ASA-intolerance: eicosanoids and the arachidonacid-metabolism
In the last decade, knowledge concerning the pathophysiologic mechanisms underlying ASA-intolerance has been focussed in several studies. Evidence was found that the pathogenesis of Aspirin-intolerance is not an IgE-mediated reaction but is related to an abnormal metabolism of arachidonic acid implicating both the Lipoxygenase (LO) and the cyclooxygenase (CO)-pathways (4, 5). This deviation results in a dysbalance of the synthesis of both eicosanoids, leukotriens and prostaglandins. Anti-inflammatory prostaglandins, especially E2, decrease and the synthesis of cysteinyl-leukotriens like leukotrien-A4, -B4, -C4 and -D4 is increased (5, 6) (Fig. 1).
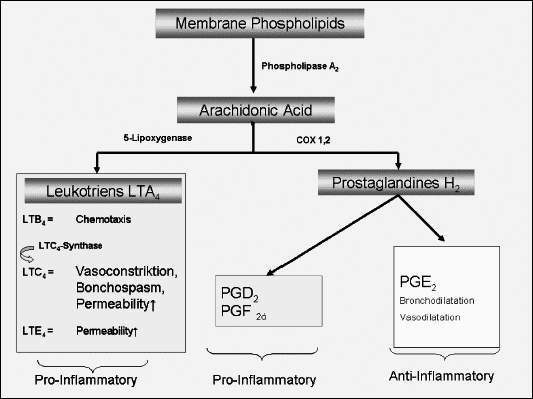 |
| Fig. 1. The exact mechanisms of aspirin intolerance as well as those of therapy by ASA-desensitization still remain unclear. Evidence has been worked out that ASA-intolerance is not an IgE-mediated reaction but is related to an abnormal metabolism of arachidonic acid implicating both the Lipoxygenase (LO) and the Cyclooxygenase (CO)-pathways. This deviation results in a dysbalance of the synthesis of the eicosanoids towards a predominance of the pro-inflammatory leukotriens (5, 6). |
Prevalence of ASA-intolerance
Aspirin intolerance is supposed to be underestimated: in a population of 500 patients with AIA studied in the European Network of Aspirin-Induced Asthma (AIANE), 18% were unaware of aspirin intolerance before having aspirin challenge tests (7). More interesting, when patients with AIA also suffered from concomitant rhinosinusitis even 34 % were unaware of their disease before testing (8, 9). Other data reveal incidence-rates from 0.6 % to 2.5% and in adult astmatics even from 4,3 % to 11% (8).
Clinical signs
In most cases sensitivity to ASA reveals a typical pattern: rhinitis often becomes the first clinical sign during the third decade, often after a viral respiratory infection. After some months concomittant chronic nasal congestion, hyposmia, chronic rhinorrhea as well as nasal polyps might reveal (8, 9). Finally, the disease results in AIA: 20% of the AIA-patients suffer from mild and intermittent asthma, 30% are moderate asthmatics who can be controlled with inhaled steroids whereas 50% of the patients have a chronic, severe corticoid-dependent asthma often accompanied by systemic anaphylactoid reactions (9).
Upper-airways: “key-area” of the ASA-intolerance
Rhinosinusitis was found to be a predominating symptom in 500 ASA-intolerant patients in a a multicenter survey of Szczeklik and Nizankowska (10). Interestingly, nasal symptoms revealed on an average age of 30 years, often as a result of a viral respiratory infection. In these cases the discharge of the nose was perennial and often watery. Hyposmic sensations were found in 55%. In average the first asthmatic symptoms appeared two years later.
Nasal polyps
In 70 % of ASA-intolerant patients nasal polyps can be found whereas in general population the overall prevalence of nasal polyps is only about 4 % (10, 11). Typical for the polyps in ASA-intolerant patients is their aggressive growth that involves all paranasal sinuses bilaterally (12). By means of CT-scans pansinus affection could be radiologically proven in almost all cases with AIA in an American study (9).
The pathogenetic link between ASA-intolerance and the origin of the nasal polyps still remains unclear: infectious agents like viruses, bacteria or fungi as potential primary factors might activate nasal epithelial cells and proinflammatory cytokines like eotaxin and growth factors and, therby, promote the inflammatory process (11, 13, 14). Further possible explanations might be differences in HLA genes (15), a reduced apoptosis-rate of local inflammation cells e.g. eosinophils (16) or a differential role of cyclooxygenase 1 and cyclooxygenase 2 having special regulator functions in the pathogenesis of nasal polyps (17).
