Most cough reactivity studies are realized in adults. Information about the influence of developmental changes during childhood and adolescence on the cough reflex pathway is missing. According to Chang and Widdicombe (6), maturation of the cough pathways from a premature infant to mature newborn is probable, but whether age-related differences in cough exist among neonates, children, and adults has by far been unexplored. Children change in many aspects during puberty. The most prominent are dimensional and hormonal changes that could play a role in the above-mentioned gender-related differences in cough reactivity in adults (1).
The aim of the present study was to investigate cough reactivity in school-age children and to find out whether gender differences exist also in children and adolescents, and whether cough reactivity to capsaicin is influenced by pubertal development.
The study was approved by the Ethics Committee of Jessenius Medical School in Martin Slovakia and the children's parents gave informed consent.
Subjects
Two hundred twenty five healthy, non-atopic, non-wheezing children and adolescent volunteers (age range 7–18 yr, median age 13 yr (interquartile range: 12-16; 103 females/122 males) from elementary and secondary schools in the Martin Region, Slovakia participated in the study. The inclusion criteria were: non-smoker, no respiratory morbidity, and no symptoms of respiratory diseases in the preceding 2 weeks before testing, no history of allergic diseases, and no history of other diseases that could modulate CT (diabetes mellitus, gastroesophageal reflux disease).
Cough sensitivity testing
Capsaicin challenge was performed in agreement with the ERS guidelines on assessment of cough (1). We used a compressed air-driven nebuliser (model 646; DeVilbiss Health Care, Inc., Somerset, PA, USA) controlled by a dosimeter (KoKo DigiDoser-Spirometer; nSpire health Inc, Louisville, CO, USA) with an inspiratory flow regulator valve added (RIFR; nSpire health Inc, Louisville, CO, USA) to assign identical inspiratory flow rate during capsaicin inhalations in all subjects. Each subject inhaled saline randomly interspersed with 12 incremental capsaicin aerosol concentrations (0.61-1250 µmol/l). Each administration of saline and capsaicin aerosol was performed at 1 min intervals with the inhalation time set at 400 ms.
Spirometry was performed before and after capsaicin challenge (KoKo DigiDoser-Spirometer; nSpire health Inc, Louisville, CO, USA). Subjects performed three forced expiratory manoeuvres from total lung capacity to residual volume and the best record was used for an analysis.
Pubertal development rating
Classification of a pubertal category was made according to Carskadon and Acebo (7) by filling in a self-administered rating scale for the pubertal development. To test the effect of a pubertal stage on CRS, the subdivision was made based on the gender for each pubertal stage according to the Puberty Category Score obtained:
- 20 girls aged 8.0 (8.0-9.5 yr) (median, interquartile range) and 14 boys aged 9.5 (8.0-11.0 yr) were classified as subjects at ‘prepubertal stage’ (1st pubertal stage - PS).
- 14 girls aged 11 (10-13) yr and 82 boys aged 13 (12-14) yr were classified as ‘early pubertal stage or midpubertal stage’ (2nd pubertal stage).
- 56 girls (aged 15 (13-16) yr and 26 boys aged 16 (15-17) yr were classified as subjects at ‘late pubertal stage’ (3rd pubertal stage).
- 13 girls aged 16 (14-17) yr, but no boys, were classified as subjects at ‘postpubertal stage’, and were not included in the statistical analysis.
Analysis was performed using Systat 11 software. C2 values were normally distributed after natural logarithm (ln) transformation and are expressed as geometric means (95% confidence interval). Two-way ANOVA was used to examine the effects of two independent variables concurrently: pubertal status and gender and to determine whether the two independent variables interact with respect to their effect on the dependent variable. One-way ANOVA was used to compare average C2 values at three pubertal stages in female and male subjects separately, followed by a post hoc Tukey test. Comparisons between the gender subgroups were performed with a two-sample t-test. Multiple linear regression was performed to assess contributions of several factors (age, height, weight, FEV1, FVC, and pubertal stage) to cough sensitivity to capsaicin. P<0.05 was regarded as statistically significant.
