Rats (n=35) received 0.1% iodoacetamide (IA) in drinking water for the 5 consecutive days (1). Slow wave recordings were performed before iodoacetamide treatment and on the 6th day in the same group of rats (n=15). The vagal nerve recordings were performed according to the same protocol but in different groups: control (n=10) and iodoacetamide pretreated group (n=10). The control group received 0.9 % saline injection according to the same protocol. This study was approved by the local Ethics Committee of Jagiellonian University Medical College.
Myoelectric activity
A single pair of unipolar silver electrodes were implanted on the distal stomach and proximal duodenum and tunneled on the neck of the rat. After surgery rats were allowed to recovery for 5 days. The slow waves were captured from the conscious, 14 hours fasted rats. The electrodes were connected to the amplifier and captured signal was filtered with the digital band pass filter 0,01-1Hz. The modified FFT (Fast Fourier Transform) spectrum analysis of 1 hour recordings for each animal was performed. The accurate dominant frequencies were read off from the FFT spectra and interpreted respectively as the gastric or the duodenal basic electrical rhythm (4).
Vagal nerve recording method
Vagal recordings were performed in anaesthetized fasted rats. The cuff electrode was placed on isolated afferent cut end of the cervical left vagus nerve in control and iodoacetamide treated rats. Recordings started 15 minutes after the cuff electrode placement and lasted 1 hour. Mass vagal discharges were amplified, recorded on hard disc then filtrated and analyzed with mathematical tools (Power Lab) (5).
Histological studies
In all experimental groups after performed recordings rats were sacrificed and stomach was taken for histological analysis. The paraffin embedded sections were prepared and the specimens were stained with hematoxilin – eosin for the routine microscopy and with toluidine blue for the mast cell evaluation. The mast cell number was assessed in 10 fields with magnification 200x.
Statistics
Statistical analyses were performed using a paired and unpaired t-test and values of p<0.05 were regarded as significant. Data were presented as the mean ± standard deviation.
Vagal activity and gastro-duodenal slow wave
