Bronchoalveolar lavage (BAL) examination belongs to the important procedures in the diagnosis of ILD (1). In the majority of cases, the result of BAL fluid examination gives only additional information and can not be the basis for definite diagnosis of ILD. However, this relatively non-invasive procedure is very useful in the differential diagnosis of ILD: normal BALF excludes an active inflammation. There are only few publications concerning the usefulness of BALF examination in the diagnosis of sr-ILD (9, 10). Most of the studies on BALF in ILD were performed before the recent ATS/ERS classification has been published and in the pre- HRCT era. The typical pathological feature of sr-ILD, DIP, and RB-ILD is an increase in inflammatory cells, mainly pigmented macrophages in the alveolar lumen (6, 11). The cellular pattern of BALF in sr-ILD (especially DIP) is very characteristic and uniform with high cellularity and alveolar macrophage number. The aim of this study was the presentation of the results of BALF examinations in patients with sr-ILD and to discuss their value in the diagnosis.
The investigation reported in this article conformed to the Helsinki Declaration for Human Research and was approved by the Ethics Committee of the Warsaw Medical University in Warsaw, Poland.
In this retrospective study, we analysed 700 BALF examinations and among them we found 18 cases of BALF with DIP reaction in 18 patients with clinical signs of ILD. These 18 cases made up 2.5% of the BALF results analyzed. The full clinical data and the results of pulmonary function tests, chest X- ray, and HRCT are presented in the Table 1. The main clinical indication for hospitalization was dyspnea and/or disseminated changes in the chest radiogram. Patients had crackles or otherwise did not present any abnormality on physical examination. HRCT images presented a variety of changes: from slight reticular, reticulonodular shadowing and ground glass to more intense interstitial alterations with emphysematous bullae, honey-combing and peribronchiolar fibrosis. The presence of mixed changes in the same patient was a common feature.
| Table 1. Anthropometrics, smoking history, results of pulmonary function tests, and arterial blood gases of patients with smoking-related interstitial lung disease and DIP reaction in the BALF. |
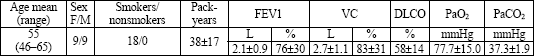 |
| Mean values ±SD, |
Mild and moderate impairments of pulmonary function (both obstructive and restrictive) were observed. The mean value of lung diffusion capacity for CO was 58 ±14% predicted. Analysis of blood gases revealed slight hypoxemia. There were no patients with respiratory failure (Table 1).
The final diagnosis was established on the basis of lung biopsy (Institute of Tuberculosis and Lung Diseases in Warsaw, Poland). They were as follows: RB-ILD in one patient, DIP in one patient, and an undefined type of interstitial fibrosis in another one. In the remaining 15 patients, we continued the observation or treatment with glucocorticosteroids was initiated mainly, because of the lack of the patients' consent for an open lung biopsy. All patients were motivated to quit smoking. In two patients, chronic obstructive lung disease (COPD), and in one asbestosis were diagnosed.
The control group consisted of 30 healthy volunteers: 10 women and 20 men, mean age 33 years (range 19-66 years). Eighteen of them were never smokers and 12 were current smokers with the mean number of pack/years: 16 ±16. All asymptomatic smokers had normal values of pulmonary function tests. None of the subjects had symptoms of infection during the investigation and did not receive any kind of immunosuppressive treatment. All the patients and healthy volunteers gave their informed consent for a bronchofiberoscopic examination with BAL.
BAL was performed using 200 ml of saline. The cell smears were prepared immediately after receiving according to the obligatory standards (12). After filtration, the fluid was centrifuged for 10 min at 400 x g. The cell pellet was resuspended in PBS. The cells were counted using a Bürker chamber. Differential cell count was performed on slides stained with the May-Grunwald-Giemsa method. Three hundred cells were counted for differential cell count analysis. The smears stained with haematoxylin-eosin served to exclude the presence of malignant cells. The classification proposed by Patricia Haslam (Brompton, Great Britain, personal contact) was used in the analysis of alveolar macrophages population. According to this classification the following types of macrophages were found: clear, pigmented, foamy, vacuolated, multinuclear, and giant cells.