Interestingly, the recurrence rate of nasal polyps after resection in ASA-intolerant patients is tremendously high: in a study of Jantii-Alanco et al. the recurrency rate is almost three times higher in AIA than in non-intolerant intrinsic asthmatics (18).
Diagnosis
Four typical findings in the patients history might correspond to intolerance to aspirin and other NSAIDs (5 - 7): i) typical symptoms of respiratory reactions after aspirin-challenge; ii) asthma attacks accompanied by chronic nasal congestion and watery, profuse rhinorrhoea; iii) high frequence of severe asthmatic attacks; iv) high frequence of nasal polyps.
So far, validated and reliable in-vitro tests are not available for the diagnosis of aspirin intolerance. Provocation testing still is the only validated diagnostic tool for aspirin sensitivity: nevertheless, challenge tests undoubtfully are harmful and should only should be carried out in specialised clinics fairly prepared for cases of anaphylactic reactions. Four types of aspirin-challenge can be performed depending on the manner of administration: oral, inhalative, nasal and intravenous (7).
In cases of predominant nasal symptoms nasal challenge test with aspirin might be performed as the method of choice because of its safety and its reliability (19). In case of a negative test result in nasal challenge testing but strong suspicions for AIA in the patients’ history, bronchial and/or oral challenge tests should follow (5, 7).
Therapy
Prevention of selective COX-1
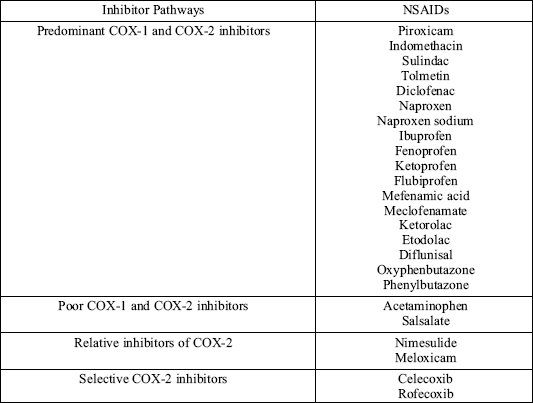
General rules concerning the treatment of AIA are related to the published guidelines on the management of asthma. Moreover, asthma-attacks in AIA often results in a severe, potentially life-threatening course. This underlines the importance of educating the patients in avoiding ASA and all other cross-reacting, non-selective COX-inhibitors (5, 6, Table 1). However, selective COX-2 might be used safely in the majority of aspirin-sensitive patients (20).
| Table 1. NSAIDs that cross-react with aspirin (5) |
 |
Surgery
Massive growth of polyps in the nasal and paranasal-sinuses are resected by endoscopical or microscopical techniques. However, the recurrence rate is quite high: in a prospective study of 227 patients operated on nasal polyposis between 1993 and 2001 a significant higher rate of recurrences has been revealed in the AIA-group compared to patients tolerant to ASA (21).
Aspirin desensitization
In 1976, Zeiss and Lockey described the paradoxical finding that ASA-intolerant patients revealed a 3-day refractory period after oral aspirin challenge (22). This report marked a new therapeutic option in the management of aspirin-sensitive patients: Could it be possible to treat the inflammatory airway-process with exactly the same medicament which undoubfully caused the symptoms? Based on these findings, various desensitization protocols and routes of administration have been elaborated in the last two decades: bronchial, endonasal, oral and, recently published, intravenous route.
i) bronchial administration
This route of administration has been developed on the premise that refractory tolerance could be achieved by repeated provocation with lysine-aspirin inhalation (23).
ii) endonasal administration
In case of predominating nasal-symtoms like rhino-nasal polys this route of administration has mainly shown efficacy. In a study of Patriarca et al. 43 patients suffering from nasal polyposis intranasal desensitization was performed with increasing doses of lysine acetylsalicylate (LAS) corresponding to 20, 200, and 2000 micrograms of aspirin until a maximum dose of 2000 micrograms weekly was reached (24). In a 5-year follow up, recurrence rates of nasal polyps were significantly lower in the aspirin-treated group compared to the control group. Recently, the first randomized, double blind, placebo controlled, cross-over trial of topical desensitization with a low-dose (16 mg) intranasal administation in 11 aspirin-sensitive nasal polyp patients has been reported (25). This study revealed only a poor clinical effect by the endonasal desensitization therapy but significant improvement at the microscopic level.
iii) oral administration
Stevenson et al. were the first to describe two cases of AIA-patients who underwent an initial desensitization with incremental doses of aspirin followed by daily therapy (26). Interestingly, both patients revealed an improvement of the aspirin-sensitive asthma as well as a reduction of the nasal polyps. Only four years later the same group published the first randomized, double-blind, placebo controlled crossover trial of aspirin desensitization in 25 patients with aspirin sensitive asthma with a daily dosage between 325 and 1300 mg over a three-month period (27). This survey could demonstrate both a significant improvement of rhino-nasal symptoms as well as a reduced need of nasal corticosteroids of the ASA-treated group compared to placebo. Nevertheless, only half of the patients experienced an improvement of their asthma-symptoms.