The vagal afferent activity was significantly increased after iodoacetamide treatment from the baseline value of 0.4 ± 0.1 to 1.9 ± 0.58 Hz (p<0.05) (Fig. 1). The gastric slow wave frequency accelerated from the baseline value of 0.08 ± 0.01 to 0.1 ± 0.02 Hz (p<0.05). The duodenal dominant slow wave frequency remained unchanged (from the baseline value of 0.64 ± 0.02 to 0.59 ± 0.1 Hz). Fig. 2 and 3 present values of dominant frequencies extracted from FFT spectrum either vagal afferents activity or the slow wave respectively. The rat ECG (about 5 Hz) and respiration rate (about 1 Hz) during experiment showed no relationship with the slow wave frequency (Fig. 4).
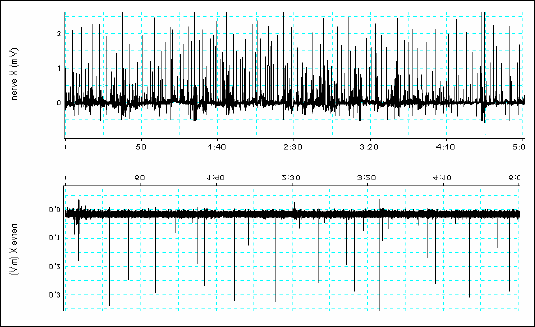 |
| Fig.1. The vagal afferents discharge in response to the gastric mucosa iodoacetamide irritation pretreatment versus control vagal afferents discharge (5 min recording) |
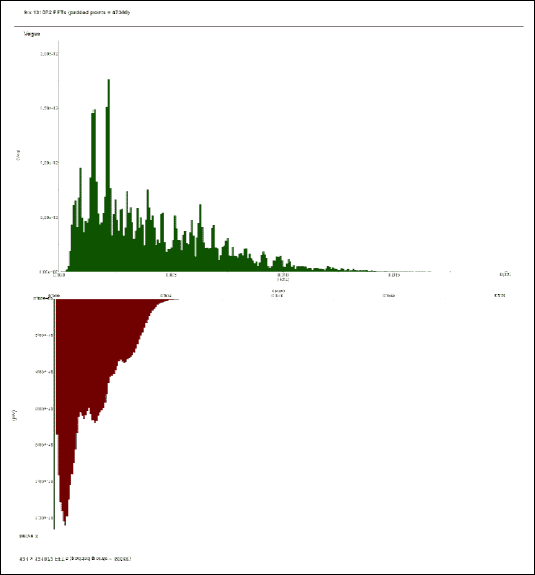 |
| Fig.2. The FFT spectra of vagal nerve recording in response to the iodoacetamide treatment versus control |
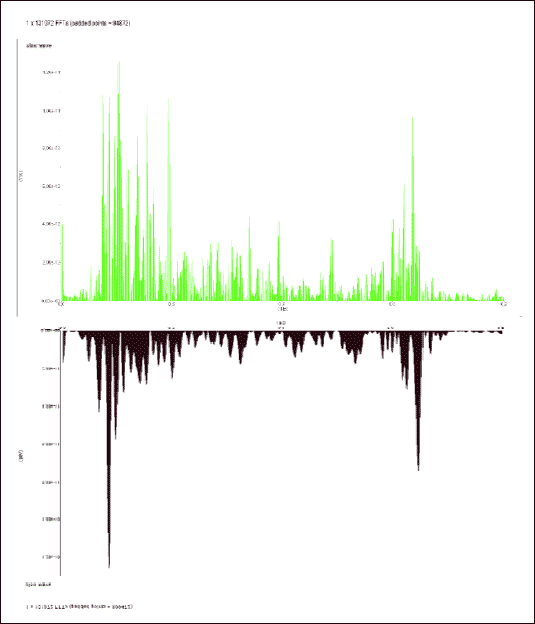 |
| Fig.3. The FFT spectra of the slow wave recording in response to the iodoacetamide treatment versus control |
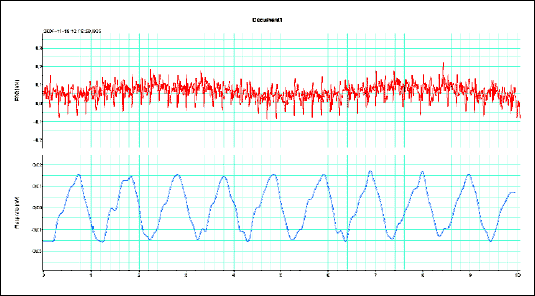 |
| Fig.4. The rat ECG (about 5 Hz) and respiration rate (about 1 Hz) during the experiment showed no relationship with the gastrointestinal slow wave frequencies. |
Histological changes
No macroscopic lesions were observed in the stomach of iodoacetamide treated rats. Microscopically iodoacetamide irritated gastric mucosa and caused the minimal inflammatory changes with the moderate thickening of the submucosal layer, the predominant submucosal mast cells infiltration, containing also some neutrophils and lymphocytes (Fig. 5). Those changes were especially visible in the antrum in multiple sections. No histological changes associated to iodoacetamide treatment were observed in other layers of the gastric wall.
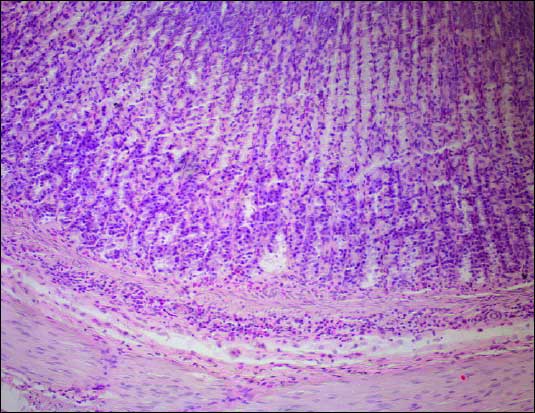 |
| Fig.5. The gastric mucosa. Mild inflammatory infiltrations in the mucosa and the submucosa. Hematoxilin - eosin staining. Magnification 200x |
The total mast cell number in the gastric wall increased in IA group (208.4 ± 53.6) in compare to control (156.6 ± 42.2) and was statistically significant (p<0.05) (Fig. 6). Mast cells were predominantly localized in the submucosa and were partially degranulated (Fig. 7).