For macrophage phenotyping an immunocytochemistry method was used with commercially available antibodies anti: CD14 and CD54 (Dako, Denmark). The alkaline phosphatase anti-alkaline phosphatase (APAAP) reaction (LSAB2 kit, Dako, Denmark) was performed on the air dried slides according to the instruction of the manufacturer. In the first step, the slide was covered by primary antibody and negative control reagent. Next, Link was added and incubation with streptavidin-alkaline phosphatase was performed for 10 min. A freshly prepared substrate chromogen-solution was added for 10 min after rinsing with distilled water and slides were counterstained with Mayer's haematoxylin. Two hundred macrophages in each reaction area were evaluated under a light microscope and the percentage of positive cells was recorded.
Values are given as means ±SD, medians, and ranges, as indicated. For data comparison, one-way ANOVA (for data normally distributed) and Kruskal-Wallis test (for data non-normally distributed) were applied. Statistical significance was set at P<0.05.
There were no significant differences in the volume of recovered BALF between the study groups. The mean cell viability was higher than 90% in all subjects. The total cell count in the BALF was elevated in the group of the patients with ILD and differed significantly compared with the healthy smokers and nonsmokers (median values 64.5 vs. 9.4 vs. 3.25 x 106, respectively). Alveolar macrophages count in the patients was as high as 55.5 x 106 and this was the main group of cells responsible for such a high total cell count in this group (Fig. 1). We found a significantly lower proportion but increased number of lymphocytes in the BALF of the patients. The proportion and number of neutrophils and eosinophils in the patients was higher compared with those in both control groups (Table 2, Fig. 1).
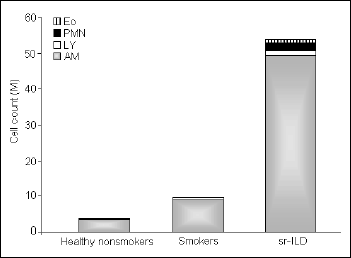 |
Fig. 1. Total and differential cell count of non-epithelial cells in the BALF of patients with smoking-related interstitial lung disease (sr-ILD), asymptomatic smokers, and healthy non-smokers. AM-alveolar macrophages, Ly-lymphocytes, PMN-neutrophils, Eo-eosinophils. |
| Table 2. Total and differential cell count in the BALF of patients with DIP reaction, asymptomatic smokers, and healthy nonsmokers. Results presented as median values, percentiles in the parentheses. |
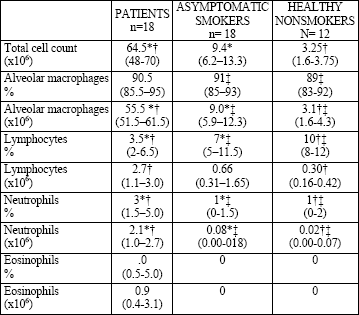 |
| *Significant difference between patients and asymptomatic smokers, †patients and healthy nonsmokers, and ‡asymptomatic smokers and healthy nonsmokers (P<0.05) |
The following proportions of alveolar macrophages subtypes in the BALF of the patients with DIP reaction were found: pigmented cells: >60%, small macrophages with condensed cytoplasm: 30%, other types were present together in less than 10%. The morphology of pigmented macrophages in BALF of the patients was comparable with those from the healthy smokers.
An analysis of macrophage surface antigens revealed some significant differences between the study groups. The intensity of the reaction with antibody anti CD54 in the cytoplasm of macrophages was strong, but the intensity of reaction with antibody anti CD14 was weak. There were no differences in the intensity of reaction with the above mentioned antibodies between the study groups. The mean proportion of CD14+ AM was 6 ±4% and CD54+ AM was 62 ±29% and did not differ compared with the normal BALF macrophage phenotype (14 ±15% and 60 ±19%, respectively). There were no malignant cells nor atypical cells in the analysed cell smears. In one patient asbestos bodies were found.
