The effect of obesity on sleep is particularly significant in OSAS patients, who, in the majority, have an abnormally high BMI (10). While in untreated patients, the effect of obesity is overshadowed by strong sleep disordering effect of respiratory events, it could be more visible in patients with apneas and hypopneas successfully abolished by CPAP. Therefore, the aim of this work was to assess the effect of obesity on sleep quality in OSAS patients before and during CPAP therapy.
This study was performed according to accepted practice concerning safety and ethics of human experimentation as set by the Declaration of Helsinki of 1975 For Human Research. The study protocol was approved by a local Ethics Committee.
Patients
We performed the retrospective analysis of clinical data from male OSAS patients, who were treated with CPAP at the Sleep Center in the Kuchwald Hospital (Chemnitz, Germany). The selected patients were divided into non-obese, obese and severely obese groups, based on their BMI. The groups were age-matched. Other inclusion criteria were the age over 18 and a successful breathing normalization under CPAP, with the amount of the respiratory events (apneas, hypopneas) per hour of total sleep time (Apnea Hypopnea Index, AHI) less than 10. Finally, only patients who at the beginning of therapy showed a CPAP use (CPAP compliance) greater than three hours per night (recorded by the CPAP-built-in-counter) were included. The exclusion criterion was the presence of other, significantly sleep co-disturbing disorders, such as periodic limb movement in sleep or narcolepsy. Excluded were also patients with underweight (BMI<18 kg/m2).
The diagnosis of OSAS was made on the basis of the presence of daily or nocturnal symptoms and the documentation of AHI>5 (11). The assessment of weight and inclusion to the proper group was done according to the guidelines of the National Institutes of Health; with non-obesity defined as BMI<25 kg/m2, obesity ranging from BMI 30 to 40 kg/m2, and severe obesity ranging from BMI 40 kg/m2 upward (12).
Diagnostics and therapy
All reviewed patients received the diagnostic polysomnography, which together with the clinical symptoms fulfilled the criteria of OSAS and warranted application of CPAP therapy. The level of the daytime sleepiness was assessed before the diagnostic polysomnography with the German version of the Epworth Sleepiness Scale (ESS) (13). The CPAP pressure was titrated manually during two nights following the diagnostic polysomnography. The control polysomnography (with CPAP-retitration if needed, therapy compliance assessment and general medical examination) was performed three months later. All recordings were performed using 16-channel polygraphs. Electroencephalogram, electrooculogram and the mentalis EMG were obtained according to standard protocols. Air flow was monitored with nasal prongs and the respiratory effort with the inductance bands, placed around thorax and abdomen. The nocturnal oxygen saturation was measured with a finger oximeter. Finally the motor leg activity was monitored with tibialis anterior EMG recorded with surface electrodes. All recordings were performed in sounds attenuated rooms, with temperature and light control.
Analysis of the polysomnographic recordings
We analyzed three polysomnographic recordings from every selected patient. The first one was the diagnostic night (Baseline), the next was the second night under CPAP (2nd CPAP), and the third one was the control polysomnography (Control). Every polysomnographic recording was scored manually for sleep stages and respiratory events using standard criteria (11, 14). The motor activity of the lower limbs was highlighted if the scorer noted its sleep disturbing effect. The polysomnographic parameters analyzed in this work included AHI, minimum oxygen saturation (min SaO2), defined as the lowest oxygen saturation during time from lights out to lights on during time-in-bed (TIB), sleep efficiency (SE), defined as the ratio of the time spent asleep to TIB, and REM%, S1%, S2%, SWS%, defined as the ratio of the time spent in a given sleep stage to TIB.
Comparisons between groups
The non-obese group was compared with the obese one, and with the severely obese group. The two latter groups were also compared between themselves. The comparisons were made for the mentioned polysomnographic parameters from Baseline, 2nd CPAP and Control nights, and ESS.
Comparisons within groups
Within every group the polysomnographic parameters from the particular nights were compared. The baseline night was compared with 2nd CPAP and with Control. The two nights with CPAP were also compared between themselves.
Statistical analysis
The Mann Whitney U test for unrelated samples was used to assess differences among the groups. Comparisons within groups were made with the Wilcoxon test for related samples. The statistical analyses were done with the SPSS statistical software (version 15.0, SPSS, Chicago, IL). The statistical confidence level for all analyses was set at P<0.05.
