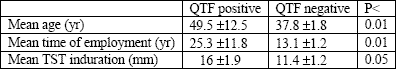Tuberculosis (TB) is a serious public health
problem in Poland (1-3). Detection and monitoring of latent
M. tuberculosis
infections are essential to control its spread. Targeted tuberculin skin testing
(TST) and drug therapy to prevent latent
M. tuberculosis infection (LTBI)
from progressing to overt disease are important TB elimination strategies in
low-incidence countries (4). In Poland, we still focus on the clinical management
of patients with active disease, but preventive programs that identify and treat
individuals with LTBI will certainly be more widely recommended in the near
future. As we approach the elimination phase of TB, due to the steadily declining
incidence in the population, the importance of preventive measures will grow.
Future TB control strategies will focus on diminishing the prevalence of LTBI
to reduce the pool of those infected, from which future cases of TB could arise.
Tuberculin skin test (TST) has been used effectively for detection of latent
M. tuberculosis infections for more than 100 years, despite many drawbacks
such as variability and subjectivity in test application and reading as well
as false positive results, especially occurring in BCG-vaccinated populations
(5). Nowadays, the advances are provided by a new generation of immunoassays,
including Quantiferon-TB-Gold (QTF) based on the detection of IFN-

secretion by peripheral T cells upon incubation with the specific
M. tuberculosis
antigens ESAT-6 and CFP-10 (6, 7). The ESAT-6 and CFP-10 proteins, secreted
by
M. tuberculosis cells, are potent T-cell antigens which have a role
in TB pathogenesis. These antigens are consider to be powerful reagents for
the precise detection of LTBI in both BCG-vaccinated and unvaccinated populations
(6-8).
As TB in the Polish population has declined, high-risk groups become more visible
(1). HCWs are at risk for TB exposure and infection when they care for patients
or process clinical samples from TB patients (9-13). The risk of transmission
of
M. tuberculosis from individuals with TB onto other patients and HCWs
has been known for many years (12, 13). Therefore, screening of workers for
LTBI remains an integral part of tuberculosis control programs in health-care
facilities (11). Bacille Calmette-Guérin (BCG) vaccination is a national policy
in Poland. The repetitive administration of BCG (and possibly hypersensitization
from multiple TST) makes the diagnosis of LTBI, using traditional tuberculin
skin tests in Poland, difficult.
The aim of this project was to estimate the prevalence of latent tuberculosis infection in high-risk populations using the TST and QTF, to determine the agreement between both tests, and to compare their relation to risk factors.
MATERIAL AND METHODS
This study was approved by a local Ethical Committee of the Institute of Tuberculosis and Lung Diseases in Warsaw, Poland. All study participants provided written informed consent. A hundred and fifty five subjects (120 women and 35 men); aged 24-69 (mean 42.1 ±9.8SD years) were enrolled, including both clinical and non-clinical health care workers. Clinical health-care workers included those with direct patient contact or contact with TB patient's sputum samples: doctors (n=31), nurses (n=19), and staff of TB labs (n=46). Nonclinical health-care workers included those who did not have direct patient contact, such as administration (n=19) and analytical laboratory employees (n=30). All TB and non-TB staff underwent chest radiological screening biannually. Neither immunocompromised nor pregnant individuals participated in this study. Patients with a history of TB were excluded. All subjects were BCG vaccinated. The study population underwent screening examination including face-to-face interview by means of a questionnaire and physical examination. Interviewers were medical doctors experienced in TB. The questionnaire contained information about age, gender, history of risk factors, degree of occupational exposure (year of training and active clinical/nursing practice in the health care service, job category), non-medical tuberculosis contact, smoking and alcohol consumption, any coexisting illness, medication(s) received, known radiographic changes and prior knowledge of sensitization to TST. Demographic and clinical data were correlated with TST and the QFT-Gold results. Everyone identified as LTBI positive within the medical service was offered information on the published sensitivity and specificity of the tests, the potential value of these tests, and counseling on the risks and benefits of chemoprophylaxis.
Tuberculin skin test (TST)
Trained health care workers performed and interpreted the results 48 h after application according to the American Thoracic Society (ATS) and Centers for Disease Control and Prevention guidelines (14). The positive interpretation of a TST is an area of induration ³10 mm.
QFT assay
Venous blood was collected into heparinized tubes provided by the manufacturer
of the test. Blood was transported to the lab in the upright position with the
use of portable blood incubator. The QFT assay was performed in accordance with
the manufacturer's instructions. The testing was conducted in two parts, an
overnight culture of blood with stimulation antigens and the subsequent quantification
of IFN-