In this study we presented the BALF picture of patients with sr-ILD. An extremely high total cell count with a significant predominance of pigmented alveolar macrophages forms a very characteristic pattern and indicates the value of BALF examination in the initial diagnosis of this kind of ILD.
The study group presented typical clinical signs of sr-ILD. The mean age was 55 years and the age range was narrow: from 46 to 65. All patients were smokers. The radiological image was complex: ground glass opacities, emphysematous bullae, and micronodules were described. Impairment of pulmonary function was mild to moderate. The clinical characteristic of patients presented above is typical for sr-ILD described in the literature (7, 8,13-15). In one patient of our group asbestosis was diagnosed. The coincidence of other lung disorders with sr-ILD was noted. Craig et al (7) observed a coincidence of occupational lung diseases with sr-ILD. In two patients of our study COPD was observed and this co-morbidity has been also described by other authors (14). Because of the insidious and probably long course of sr-ILD, the patient group was not uniform, and the HRCT image differed. Even at the time of initial diagnosis, before treatment, the patients are in very different stages of the disease (this is enhanced by active smoking). This needs to be taken into consideration in the analysis of the clinical signs of in studies on patients with sr-ILD.
The results of this study showed that BALF picture of sr-ILD is typical and uniform. As high total cell count as about 50 million cells with narrow range was not observed in other types of ILD or in asymptomatic smokers. The number of macrophages was extremely high and the population of these cells consisted mainly of pigmented cells. The presence of such a high number of AM was not usual for BALF of asymptomatic smokers. BALF of smokers is characterized by an elevated number of cells and increased macrophages proportion and number as well (12). However, the total cell count is rarely higher than 20 x 106 (12). In our study, the range of TCC in patients was: 28 to 135 x 106 . Therefore, our results indicate that TCC higher than 30 x 106 with macrophage predominance are suggestive of sr-ILD. The BALF in ILD is commonly rich in cells, but such a high number of AM is not typical. In a Japanese study of a large group of 80 patients with IPF/UIP, the mean number of recovered AM in smoking patients was about 20 x 106 , which was much lower that in sr-ILD (16)
The influence of cigarette smoke (CS) on airways inflammation and respiratory bronchiolitis (BR) has been elucidated (17, 18), but the pathogenesis of sr-ILD remains unclear. Profibrotic role of CS was found in experimental studies (19). A role of dendritic cells in sr-ILD pathogenesis was postulated (20). In the present study we analyzed the morphology of AM and we investigated the expression of CD14 and CD54 on AM to elucidate the origin of AM augmentation. The population of AM consisted mainly of pigmented cells and those with condensed cytoplasm. These two populations of AM are characteristic for sr-ILD (6, 11) and differ markedly from those which predominate in IPF/UIP: foamy and vacuolated cells (12). We have found only few percent of the latter cells, which may be important in the differential diagnosis of sr- ILD with other types of ILD.
CD14 is a receptor for lipopolisaccharide (LPS) binding protein. This marker is expressed to a higher degree in blood monocytes than in tissue macrophages (21, 22). We found about 30% of small macrophages, the population probably representative for young cells which originated from monocytes in patients with sr-ILD. However, the small number of CD14 positive cells did not confirm the influx of young forms from the circulation. We have analyzed the macrophages with expression of CD54 (ICAM 1) which are known be over-expressed in smokers (23, 24) and ILD (25). The role of adhesion molecules in the inflammatory reaction has been taken into consideration by others (25, 26). The correlation of CD54 positive AM with increased proportion of other inflammatory cells: neutrophils, lymphocytes, and eosinophils in ILD has been found by Taylor et al (25). In the study of Scold et al (23) the expression of CD54 on AM was lower in smokers than in nonsmokers (23); the explanation may be that AM in smokers have lower properties of antigen presenting cells (APC). CS changes the AM function from APC to phagocytic properties. In the present study, we did not confirm any changes in the proportion of CD54+ AM in patients with sr-ILD when compared with normal values. The precise characteristics of the AM phenotype and function in sr-ILD needs further studies.