Demographic and clinical data
13 non-obese, 13 obese, and 12 severely obese patients fulfilling the inclusion criteria were selected from the database. The mean age was 52.3 ±9.4, 53.2 ±9.5, and 52.2 ±8.5 years, respectively, with no significant differences between the groups. The mean BMI of the non-obese subjects was lower than those of the other two groups (26.3±1.0 vs. 33.9 ±3.2 kg/m2, P<0.01, and vs. 44.2 ± 3.3 kg/m2; P<0.01). The average BMI of the two obese groups were also significantly different (P<0.01). The ESS score (non-obese - 9.6 ±3.3, obese - 9.8 ±5.1, and severely obese - 10.8 ±5.0), and the CPAP compliance (non-obese 6.1 ±1.3, obese 6.3 ±1.2, and severely obese 6.3 ±1.3 hours/night) did not differ between the groups.
Comparison of polysomnography between groups
Respiratory parameters. At baseline, obese and severely obese patients showed an increased AHI, when compared with the non-obese (73.7 ±17.4 and 83.6 ±28.1 vs. 27.7 ±20.3; P<0.01 for both). At the 2nd CPAP and at the control night, the AHI did not differ between the groups (Fig. 1). In concordance with the severity of the respiratory events, the min SaO2 at baseline was lower in the severely obese and obese groups than in the non-obese (68.1 ±8.3 and 74.4 ±9.5 vs. 83.2 ±6.9 %; P<0.01 and P<0.02, respectively), but there was also a significant difference between both obese groups (P<0.05) (Fig. 2). At the 2nd CPAP night, the min SaO2 was still lower in the severely obese and obese than in the non-obese patients (87.2 ±2.5 and 87.4 ±2.4 vs. 90.7 ±2.1%; P<0.01 for both). The only difference at the control night was found between the obese and non-obese (88.5 ±3.2 vs. 91.5 ±2.3%; P<0.02) (Fig. 2).
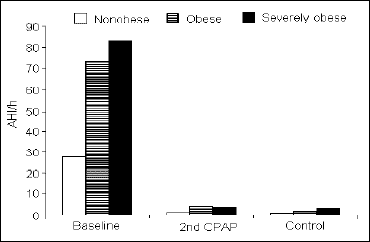 |
Fig. 1. Apnea-hypopnea index (AHI), expressed as the number of the respiratory events per hour of time-in-bed, during consecutive study nights in the three groups of subjects studied. |
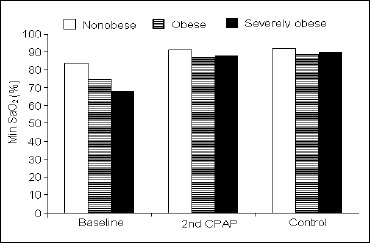 |
Fig. 2. Minimum SaO2 during time-in-bed during the consecutive nights in the three groups of subjects studied. |
Sleep efficiency and sleep stages. Comparison of sleep efficiency revealed no differences between the groups at any recorded night (Table 1). The light sleep (1NREM) prevailed at baseline in the severely obese and obese groups (35.3 ±18.0 and 33.3 ±13.1 vs. 21.1 ±10.4%; P<0.04 and P<0.02, respectively), while at the 2nd CPAP it was less in the severely obese than in the obese and non-obese (7.2 ±3.5 vs. 12.5 ±8.4 and 13.9 ±7.6%; P<0.04 and P<0.01, respectively). Finally, after three months, the 1NREM% was approximately equal in all three groups (Fig. 3). The amount of 2NREM stage did not differ between the groups at any night (Table 1). The differences in the amount of SWS were found only between the non-obese and severely obese groups, with the latter presenting less SWS at baseline (7.6 ±11.1 vs. 14.5 ±9.8%; P<0.03). At the 2nd CPAP night, the overall increase in SWS was far greater in the severely obese than in the nonobese resulting in a significant difference in SWS% in favor of the severely obese group (33.1 ±11.0 vs. 23.5 ±9.0%; P<0.05). At the control night, no differences in SWS% were observed (Fig. 4). Finally, the differences in REM% between the groups showed similar pattern like that in SWS%: at baseline the obese and the severely obese presented less REM sleep than the non-obese (5.3 ±4.5 and 5.2 ±5.8 vs. 8.5 ±4.0%; P<0.05 and P<0.04, respectively). This relation inverted at the 2nd CPAP night, with the REM sleep prevailing much more in the severely obese than in the non-obese (21.2 ±6.6 vs. 12.4 ±3.3%; P<0.01). At the control night, the groups did not differ significantly in REM% (Fig. 5).