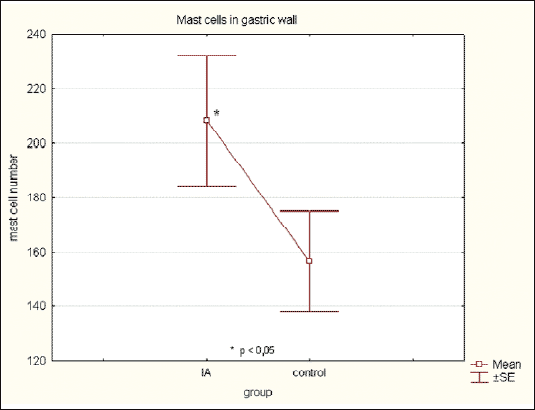 |
| Fig.6. The mast cells number in the gastric wall in control and iodoacetamide treated rats. (p<0.05) |
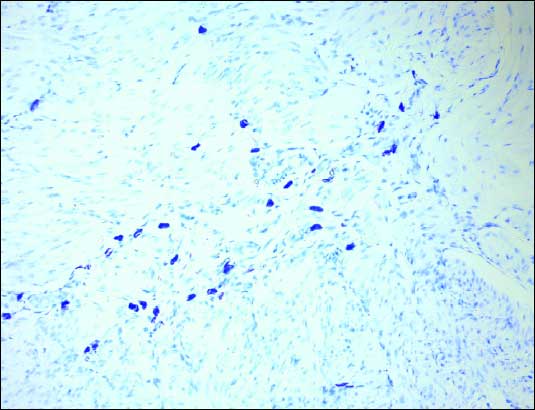 |
| Fig.7. The mast cells (pink – blue cells) in gastric wall. Toluidine blue staining. Magnification 200x |
Usefulness of iodoacetamide in inducing gastric hyperalgesia in rats has been reported by Ozaki et al. (1). IA is a sulfhydryl blocker and reduction of this substance in gastric mucosa affects its integrity and causes oxidative damage. IA treatment increased mucosal PGE2 content with apparent expression of COX-2 mRNA in the stomach (6). The inflammatory cytokines generated during this process are believed to sensitize the primary afferent fibers and the complex chain reaction leads to prolonged hyperalgesia and visceral hypersensitivity (2). IA model of the gastritis has the neurogenic inflammation character and involves capsaicin-sensitive afferent nerves (7). We used the vagal afferent discharge as the marker of visceral hypersensitivity. Our results showed the increased vagal afferent discharge in response to the IA treatment. Similarly, previous studies on gastric ulcers reported increased the resting vagal discharge and enhanced response to the gastric distension explained by the sensitization in inflamed mucosa (8). Recent studies provided evidence that the local gut inflammation activates vagal sensory neurons (9). This finding correlates with the enhanced visceromotor response to the gastric distension observed in different models of gastric insult (10). The particular cytokines (for example IL-1 or PAF) were found to be a very potent activator of vagal afferents (11, 12). This suggests an important role of vagal nerve in conveying inflammatory information from the gut. Going into details the most abdominal vagal afferents conduct the sensory information in the C-fiber range (13). Capsaicin deaferentation was shown to alleviate inflammatory parameters in experimental IA induced gastritis (7). C-fibers were suggested as crucial in the process of inflammatory hyperalgesia (14). In consequence, inflammation induced changes of the visceral sensation can elicit dyspeptic symptoms (15). We provide the first description of slow wave disturbances by the IA induced mucosal inflammation. This evidence may partially explain the gastric motility disturbances as an effect of persistent inflammation running in mucosal layer. Our findings reinforce the idea that inflammatory mediators induce the excitability of vagal sensory neurons and may contribute to the functional dyspepsia (16). The link between the functional dyspepsia and the slow wave disorganization was established in different studies (17). In the study of Piqueras et al. IA increased the gastric acid secretion response but did not influence the gastric emptying in mice (18). Prior studies have demonstrated some gastric motility abnormalities in gastritis or in response to particular inflammatory mediators (3, 12). The inflammation caused by Helicobacter pylori may affect the proximal stomach motility but recent reports are inconsistent (19, 20). A group of researches: Kang and Bielefeldt have suggested that the stomach inflammation directly affects the gastric sensory and motor function. According to them the inflammation impairs vagovagal reflexes and in consequence changes the gastric motility (3). Thus afferent and efferent pathways both contribute to the development of the dyspeptic symptoms. The outcome of this activation may be the deterioration of the slow wave seen in our gastritis model. The slow wave generators - interstitial cells of Cajal have been found to be active in the mechanosensitive function. Stretching gastric muscles fragments caused ICC membrane depolarization and increased the slow-wave frequency (21). In the inflammation induced hypersensitive state threshold for mechanoreceptors activation is decreased. This evidence provides a link between the hypersensitivity and the slow wave deterioration in the gastritis. In conclusion, we have demonstrated that mild gastric mucosa irritation sensitizes the vagal afferents and alters the gastric but not duodenal pacemaker activity which may contribute to the dyspeptic sensations.