The discussion on the usefulness of BALF in the diagnosis and prognosis of ILD goes on (10, 16, 26). In the authors' opinion, the value of BALF examination in the initial differential diagnosis of ILD is essential. BALF may show an active inflammatory process, not fibrosis. There is a marked difference between DIP pattern typical for sr-ILD and UIP and NSIP in the BALF; the latter (UIP, NSIP) are characterized by a much higher percentage of neutrophils and lymphocytes (10, 12, 16). The value of surgical biopsies as a gold standard in ILD has been established (1). However, BALF is an appropriate representative material from distal airways and alveoli, comes from a larger area than small bronchial biopsy, and is less invasive. Cells in BALF represent exfoliated population and it is not sure if they are representative for inflammation in the lung interstitium.
In spite of the uniform BALF results, the final diagnosis in the lung biopsy of our patients was not uniform: in one RB-ILD was confirmed, in the second NSIP suspected, and only one had DIP. There is no agreement in the literature if DIP and RB-ILD are the same or different diseases. Some authors present them as different ILD (27). Others argue that they are two forms of one disease, and the clinical and morphological picture may be mixed (15). The third opinion is that both may cause non-specific interstitial pneumonia NSIP (7). In the HRCT, differential diagnosis of these three forms of ILD are taken into consideration (1). The relation of PLCH and RB-ILD /DIP remains uncertain. The histological pattern of RB-ILD is characterized by: bronchiolocentric distribution of changes, submucosal infiltration of PMN and lymphocytes, mild peribronchial fibrosis, and hyperplasia of type II pneumocytes. The typical features of DIP are as follows: thickening of alveolar septa and peribronchial lymphoid hyperplasia (6, 11, 14). The common feature is an increased number of macrophages. The microscopic structural changes of airway wall and interstitium can not be reflected by lavage, so it is difficult or impossible to distinguish RB-ILD and DIP in the BALF. When we compared our results with those of Coredeiro et al (9), we found in BALF of patients with sr-ILD a much higher TCC and a lower proportion of lymphocytes. The final diagnosis in those autors’ study was RB-ILD. Thus, an increased proportion of lymphocytes may distinguish RB-ILD from DIP, which needs further confirmation.
Our study could confirm the opinion that BALF with a very high number of cells and pigmented macrophages is non-specific and is a form of DIP-like reaction. DIP-like reaction is a consequence of cigarette smoking, characterized by intraalveolar macrophage accumulation and focal distribution (2, 5, 11). DIP-like reaction may be observed in other ILD, like: UIP, NSIP, RB, eosinophilic pneumonia, and chronic haemorrhage (1). In our study diffuse, changes in HRCT were an indication for BALF which was taken from the right middle lobe in all patients, and still we detected typical changes. This excludes focal (DIP-like) reaction and supports the diagnosis of DIP, which is a diffuse process.
What are the possible clinical implications of finding changes typical for sr-ILD in BALF? Most experts would advise performing a lung biopsy. The management of the three forms of sr-ILD (DIP, RB-ILD, PLCH) is similar - smoking cessation. In some cases the pathological confirmation may be needed for exclusion of UIP and to establish the exact prognosis. The group of sr-ILD is characterized by a relatively good prognosis, smoking cessation, and glicocorticosteroids treatment; the latter has been reported to be effective (2, 3, 14). The development of HRCT technique helps in the differential diagnosis and together with BALF may identify the type of ILD. Thus, the place of BALF in the diagnosis of sr-ILD may be established.