Comparison of polysomnography within groups
Respiratory parameters. In each group, the AHI from the 2nd CPAP and from the control night were significantly lower, when compared with the baseline night. No differences were found between the 2nd CPAP and control nights (Fig. 1). The min SaO2 from the 2nd CPAP and from the control nights were significantly higher in each group, when compared with the baseline night. Unlike the AHI, there was a significant increase in min SaO2 from the 2nd CPAP to the control night in the severely obese group (87.2 ±2.5 vs. 89.3 ±3.0%; P<0.03) (Fig. 2).
Sleep efficiency and sleep stages. The only significant change in sleep efficiency across the investigated nights was the improvement from the baseline night to the 2nd CPAP night in the severely obese group (71.6 ±17.1 vs. 87.5 ±8.2%; P<0.01) (Table 1). In all groups, 1NREM% decreased from the baseline night to the 2nd CPAP and did not change significantly later on, to the control night (Fig. 3). All groups tended to have a decrease in 2NREM% during the 2nd CPAP night and an increase thereafter, but the statistical significance has been achieved only with respect to the increase in 2NREM% from the 2nd CPAP night to the control night in the severely obese group (26.0 ±8.0 vs. 32.6 ±6.5%; P<0.03) (Table 1). The SWS% increased significantly from baseline to the 2nd CPAP night in all groups. In the severely obese, it decreased then from the 2nd CPAP to the control night (33.1 ±11.0 vs. 23.4 ±9.3%; P<0.03) (Fig. 4). Similar pattern was observed in REM% with an overall increase in the 2nd CPAP and a decrease in the control night in the severely obese (21.2 ±6.6 vs. 15.6 ±6.9%; P<0.03) (Fig. 5).
| Table 1. Sleep efficiency and procentage of NREM2 during the consecutive nights. |
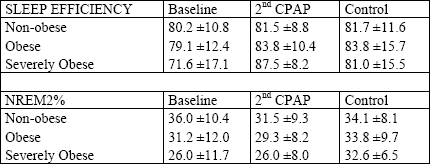 |
| All values are means ± SE. Sleep efficiency indicates the percentage of the time-in-bed (TIB) spent asleep and NREM2% indicates the percentage of TIB spent in NREM2. In sleep efficiency and NREM2% all differences between the groups were insignificant. Within the groups, there was a significant increase in sleep efficiency from baseline to 2nd CPAP (P<0.03) and in NREM2% from 2nd CPAP to the control night in the severely obese group (P<0.03) |
 |
Fig. 3. Percentage of 1NREM sleep during consecutive nights in the three groups of subjects studied. |
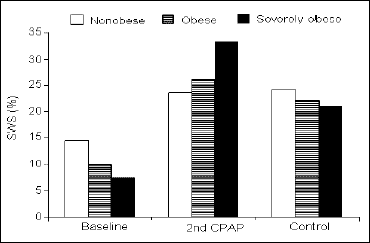 |
Fig. 4. Percentage of time-in-bed spent in SWS sleep during consecutive nights in the three groups of subjects studied. |
 |
Fig. 5. Percentage of time-in-bed (TIB) spent in REM sleep during consecutive nights in the three groups of subjects studied. |
This study documents the influence of obesity on sleep quality in male OSAS patients across the therapy course. Prior to CPAP, obese OSAS patients presented worse sleep quality than the non-obese. Directly after commencing the CPAP therapy, all OSAS patients experience a rebound of the SWS and REM sleep, which is strongly pronounced in the severely obese group. Finally, during the long-term CPAP therapy, obesity seems not to have a significant effect on the sleep quality in male OSAS patients. This finding is unexpected, since previous studies found that obesity decreases the sleep quality independently of the sleep related breathing disorders (4, 5).
Untreated OSAS
Before CPAP, the elevated light sleep, diminished REM, and deep sleep (SWS) result in worse sleep quality in obese and severely obese patients. The main cause of this effect is the greater number of apneas and hypopneas in both obese groups than in non-obese. The respiratory events are known to decrease the sleep quality and to alter its architecture through frequent awakenings and arousals (2, 15). A higher AHI in obese and severely obese patients confirms the previously known association of AHI and BMI in OSAS (16). Surprisingly, the differences between the obese and severely obese groups in BMI were not followed in the present study by the differences in AHI, SE, and sleep stages. This finding suggests that the association between body mass and severity of breathing disorders disappears, when the BMI reaches the extreme values (>40 kg/m2), or that it becomes weaker or not detectable in our relatively small sample. The study of Quera-Salva et al (16) did not report such finding. The BMI of the subjects in that study ranged, however, from 18.9 to 46.9 kg/m2, and consequently there might have been not enough severely obese patients for which this effect could be pronounced.