Conflicts of interest statement: None declared.
- Ozaki N, Bielefeldt K, Sengupta JN, Gebhart GF. Models of gastric hyperalgesia in the rat. Am J Physiol Gastrointest Liver Physiol 2002; 283: G666-G676.
- Bueno L, Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol 1999; Suppl A: 42A-46A.
- Kang YM, Lamb K, Gebhart GF, Bielefeldt K. Experimentally induced ulcers and gastric sensory-motor function in rats. Am J Physiol Gastrointest Liver Physiol 2005; 288: G284-G291.
- Krolczyk G, Czupryna A, Sobocki J, et al. Effect of bowel decontamination with metronidazole and vancomycin on gastro-duodenal myoelectric activity. Folia Med Cracov 2004; 45: 45-53.
- Krolczyk G, Zurowski D, Sobocki J, Laskiewicz J, Thor PJ. Encoding meal in integrated vagal afferent discharge. J Physiol Pharmacol 2004; 55(1 Pt 1): 99-106.
- Takeeda M, Hayashi Y, Yamato M, Murakami M, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase izoenzymes in mucosal defense of inflamed rat stomach. J Physiol Pharmacol 2004; 55(1 Pt 2): 193-205.
- Larauche M, Anton PM, Peiro G, Eutamene H, Bueno L, Fioramonti J. Role of capsaicin-sensitive afferent nerves in different models of gastric inflammation in rats. Auton Neurosci 2004; 110: 89-97.
- Kang YM, Bielefeldt K, Gebhart GF. Sensitization of mechanosensitive gastric vagal afferent fibers in the rat by thermal and chemical stimuli and gastric ulcers. J Neurophysiol 2004; 91: 1981-1989.
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 2005; 19: 334-344.
- Gebhart GF, Bielefeldt K, Ozaki N. Gastric hyperalgesia and changes in voltage gated sodium channel function in the rat. Gut 2002; 51 Suppl 1: 15-18.
- Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci 1998; 18: 9471-9479.
- Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol 1999; 82: 2210-2220.
- Bielefeldt K, Zhong F, Koerber HR, Davis BM. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol 2006; 291: G987-G997.
- Katz EJ, Gold MS. Inflammatory hyperalgesia: a role for the C-fiber sensory neuron cell body?. Pain 2006; 7: 170-178.
- Kang YM, Bielefeldt K, Gebhart GF. Sensitization of mechanosensitive gastric vagal afferent fibers in the rat by thermal and chemical stimuli and gastric ulcers. J Neurophysiol 2004; 91: 1981-1989.
- Choung RS, Talley NJ. Novel mechanisms in functional dyspepsia. World J Gastroenterol 2006; 12: 673-677.
- Zhang H, Xu X, Wang Z, Li C, Ke M.Correlation between gastric myoelectrical activity recorded by multi-channel electrogastrography and gastric emptying in patients with functional dyspepsia. Scand J Gastroenterol 2006; 41: 797-804.
- Piqueras L, Corpa JM, Martinez J, Martinez V. Gastric hypersecretion associated to iodoacetamide-induced mild gastritis in mice. Naunyn Schmiedebergs Arch Pharmacol 2003; 367: 140-150.
- Van der Schaar PJ, Straathof JW, Veenendaal RA, Lamers CB, Masclee AA. Does Helicobacter pylori gastritis affect motor function of proximal stomach in dyspeptic patients? Dig Dis Sci 2001; 46: 1833-1838.
- Riezzo G, Chiloiro M, Russo F, et al. Gastric electrical activity and gastrointestinal hormones in dyspeptic patients. Digestion 2001; 63: 20-29.
- Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA 2005; 102: 14913-1498.