production. Following an overnight incubation at 37°C in humidified atmosphere,
the supernatant plasma was harvested and then stored at -80°C until the analysis.
All samples were assayed for IFN-

in a single ELISA run. Results were calculated and interpreted according to
the manufacturer's instructions. Calculations were performed using software
provided by the manufacturer's kit (Analysis Software v1.51 Cellestis).
Statistical analysis
Differences between the examined groups were compared using the Mann-Whitney
U test. Correlation between TST and INF-

level was assessed with Spearman's correlation test. Statistical significance
was accepted at P<0.05. All analyses were performed with a Statistica software
program.
RESULTS
The prevalence of LTBI among HCWs was, on average, 27.1%. A higher risk of acquiring
LTBI disease was associated with certain work locations (TB lab workers - prevalence
50%, TB ward clinicians - 34%, nurses - 30%). The prevalence in analytical lab
workers group was of 20% and in administration staff group of 15%. All microbiologists
processing sputum from TB patients without modern safety facilities were infected.
Gender was not associated with a positive QTF test result. The HCWs with positive
QTF test results were older and worked longer than individuals with negative
results (
Table 1). The mean TST induration was bigger in the QTF positive
than negative group (
Table 1). The prevalence of LTBI in HCWs older than
40 years was 62%, and in the group younger than 40 years, it was 22%. Among
HCWs working less than 10 years, infection rate was 33% and in those working
longer than 10 years, it was 56%. All employees of TB lab equipped with modern
safety facilities were free from infection. The presence of a BCG scar was not
significantly associated with a higher prevalence of LTBI.
| Table 1.
Age, length of employment, and magnitude of TST induration in the HCW
groups with positive and negative QTF test results. |
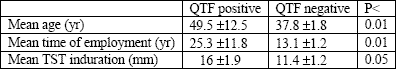 |
The correlation between the diameter of the skin test induration and the magnitude
of the INF-

response was highly significant (P<0.001) (
Fig.1). Consistency of both
tests was 82%. There also were correlations between the level of IFN-

,
on one side, and age (P<0.001) (
Fig. 2) or length of employment (P<0.01)
(
Fig. 3), on the other.
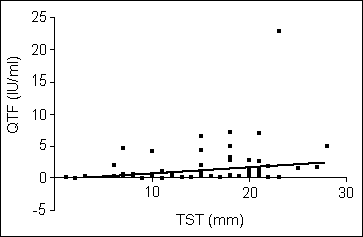 |
Fig. 1. Correlation between
INF- production and the magnitude of TST induration in health care workers.
production and the magnitude of TST induration in health care workers. |
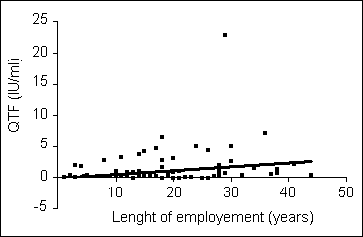 |
Fig. 2. Correlation between
INF- production and the length of employment in health care workers.
production and the length of employment in health care workers. |
 |
Fig. 3. Correlation between
INF- production and the age of health care workers.
production and the age of health care workers. |
DISCUSSION
The rate of TB in the general population in Poland was 24.3/100000 in 2004 (1),
but TB incidence among HCWs staff in contact with TB was 204-721/100000 in 2001
(2). There is little information available on the rate of LTBI infection in
HCWs, due largely to the methodological problems associated with interpreting
TST in a population that is universally immunized for BCG and have had multiple
TST examinations. TST has been used for many years to screen contacts of patients
with tuberculosis and other populations of high TB infection risk (5, 11). However,
TST is based on a crude mixture of poorly defined mycobacterial antigens, some
of which are shared with the
M. tuberculosis complex, environmental non-tuberculous
mycobacterial strains, and the vaccine substrain
M. bovis BCG (5).
In the present study, we attempted to establish the rate of LTBI infection in
HCWs using a novel
in vitro IFN-