Acute CPAP effects (results from the 2nd CPAP night)
In all three groups, the polysomnographic recordings from the 2nd CPAP night showed normalization of AHI followed by a decrease of 1NREM% and an increase in REM% and SWS%, when compared with the baseline. These changes reflect a rebound phenomenon after beginning on CPAP, described previously by Fietze et al (2). In our study, the rebound phenomenon was more pronounced in the severely obese group than in both other groups; the severely obese group presented significantly more REM% and SWS% than the non-obese and significantly less S1% than both the obese and non-obese at the 2nd CPAP night. Moreover, the severely obese group was the only one to show an increase in sleep efficiency from baseline to 2nd CPAP. These findings seem to be associated primarily with a high BMI, since it was the only parameter which differentiated the severely obese group from the other two groups. The poor sleep quality and AHI prior to CPAP seem to influence the rebound magnitude to a lesser extent than the BMI, since the sleep quality and AHI prior to CPAP were similar in the obese and severely obese groups. This finding indicates that severe obesity can augment the sleep deprivating effect of apneas and hypopneas, e.g., by increasing their duration or by enhancing arousals due to increased respiratory effort. This hypothesis requires more detailed analysis of the respiratory events.
Effects of the long-term CPAP therapy (results from the control night)
After three months of CPAP therapy, the AHI and the sleep patterns showed no differences between the groups. These results are at variance with previous studies on obese subjects without SRBD mentioned above (4, 5). Three factors will be proposed to explain our results. The first one is a loud snoring in obese subjects without apneas and hypopneas. In the study of Resta et al (5), it was present in 46.7% of obese and only in 8.1% of the control non-obese subjects. Vgontzas et al (4) do not report the detailed snoring prevalence. The presence of snoring differentiates the subjects of Resta et al (5) and ours, who being under CPAP therapy expressed neither significant apneas and hypopneas nor significant snoring. Snoring is associated with decreased sleep efficiency and increased arousals, but its influence on sleep stages was confirmed up to now only in adolescents (17) and not in adults (18). Available data in adults include cohorts not well specified for the BMI and, therefore, give no exact information on the influence of snoring on sleep architecture in obese adult population. For this reason, it is possible that the abolition of snoring by CPAP is one of the explanations of the better sleep quality of our obese and severely obese patients than the patients of Resta et al (5). The second factor can be the sleep disordering effect of the CPAP-device, which by affecting all three groups can overshadow that of the obesity. Polysomnographic data on this topic are not available, but the subjective patients' reports on disordered sleep under CPAP are abundant. Typical complaints include nocturnal awakenings, facial pain, upper airway dryness, allergic rhinitis, and even skin lesions (19, 20). Although the mean compliance among our patients was high reflecting the good tolerance of the device, the changes in sleep architecture in all groups can not be excluded since no healthy control was investigated. The third factor is the relatively small sample size in our study. Vgontzas et al (4) and Resta et al (5) examined 73 and 78 obese vs. 45 and 40 non-obese subjects, respectively. Their groups oversized those in the present study three to six times and had much greater sensitivity concerning the potential effect of obesity on sleep.
CPAP failure in improving the minimum SaO2 in obese and severely obese groups
The decreased min SaO2 persisted in both obese groups, despite normalized AHI under CPAP therapy. This decrease resulted in a significant difference in min SaO2 between the 2nd CPAP and the control night between the obese and non-obese. Between the severely obese and non-obese this difference was significant only during the 2nd CPAP night. Similar results were observed by Vgontzas et al (4). Resta et al (5) used a percentage of the total sleep time with oxyhemoglobin saturation less than 90% and not a min SaO2. This parameter also showed a worse nocturnal oxygenation in the obese group (without SRBD), but the difference did not reach the statistical significance. According to previous findings in extremely obese OSAS patients with persistent hypoxemia under CPAP (21), our results may reflect the coexistent obesity-related hypoventilation syndrome. However, in our study persistent low min SaO2 under CPAP had apparently no effect on sleep quality, the hypoventilation related hypoxemia and hypercapnia can significantly decrease the alertness level (22) and according to some studies also the sleep quality (23). A future study concerning the sleepiness and disordered sleep in obese population should include the nocturnal PaCO2 monitoring, which is considered to be crucial in diagnosing and management of hypoventilation.