Spirometric results, median age, height, and weight of studied subjects divided according to the pubertal development rating are shown in Table 1. FEV1 and FVC were significantly lower in girls compared with boys at each pubertal stage. At the late pubertal stage, girls had significantly lower FVC and FEV1 expressed in %predicted. Girls at each pubertal level were significantly younger and shorter compared with boys.
| Table 1. Proband characteristics. |
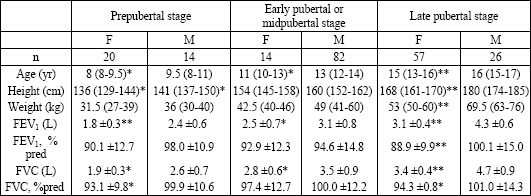 |
| Data are expressed as median (IQR) (for categories: age, height, weight) or mean ±SD (for categories: FEV1,FVC). *P<0.05 and **P<0.001 compared with the corresponding values for boys. |
Two-way ANOVA using the lnC2 as the dependent variable, and pubertal stage and gender as between-group factors revealed that gender showed no significant effect on the cough threshold (P=0.33), pubertal status showed a significant effect (P=0.001), and there was no interaction of the two on the cough threshold (P=0.2).
One-way ANOVA table with the pubertal stage as a factor and lnC2 as a dependent variable showed a significant difference among the three lnC2 means when all subject were taken into account (P=0.002); and also in female (P=0.035) and male subjects (P=0.009) separately (Fig. 1). The results of the post hoc Tukey test are described in Table 2.
 |
Fig. 1. Cough threshold to inhaled capsaicin expressed in natural logarithm values in boys and girls at separate pubertal stages. Results of boys are represented by crosses linked with full lines and girls are represented by dots linked with dotted line. *Significant difference in lnC2 between boys and girls at the late pubertal stage. |
| Table 2. Post hoc Tukey test - matrix of pairwise comparison probabilities in lnC2 among different pubertal stages. |
 |
| PS – pubertal stage |
A two-sample t-test, performed between boys and girls at consecutive pubertal stages revealed that CS was similar in both groups at the ‘prepubertal stage [19.53 (7.94-47.99) µmol/l vs. 26.28 (8.78-78.65) µmol/l; P=0.66]. Girls at the ‘early pubertal or midpubertal’ stage had a lower cough sensitivity compared with boys, but the difference was insignificant [74.37 (27.38-201.88) µmol/l vs. 55.70 (40.12-77.32) µmol/l; P=0.56]. In contrast the ‘late pubertal’ girls had a significantly higher cough sensitivity compared with the boys in this group [53.89 (35.55-81.69) µmol/l vs. 119.7 (70.74-202.55) µmol/l; P=0.02] (Fig. 1, Table 3).
| Table 3. C2 (geometric mean (95%CI)) values in separate subgroups of subjects. |
 |
| PS – pubertal stage |
As none of boys were at the post-pubertal stage at the time of testing, we were not able to follow whether the sex-related differences determined in our study had a tendency to accentuate after puberty had ended. The information obtained from a small sample of post-pubertal girls (n=13) suggests a further significant reduction of the cough threshold compared with the late pubertal girls (P=0.04) (Table 3).
A stepwise multiple regression analysis was performed with lnC2 as a dependent variable and age, height, weight, FVC, FEV1, and pubertal stage as independent variables in girls and boys separately. In the boys, the pubertal stage was the most important predicting factor with its significant contribution to capsaicin cough sensitivity (partial regression coefficient was 0.754; P=0.002). In the girls, both age and pubertal stage significantly contributed to capsaicin cough sensitivity (partial regression coefficient for age was 0.296 (P=0.0001) and for the pubertal stage -0.571 (P=0.009). Other independent variables did not significantly contribute to cough threshold.
The aim of this study was to clarify whether there are gender differences in cough reactivity during childhood and adolescence and to test the impact of the pubertal development on capsaicin cough threshold.
According to the ERS recommendations we tested the cough threshold to capsaicin using standardized methodology in terms of equipment, method of administration, nebulizer output, and inspiratory flow rate (1). Two exceptions were made, each arising from the differences between children and adults: 1) we used different capsaicin concentrations, first applied by Chang et al (8) for cough sensitivity testing in children; 2) we applied a different dose of aerosol per breath, necessitated by inhalation time reduction to 400 ms. These changes were made according to our previous experience with cough reactivity testing in children and were proved to be adequate for CT testing in comparative clinical studies in children (9-11).
Our results show that changes represented by puberty had a strong impact on capsaicin cough sensitivity occurring throughout childhood. A highly significant increase in cough threshold to capsaicin was observed in children at the 2nd PS (P=0.005) and 3rd PS (P=0.001) compared with children at the 1st PS. Therefore, it is likely that the cough reflex pathway undergoes a maturation process toward a lower cough reactivity in adolescence.