107 patients with diagnosed aspirin-sensitivity with both rhinosinusitis-symptomas and AIA were involved in a retrospective survey published 6 years later (28). In this study, 65 patients were treated by aspirin-desensitization whereas a control-group of 42 patients avoided all NSAID. Taken together, these data clearly demonstrate the clinical benefit of the desensitization therapy to aspirin-sensitive patients concerning a reduction in the number of hospitalizations and emergency room visits, upper airway tract infections and sinus operations as well as an improvement in olfactory sense. Interestingly, 20% of the 65 patients of the therapy group reported gastritic complaints.
Between 1988 and 1994 a long term follow-up study on aspirin-desensitization with a daily peroral dose of 1300 mg has been inaugurated (29). The outstanding importance of this study was the finding that aspirin-desensitization in fact reduces the aggressive growth and recurrence rate of sinunasal polyps in aspirin-sensitive patients over a longer time period. The necessity for a sinunasal surgery declined from one operation per 3 years to one operation per 9 years. However, the number of emergency room visits and need of inhalative corticosteroids remained unchanged in this study.
A subsequent study on 172 patients with a similar study design demonstrated a reduction of purulent-sinusitis rate from an average of 5 times per year before therapy to less than half of that (30). Interestingly, a clinical response to treatment rate of 67% was evident as early as 6 months which indicates a relative early therapeutic effect of the desensitization therapy. Furthermore, these results persisted for 1 to 5 years.
On the other hand, 9% discontinued the long-term therapy due to gastritic symptoms which was lower compared to the data of the same group from 1996 (29). The authors related these findings to the use of misoprostol and proton pump inhibitors, both of which became available in recent years.
iv) i.v. administration
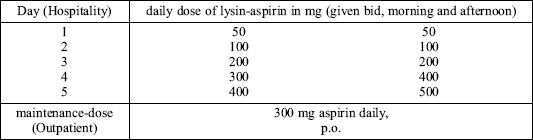
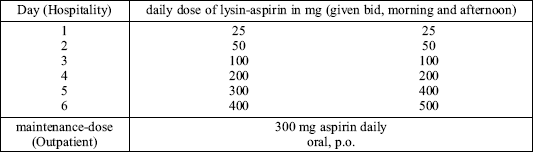
In a recently published study possible side effects by using the intravenous route for ASA desensitization were studied (31). 36 patients with a clear positive-history of ASA-intolerance, (recurrent) sinunasal polyps and a positive test-result using peripheral white blood cells (32) were treated by ascending doses of intravenous lysine-aspirin under hospital conditions (Table 2a and Table 2b). The total amount of systemic reactions observed was n = 27 or 6.6% of all therapeutic doses. Of all systemic reactions, 81% were 1st degree reactions and 19% 2nd degree reactions. Systemic reactions of the 3rd or 4th degree have not been revealed.
| Table 2a. Adaptive desensitization by i.v. application on patients with low-grade-intolerance (31) |
 |
| Table 2b. Adaptive desensitization by i.v. application on patients with high-grade-intolerance (31) |
 |
Taken together, this survey indicated that the intravenous route for the initial ascending phase of ASA desensitization might be a safe procedure without severe complications comparable to the peroral route. In contrast to the peroral application, the intravenous procedure might have the advantage of interrupting the ASA application by stopping the infusion therapy in case of (beginning) systemic reactions. Undoubtfully, more studies are necessary to investigate the intravenous application concerning its safety aspects and comparability to the peroral application in the early phase of therapy.
- Hirschberg VG. Anaphylactoid reaction to aspirin (1902) (classical article). Allergy Proc 1990; 11: 249-252.
- Widal MF, Abrami P, Lenmoyez J. Anaphylaxie et idiosyncrasie. Presse Med 1922; 30: 189-192.
- Samter M, Beers RF. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med 1968; 68: 975-983.
- Szczeklik A, Stevenson DD. Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol 2003; 111: 913-921.
- Babu KS, Salvi S. Aspirin and Asthma. Chest 2000; 118: 1470-1478.
- Pfaar O, Klimek L. Aspirin desensitization in aspirin intolerance: update on current standards and recent improvements. Curr Opin Allerg Clin Immunol 2006; 6: 161-166.
- Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J 2000; 16(3): 432-436.
- Szczeklik A, Sanak M, Niz E, Kielbasa B. Aspirin intolerance and the cyclooxygenase–leukotriene pathways. Curr Opin Pulm Med 2004; 10: 51-56.
- Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 2002; 89(5): 474-478.
- Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax 2000; 55 (Suppl 2): 42-44.
- Bachert C, Watelet JB, Gevaert P, Van Cauwenberge P. Pharmacological management of nasal polyposis. Drugs 2005; 65(11): 1537-1552.
- Picado C, Mullol J. The nose in aspirin-sensitive asthma. In Eicosanois, aspirin and asthma. A Szczeklik, RJ Gryglewski, J Vane (eds). New York, Marcel Dekker 1998 pp. 493-505.
- Pawliczak R, Lewandowska-Polak A, Kowalski ML. Pathogenesis of nasal polyps: an update. Curr Allergy Asthma Rep 2005; (6): 463-471.
- Min JW, Jang AS, Park SM, et al. Comparison of plasma eotaxin family level in aspirin-induced and aspirin-tolerant asthma patients. Chest 2005; 128(5): 3127-3132.
- Molnar-Gabor E, Endreffy E, Rozsasi A: HLA-DRB1, -DQA1, and -DQB1 genotypes in patients with nasal polyposis. Laryngoscope 2000; 110(3 Pt 1): 422-425.
- Kowalski ML, Grzegorczyk J, Pawliczak R, Kornatowski T, Wagrowska-Danilewicz M, Danilewicz M. Decreased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivity. Allergy 2002; 57(6): 493-500.
- Gosepath J, Brieger J, Mann WJ. New immunohistologic findings on the differential role of cyclooxygenase 1 and cyclooxygenase 2 in nasal polyposis. Am J Rhinol 2005; 19(2): 111-116.
- Jantii-Alanco S, Holopainen E, Malmberg H. Recurrence of nasal polyps after surgical treatment. Rhinology 1989; 8: 59-64.
- Milewski M, Mastalerz L, Nizankowska E. Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol 1998; 101: 581-586.
- Stevenson DD, Simon RA. Lack of cross reactivity between Rofecoxib and aspirin-sensitive patients with asthma. J Allergy Clin Immunol 2001; 108: 47-51.
- Albu S, Tomescu E, Mexca Z, Nistor S, Necula S, Cozlean A. Recurrence rates in endonasal surgery for polyposis. Acta Otorhinolaryngol Belg 2004; 58(1): 79-86.
- Zeiss CR, Lockey RF. Refractory period to aspirin in a patient with aspirin-induced asthma. J Allergy Clin Immunol 1976; 57(5): 440-448.
- Schmitz-Schumann M, Schaub E, Virchow C. Inhalation provocation test with lysine-acetylsalicylic acid in patients with analgetics-induced asthma. Prax Klin Pneumol 1982; 36(1): 17-21.
- Patriarca G, Schiavino D, Nucera E, Papa G, Schinco G, Fais G. Prevention of relapse in nasal polyposis. Lancet 1991; 337(8755):1488.
- Parikh AA, Scadding GK. Intranasal lysine-aspirin in aspirin-sensitive nasal polyposis: a controlled trial. Laryngoscope 2005; 115(8): 1385-1390.
- Stevenson DD, Simon RA, Mathison DA. Aspirin-sensitive asthma: tolerance to aspirin after positive oral aspirin challenges. J Allergy Clin Immunol 1980; 66: 82-88.
- Stevenson DD, Pleskow WW, Simon RA, Mathieson DA, Lumry WR, Schatz M. Aspirin-sensitive rhinosinusitis asthma: a double blind cross over study of treatment with aspirin. J Allergy Clin Immunol 1984; 73: 500-507.
- Sweet J, Stevenson DD, Simon RA, Mathison DA. Long-term effects of aspirin desensitization - treatment for aspirin-sensitive rhinosinusitis-asthma. J Allergy Clin Immunol 1990; 85: 59-65.
- Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol 1996; 98(4): 751-758.
- Berges-Gimeno P, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2003; 111: 180-186.
- Pfaar O, Spielhaupter M, Wrede H, et al. Aspirin desensitization on patients with aspirin intolerance and nasal polyps – a new therapeutic approach by the intravenous route. Allergologie 2006; 8: 322-331.
- Schaefer D, Lindenthal U, Wagner M, Bolcskei PL, Baenkler HW. Effect of prostaglandin E2 on eicosanoid release by human bronchial biopsy specimens from normal and inflamed mucosa. Thorax 1996; 51 (9): 919-923.