assay. The QuantiFERON-TB-Gold test has been recently developed to overcome
some of the limitations of the TST (6, 7). This provides an opportunity for
targeted intervention. An effective program for high-risk groups in low-incidence
countries includes chemoprophylactic treatment (11). The common policy in Poland
is to apply chemoprophylaxis only in children and in dual HIV and TB infected
patients. However, if we have a more accurate tool to detect latent TB in BCG
vaccinated subjects, a national policy of preventive therapy may also include
other risk groups, including HCWs. The goal of the present study was to compare
an
in vitro response, as measured by QFT test results, with the size
of the TST induration in HCWs. Both tests were simultaneously performed in 155
subjects divided into the different subgroups to estimate the prevalence of
latently infected clinical staff within the TB medical services (high-exposure
group), in comparison with other medical staff, not primarily working with TB
patients (low exposure). The study supports the hypothesis that there is a higher
prevalence of LTBI in HCWs within the TB services, including those working in
TB labs. There was considerable heterogeneity in the risk of LTBI between different
occupations: doctors and nurses in TB wards and TB laboratory personnel had
a high incidence of TB disease, while the incidence of LTBI was lowest in administrative
staff. The analysis demonstrates that work in TB services was the most important
risk factor for LTBI, reflecting extensive exposure to
M. tuberculosis.
Our results are consistent with those of other studies in HCWs from a high (India,
Russia, and Georgia) and middle (Japan) incidence countries (13, 15-18). In
our study, the prevalence of LTBI in senior years was two times greater than
that in junior years. A study from India reported a 4-fold higher prevalence
in medical students who were more than 23 years of age than in those aged 18-20
years (13).
The IFN-

based assays appear to offer a rapid
in vitro testing requiring a single
visit, which is less subjective than TST and can be repeated as an annual occupational
screening. Nevertheless, larger cohort studies are necessary to assess the value
of these tests, risks of active TB development in positive individuals, and
effectiveness of preventive therapy based on IFN-

test results. The results may modify institutional infection control and occupational
health policies. Negative-pressure facilities are available in Polish hospitals,
including those centers dealing exclusively with TB (2). In Poland, unlike in
Western Europe, a common feature of TB control is the use of ultraviolet germicidal
radiation and daily surface disinfection (2). However, continuous effort leading
to larger implementation of laminar-flow chambers (present nowadays in 41% of
TB labs) may reduce the number of nosocomial transmissions in TB laboratories
in Poland.
Preventive therapy given to those with a positive tuberculin test substantially
decreases the risk of development of active TB (9). Chemoprophylaxis following
a positive TST in Poland is limited largely to younger age groups. All HCW staff
is radiologically screened for TB annually with referral to the TB services
for full treatment if appropriate. Within the TB services, isoniazid prophylaxis
is not widely used, as there is an argument that it would be of limited value
due to continued exposure. On the other hand, a reduction of exposure risk should
be a high priority, because occupational TB leads to the loss of skilled HCWs,
who are essential in the fight against TB. Unfortunately, there are no guidelines
to indicate how this should be done. In the United States and other high-income
countries, the risk of nosocomial transmission of TB declined with the reduction
in incidence of TB disease in the population (9, 19). This has led to recommendations
for a comprehensive set of infection-control practices to protect HCWs and reduce
nosocomial transmission (20). In the years following the publication of those
recommendations, there was a dramatic decline in the burden of TB among HCWs
(9, 20-22). In a recent review of TB among HCWs in high-income countries, the
overall incidence of TB disease in the general population and native-born HCWs
was less than 10 and 25 per 100,000 per year, respectively (9, 23). The situation
is different in Poland, as we still focus largely on case detection and treatment
of active cases. At present, IFN-

tests are not licensed in the Poland, and there are no guidelines on the use
of these tests. HCWs in Poland have a higher risk of TB infection than the estimates
of risk in the general population (1). In our study, more years of clinical
training and greater exposure to TB patients were risk factors for LTBI, and
this provides additional prove for nosocomial transmission. Our study provides
evidence that a reduction in the risk of LTBI in TB lab can be achieved with
implementation of modern safety facilities, but this needs to be evaluated in
larger, better-controlled studies.
In summary, there is consistent epidemiologic evidence that TB is an important
occupational disease in HCWs. In our study, the infection rate correlated with
indicators of exposure, including longer time of employment and workplace that
has been identified as high risk. Finally, we noticed that the incidence of
infection is lower in TB laboratories which implemented infection-control measures.
With the recent emergence of extensively drug-resistant tuberculosis, the need
to implement infection-control measures has been reemphasized by global agencies
such as the WHO and the Stop TB Partnership (24). Currently available evidence
is limited, but it suggests that relatively simple interventions, such as early
diagnosis of TB, segregation of infectious TB patients, or education and training
of HCWs, might be effective. High rates of LTBI would support the need for the
review and reinforcement of institutional cross-infection measures. Additional
low-cost measures could include engineering controls, such as exhaust ventilation,
improved natural ventilation, or sunlight (24). In high-income countries, there
are guidelines to minimize the transmission of TB in health-care facilities
(9, 16, 25). Administrative controls help reduce the exposure of HCWs to people
with TB. Environmental controls (isolation rooms - present in 50% of Polish
hospitals dealing with TB patients) aim to prevent the spread and to reduce
the concentration of infectious droplets in the air. Respiratory-protection
controls (personal devices with HEPA filters - present only in 12.5% of TB centers
in Poland) aim to reduce the risk of infection when exposure to
M. tuberculosis
is unavoidably high (9, 16).
We conclude that latent TB is an important occupational problem among HCWs,
in particular those working in the TB services in Poland, as a consequence of
lack of isolation facilities in many TB centers and weak infection control measures.
These observations suggest that appropriate preventive strategies and staff
health monitoring should be improved. Moreover, extensive studies evaluating
the cost, feasibility, and effectiveness of these interventions are urgently
needed. IFN-