Acknowledgements: Authors thanks the students: Anna Jachimiak and Marta Kurzeja for their help in preparing the manuscript.
Conflicts of interest: No conflicts of interests were declared in relation to this article.
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002; 165: 277-304.
- Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases: a concise review. Eur Respir J 2001; 17: 122-132.
- Flaherty KR, Martinez FJ. Cigarette smoking in interstitial lung disease: concepts for the internist. Med Clin North Am 2004; 88: 1643-1653
- Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003; 29: S93-S97.
- Fraig M, Shreesha U, Savici D, Katzenstein AL. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathol 2002; 26: 647-653.
- Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006; 3: 322-329.
- Craig PJ, Wells AU, Doffman S et al. Desquamative interstitial pneumonia, respiratory bronchiolitis and their relationship to smoking. Histopathology 2004; 45: 275-282.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127: 178-184.
- Cordeiro CR, Freitas S, Rodrigues B et al. Diagnosis of Respiratory Bronchiolitis associated interstitial lung disease. Monaldi Arch Chest Dis 2006; 2: 96-101
- Veeraraghavan S, Latsi PI, Wells AU et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J 2003; 22: 239-244.
- Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998; 157: 1301-1315.
- Costabel U. Atlas of Bronchoalveolar Lavage. Chapman & Hall Medical, 1998.
- Mavridou D, Laws D. Respiratory bronchiolitis associated interstitial lung disease (RB-ILD): a case of an acute presentation. Thorax 2004; 59: 910-911.
- Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax 1999; 54: 1009-1014.
- Vassallo R, Jensen EA, Colby TV et al. The overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans cell histiocytosis: high-resolution CT, histologic, and functional correlations. Chest 2003; 124: 1199-1205.
- Tabuena RP, Nagai S, Tsutsumi T et al. Cell profiles of bronchoalveolar lavage fluid as prognosticators of idiopathic pulmonary fibrosis/usual interstitial pneumonia among Japanese patients. Respiration 2005; 72: 490-498.
- Poletti V, Zompatori M, Cancellieri A. Clinical spectrum of adult chronic bronchiolitis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16: 183-196.
- Domagala-Kulawik J. The nature of immunological reaction in the peripheral airways of cigarette smokers. Current Respir Med Rev 2007; 3: 117-127
- Cisneros-Lira J, Gaxiola M, Ramos C, Selman M, Pardo A. Cigarette smoke exposure potentiates bleomycin-induced lung fibrosis in guinea pigs. Am J Physiol Lung Cell Mol Physiol 2003; 285: L949-L956.
- Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 2005; 175: 2684-2691.
- Frankenberger M, Menzel M, Betz R et al. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol 2004; 138: 507-516.
- Wahlstrom J, Berlin M, Skold CM, Wigzell H, Eklund A, Grunewald J. Phenotypic analysis of lymphocytes and monocytes/macrophages in peripheral blood and bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Thorax 1999; 54: 339-346.
- Skold CM, Lundahl J, Hallden G, Hallgren M, Eklund A. Chronic smoke exposure alters the phenotype pattern and the metabolic response in human alveolar macrophages. Clin Exp Immunol 1996; 106: 108-113.
- Domagala-Kulawik J, Maskey-Warzechowska M, Hermanowicz-Salamon J, Chazan R. Expression of macrophage surface markers in induced sputum of patients with chronic obstructive pulmonary disease. J Physiol Pharmacol 2006; 57 Suppl 4: 75-84.
- Taylor ML, Noble PW, White B, Wise R, Liu MC, Bochner BS. Extensive surface phenotyping of alveolar macrophages in interstitial lung disease. Clin Immunol 20000; 94: 33-41.
- Welker L, Jorres RA, Costabel U, Magnussen H. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur Respir J 2004; 24: 1000-1006.
- Yousem SA, Colby TV, Gaensler EA. Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc 1989; 64: 1373-1380.