 003 25). Associate Professor Stefan Chlopicki is the recipient of a Professorial grant from the Foundation for Polish Science (SP/04/04).
- Faint RW. Platelet-neutrophil interactions: their significance. Blood Rev 1992;6:83-91.
- Geng JG, Chen M, Chou KC. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr Med Chem 2004;11:2153-2160.
- Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem 1995;64:113-139.
- Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest 1996;97:1525-1534.
- Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med 1991;174:785-790.
- von HP, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 2001;103:1772-1777.
- Nagata K, Tsuji T, Todoroki N, Katagiri Y, Tanoue K, Yamazaki H, Hanai N, Irimura T. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62). J Immunol 1993;151:3267-3273.
- Chlopicki S, Olszanecki R, Janiszewski M, Laurindo FR, Panz T, Miedzobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxid Redox Signal 2004;6:691-698.
- Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol 1999;106:391-399.
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003;9:61-67.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002;8:1227-1234.
- Song C, Suzuki S, Kubo H, Chida M, Hoshikawa Y, Tabata T, Kondo T. Effects of antiplatelet agents on pulmonary haemodynamic response to fMLP in endotoxin primed rats. Thorax 2004;59:39-44.
- Bevilacqua MP, Nelson RM. Selectins. J Clin Invest 1993;91:379-387.
- Brown KK, Henson PM, Maclouf J, Moyle M, Ely JA, Worthen GS. Neutrophil-platelet adhesion: relative roles of platelet P-selectin and neutrophil beta2 (DC18) integrins. Am J Respir Cell Mol Biol 1998;18:100-110.
- Evangelista V, Manarini S, Rotondo S, Martelli N, Polischuk R, McGregor JL, de GG, Cerletti C. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the beta 2 integrin CD11b/CD18. Blood 1996;88:4183-4194.
- Spangenberg P, Redlich H, Bergmann I, Losche W, Gotzrath M, Kehrel B. The platelet glycoprotein IIb/IIIa complex is involved in the adhesion of activated platelets to leukocytes. Thromb Haemost 1993;70:514-521.
- Chlopicki S, Lomnicka M, Gryglewski RJ. Obligatory role of lipid mediators in platelet-neutrophil adhesion. Thromb Res 2003;110:287-292.
- Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy 2005;60:13-22.
- Jawien J. A new insight into aspirin-induced asthma. Eur J Clin Invest 2002;32:134-138.
- Jawien J, Chlopicki S, Gryglewski RJ. Interactions between human platelets and eosinophils are mediated by selectin-P. Pol J Pharmacol 2002;54:157-160.
- Jungi TW, Spycher MO, Nydegger UE, Barandun S. Platelet-leukocyte interaction: selective binding of thrombin-stimulated platelets to human monocytes, polymorphonuclear leukocytes, and related cell lines. Blood 1986;67:629-636.
- Hansel TT, De V, I, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods 1991;145:105-110.
- Radomski M, Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res 1983;30:383-389.
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77-89.
- Yazdanbakhsh M, Eckmann CM, Koenderman L, Verhoeven AJ, Roos D. Eosinophils do respond to fMLP. Blood 1987;70:379-383.
- Teixeira MM, Hellewell PG. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br J Pharmacol 1997;120:1059-1066.
- Aristides M, Gliksman M, Rajan N, Davey P. Effectiveness and cost effectiveness of single bolus treatment with abciximab (Reo Pro) in preventing restenosis following percutaneous transluminal coronary angioplasty in high risk patients. Heart 1998;79:12-17.
- de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood 1992;80:134-142.
- Ulfman LH, Joosten DP, van Aalst CW, Lammers JW, van de Graaf EA, Koenderman L, Zwaginga JJ. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol 2003;28:512-519.
- Jirillo E, Miragliotta G, Fumarola D. Effects of bacterial lipopolysaccharides (LPS) on leukocytes and platelets. Recent views on the pathophysiological role of LPS in the host. Boll Ist Sieroter Milan 1982;61:285-293.
- Castro-Faria-Neto HC, Penido CM, Larangeira AP, Silva AR, Bozza PT. A role for lymphocytes and cytokines on the eosinophil migration induced by LPS. Mem Inst Oswaldo Cruz 1997;92 Suppl 2:197-200.
- Dembinska-Kiec A, Zmuda A, Wenhrynowicz O, Stachura J, Peskar BA, Gryglewski RJ. Selectin-P-mediated adherence of platelets to neutrophils is regulated by prostanoids and nitric oxide. Int J Tissue React 1993;15:55-64.
- McCarty OJ, Tien N, Bochner BS, Konstantopoulos K. Exogenous eosinophil activation converts PSGL-1-dependent binding to CD18-dependent stable adhesion to platelets in shear flow. Am J Physiol Cell Physiol 2003;284:C1223-C1234.
- Yachie A, Toma T, Miyawaki T, Taniguchi N. Expression of surface CD11b antigen and eosinophil activation. Nippon Rinsho 1993;51:593-597.
- Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood 1996;88:146-157.
- Gryglewski RJ. Transcellular biosynthesis of eicosanoids. Pol J Pharmacol 1999;51:113-117.
- Bussolati B, Mariano F, Cignetti A, Guarini A, Cambi V, Foa R, Piccoli G, Camussi G. Platelet-activating factor synthesized by IL-12-stimulated polymorphonuclear neutrophils and NK cells mediates chemotaxis. J Immunol 1998;161:1493-1500.
- Nieuwenhuys CM, Feijge MA, Offermans RF, Kester AD, Hornstra G, Heemskerk JW. Modulation of rat platelet activation by vessel wall-derived prostaglandin and platelet-derived thromboxane: effects of dietary fish oil on thromboxane-prostaglandin balance. Atherosclerosis 2001;154:355-366.
- Nusing R, Lesch R, Ullrich V. Immunohistochemical localization of thromboxane synthase in human tissues. Eicosanoids 1990;3:53-58.
- Mita H, Ishii T, Akiyama K. Generation of thromboxane A2 from highly purified human sinus mast cells after immunological stimulation. Prostaglandins Leukot Essent Fatty Acids 1999;60:175-180.
- Jawien J, Olszanecki R, Lorkowska B, Korbut R. Effect of aspirin on cysteinyl leukotrienes production by eosinophils co-cultured with epithelial cells. J Physiol Pharmacol 2004;55:765-772.
- Jawien J, Chlopicki S, Olszanecki R, Lorkowska B, Gryglewski RJ. Eosinophil-epithelial cell interaction augments cysteinyl leukotrienes synthesis. J Physiol Pharmacol 2002;53:127-132.
- Page CP, Coyle AJ. The interaction between PAF, platelets and eosinophils in bronchial asthma. Eur Respir J Suppl 1989;6:483s-487s.
- Sala A, Buccellati C, Zarini S, Bolla M, Bonazzi A, Folco GC. The polymorphonuclear leukocyte: a cell tuned for transcellular biosynthesis of cys-leukotrienes. J Physiol Pharmacol 1997;48:665-673.
- Kazura JW. Platelet-neutrophil interaction: modulation of the inflammatory response. J Lab Clin Med 1989;114:469-470.