tests are useful tools in detecting LTBI cases in a country where BCG vaccination
is a national policy.
Acknowledgments:
Supported in part by a grant from the Polish Committee for Research (KBN) No
ZPO5D04530.
Conflicts of interest: The authors had no conflicts of interest to declare
in relation to this article.
REFERENCES
- Gruzlica i Choroby Ukladu Oddechowego w Polsce w 2004. I Szczuka (ed). Warszawa, Instytut Grozlicy i Choroby Pluc, 2005.
- Zwolska Z, Augustynowicz-Kopec E, Domagala-Krzewniak A et al. Ryzyko narazenia na gruzlice jako chorobe zawodowa u personelu medycznego. Zakazenia 2001; 2: 17-22 (in Polish).
- Demkow U, Filewska M, Michalowska-Mitczuk D et al. Heterogeneity of antibody response to myobacterial antigens in different clinical manifestations of pulmonary tuberculosis. J Physiol Pharmacol 2007; 58 Suppl 5: 117-127.
- Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis 2006; 6: 710-725.
- Al Zaharani K, Al Jahdali H, Menzies D. Does size matter? Utility of size of tuberculin reactions for the diagnosis of mycobacterial disease. Am J Respir Crit Care Med 2000; 162: 1419-1422.
- Dheda K, Chang JS, Kim LU, Huggett JF, Johnson MA et al. Interferon gamma assays for tuberculosis. Lancet Infect Dis 2005; 5: 324-325.
- Lalvani A, Richeldi L, Kunst H. Interferon gamma assays for tuberculosis. Lancet Infect Dis 2005; 5: 322-324.
- Pai M, Dheda K, Cunningham J et al. T-Cell Assays for the diagnosis of latent tuberculosis infection: Moving the research agenda forward. Lancet Infect Dis 2007; 7: 428-438.
- Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med 2006; 3: e494.
- Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 2007; 11: 593-605.
- American Thoracic Society and the Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000; 161: S221-S247.
- Pai M, Joshi R, Dogra S et al. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med 2006; 174: 349-355.
- Pai M, Gokhale K, Joshi R et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 2005; 293: 2746-2755.
- Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000; 161: 1376-1395.
- Mori T, Sakatani M, Yamagishi F et al. Specific detection of tuberculosis infection: An interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 2004; 170: 59-64.
- Drobniewski FA, Tayler E, Ignatenko N et al. TB in Siberia 2- diagnosis, chemoprophylaxis and treatment. Tuber Lung Disease 1996; 77: 297-301.
- Mirtskhulava V, Kempker R, Shields KL et al. Prevalence and risk factors for latent tuberculosis infection among health care workers in Georgia. Int J Tuberc Lung Dis 2008; 12: 513-519.
- Drobniewski F, Balabanova Y, Zakamova E et al. Rates of latent tuberculosis in health care staff in Russia. Plos Med 2007; 4: e55.
- Harries AD, Maher D, Nunn P. Practical and affordable measures for the protection of health care workers from tuberculosis in low-income countries. Bull World Health Organ 1997; 75: 477-489.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm Rep 2005; 54: 1-141.
- Fella P, Rivera P, Hale M et al. Dramatic decrease in tuberculin skin test conversion rate among employees at a hospital in New York City. Am J Infect Control 1995; 23: 352-356.
- Seidler A, Nienhaus A, Diel R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration 2005; 2: 431-446.
- Raviglione M. XDR-TB. Entering the post-antibiotic era? Int J Tuberc Lung Dis 2006; 10: 1185-1187.
- Granich, R, Binkin, NJ, Jarvis WR et al. Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. WHO/CDS/TB/99.269.Geneva, Switzerland: World Health Organization; 1999; 51.
- Mazurek GH, Jereb J, Lobue P et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54: 49-55.