Gender differences in cough reactivity, however, were not present when comparing the whole group of tested children. These results are similar to those previously published (12). After dividing subjects according to the pubertal stage, our analysis revealed that cough threshold was similar in boys and girls before the start of puberty. In the course of puberty, a 2-fold increase of the cough threshold occurred in boys and almost a 4-fold increase was noted in girls at the 2nd PS compared with the corresponding gender at the prepubertal stage.
Analysis of the late pubertal stage determined a further increase in the cough threshold in boys, whereas there was a tendency to reduce it in girls. This divergence caused a statistically significant difference in cough reactivity observed between boys and girls at the late pubertal stage.
There is no definite answer why there are gender differences in airway behavior, although according to review of Becklake and Kauffmann (13), the determinants of biological nature (dimensional, immunological, and hormonal), and also environmental and sociocultural factors are likely to play a role.
In agreement with other published studies (3, 4, 14), we observed that forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) did not significantly contribute to the cough threshold. Even though there are significant differences in spirometric parameters between boys and girls, such differences in the airway size are not likely to affect CT.
The most tempting explanation for the sex-related differences in cough reactivity to capsaicin would be an influence of different sexual hormones. As the late pubertal stage in females is characterized by starting of menarche, female sexual hormones could play a role in reduction of the cough threshold that was observed in our study. On the other hand, no significant difference in CT between girls at pre-menarche pubertal stages (1st and 2nd PS) compared with girls with menarche (late pubertal and postpubertal stage) (P=0.45; results not shown) argues against this hypothesis. Similarly, an analysis of the cough threshold in postmenopausal women showed significantly reduced CT compared with pre-menopausal women (3). According to these observations we could conclude that female sexual hormones are not likely to cause a decrease in cough reactivity in female gender.
The question about gender differences in cough reactivity to tussigenic agents still remains unexplained. According to our results we can only conclude that the pubertal development plays a significant role in changing the cough threshold during childhood and adolescence. This change has, however, an opposite trend in females (towards enhancement of cough reactivity) and in males (towards reduction of cough reactivity) resulting in sex-related differences in cough reflex sensitivity in adulthood.
Acknowledgements: This work was supported by Science and Technology Assistance Agency under contract No. APVT-20-005304 and by Project of European Social Fond No. SOP LZ 20005/NP1-027, 11230100433.
Conflicts of interest: The authors had no conflicts of interest to declare in relation to this article.
- Morice AH, Fontana GA, Belvisi MG et al. ERS guidelines on the assessment of cough. Eur Respir J 2007; 29: 1256–1276.
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Sex difference in the inhaled tartaric acid cough threshold in non-atopic healthy subjects. Thorax 1990; 45: 633–634.
- Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J 1996; 9: 1624–1626.
- Kastelik JA, Thompson RH, Aziz I, Ojoo JC, Redington AE, Morice AH. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med 2002; 166: 961–964.
- Coulter DM, Edwards IR. Cough associated with captopril and enalapril. Br Med J 1987; 294: 1521–1523.
- Chang AB, Widdicombe JG. Cough throughout life: Children, adults and the senile. Pulm Pharm Ther 2007; 20: 371-382.
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health, 1993; 14: 190-195.
- Chang AB, Phelan PD, Roberts RGD, Robertson CF. Capsaicin cough receptor sensitivity test in children. Eur Respir J 1996; 9: 2220–2223.
- Varechova S, Plevkova J, Javorka M, Hanacek J. Reliability of the capsaicin cough reflex sensitivity test in healthy children. J Physiol Pharmacol 2006; 57 Suppl 4: 365-373.
- Varechova S, Mikler J, Murgas D, Dragula M, Banovcin P, Hanacek J. Cough reflex sensitivity in children with suspected and confirmed gastroesophageal reflux disease. J Physiol Pharmacol 2007; 58 Suppl 5 (Pt 2): 717-727.
- Varechova S, Durdik P, Cervenkova V, Ciljakova M, Banovcin P, Hanacek J. The influence of autonomic neuropathy on cough reflex sensitivity in children with diabetes mellitus type 1. J Physiol Pharmacol 2007; 58 Suppl 5: 705-715.
- Chang AB, Phelan PD, Sawyer SM, Del Brocco S, Robertson CF. Cough sensitivity in children with asthma, recurrent cough, and cystic fibrosis. Arch Dis Child 1997; 77: 331-334.
- Becklake MR, Kauffmann F. Gender Differences in airway behaviour over the human life span. Thorax 1999; 54: 1119-1138.
- Thompson RH, Wright C, Morice AH. Female gender and enhanced citric acid induced cough response. Thorax 1999; 54: A75 (Abstract).