Study limitations
Due to retrospective character of the study only the data collected from the database were available, which causes shortcomings. The most important one is the lack of documentation of the daytime sleepiness level during the therapy course. This would be interesting, since previous studies suggested obesity is an independent factor of the daytime sleepiness (4, 5, 9). Another limitation is the lack of the arousal index (AI) among the sleep parameters measured in the present study. This is an important parameter reflecting well the restorative function of the sleep (24, 25). We assume, however, that it cannot have a great impact on the sleep of our patients, since in the study of Resta et al (5) no differences in AI between the obese and non-obese were observed, while the rest of the sleep parameters showed differences. A third limitation is the lack of analysis of the first night under CPAP, when the rebound is often most pronounced (2). According to clinical experience, however, the reliability of the first night with CPAP could be limited, since the pressure titrating is in process and sufficient abolition of the respiratory events is often, especially in the first half of the night, not reached.
In conclusion, after long-term CPAP therapy, no deleterious effects of obesity on sleep quality is apparent in sleep apnea patients.
Acknowledgements: This work was supported by an intramural grant No 56 of the Institute of Psychiatry and Neurology in Warsaw, Poland.
Conflicts of interest: No conflicts of interest were declared with relation to this work.
- Glebocka A, Kossowska A, Bednarek M. Obstructive sleep apnea and the quality of life. J Physiol Pharmacol 2007; 58 Suppl 5: 685-690.
- Fietze I, Quispe-Bravo S, Hansch T, Rottig J, Baumann G, Witt C. Arousals and sleep stages in patients with obstructive sleep apnea syndrome: Changes under nCPAP treatment. J Sleep Res 1997; 6: 128-133.
- Wierzbicka A, Rola R, Wichniak A, Richter P, Ryglewicz D, Jernajczyk W. The incidence of sleep apnea in patients with stroke or transient ischemic attack. J Physiol Pharmacol 2006; 57 Suppl 4: 385-390.
- Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 1998; 158: 1333-1337.
- Resta O, Foschino Barbaro MP, Bonfitto P et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med 2003; 253: 536-543.
- Birketvedt GS, Florholmen J, Sundsfjord J et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 1999; 282: 657-663.
- Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab 2001; 86: 90-96.
- Chorostowska-Wynimko J, Plywaczewski R, Jonczak L et al. Leptin measurement in urine is a reliable method of monitoring its secretion in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol 2007; 58 Suppl 5: 105-115.
- Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep 2006; 29: 645-649.
- Sasanabe R, Banno K, Otake K et al. Metabolic syndrome in Japanese patients with obstructive sleep apnea syndrome. Hypertens Res 2006; 29: 315-322.
- American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22: 667-689.
- National Institute of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr 1998; 68: 899-917.
- Bloch KE, Schoch OD, Zhang JN, Russi EW. German version of the epworth sleepiness scale. Respiration 1999; 66: 440-447.
- Rechtschaffen A. Kales A. A manual of standarized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, Government Printing Office, Washington D.C. 1968.
- Collard P, Dury M, Delguste P, Aubert G, Rodenstein DO. Movement arousals and sleep-related disordered breathing in adults. Am J Respir Crit Care Med 1996; 154: 454-459.
- Quera-Salva MA, Guilleminault C, Partinen M, Jamieson A. Determinants of respiratory disturbance and oxygen saturation drop indices in obstructive sleep apnoea syndrome. Eur Respir J 1988; 1: 626-631.
- Fuentes-Pradera MA, Botebol G, Sanchez-Armengol A et al. Effect of snoring and obstructive respiratory events on sleep architecture in adolescents. Arch Pediatr Adolesc Med 2003; 157: 649-654.
- Guilleminault C, Stoohs R, Duncan S, Snoring I. Daytime sleepiness in regular heavy snorers. Chest 1991; 99: 40-48.
- Guilleminault C, Philip P. Tiredness and somnolence despite initial treatment of obstructive sleep apnea syndrome (what to do when an OSAS patient stays hypersomnolent despite treatment). Sleep 1996; 19: S117-S122.
- Hoffstein V, Viner S, Mateika S, Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis 1992; 145: 841-845.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131: 1678-1684.
- Nowbar S, Burkart KM, Gonzales R et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116: 1-7.
- Fleetham J, West P, Mezon B, Conway W, Roth T, Kryger M. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. The effect of oxygen therapy. Am Rev Respir Dis 1982; 126: 429-433.
- Bonnet MH, Sleep fragmentation as the cause of daytime sleepiness and reduced performance. Wien Med Wochenschr 1996; 146: 332-334.
- Punjabi NM, O'hearn DJ, Neubauer DN et al. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med 1999; 159: 1703-1709.