In the periphery BDNF is found in the plasma, serum and platelets (10) and it is formed by vascular endothelial cells and by peripheral blood mononuclear cells (11). Despite the size of the protein (27 kDa) BDNF can cross the blood-brain barrier (12, 13) in both directions from brain to the periphery and from the periphery to the brain (13), via high capacity saturable transporter system (13). A positive correlation between BDNF levels in the brain and serum was described (14), therefore the blood levels of BDNF may reflect the brain levels and vice-versa. It should be mentioned however, that some authors (15, 16) challenged the finding by Poduslo and Curran (12), and by Pan et al. (13).
Physical exercise is not only beneficial in preventing cardiovascular diseases, it also reduces the risk of developing some types of cancers including colon, breast and prostate cancer (17-20), and it is also potent to enhance brain health and its plasticity (21). Physical activity increases the expression of BDNF in the rat brain (22-25). It was shown that voluntary wheel running in rats leads to an increase in expression of BDNF mRNA level in the hippocampus (26) and this increase is sustained for several weeks after training (21). Also in humans serum BDNF levels [BDNF]s are significantly elevated in response to exercise. It was described that 30 min of moderate exercise (bicycle ergometry) increases [BDNF]s (27). An increase in [BDNF]s during the ramp test to exhaustion was observed (28) and the magnitude of its increase is exercise intensity dependent (29). On one hand, exercise improves neurological health and decreases negative mood (30, 31) also in depressed patients voluntary exercise produced antidepressant effect (32) and the positive effect was increased after acute exercise (33). On the other hand, a role for BDNF in pathogenesis of depression was proposed (34) and decreased [BDNF]s was found in depressed patients (35). Moreover, it was recently reported that low levels of [BDNF]p in humans accompanies impaired glucose metabolism as well as it was postulated that decreased [BDNF]p may be a pathogenetic factor involved not only in dementia and depression, but also in type 2 diabetes (36). It should be also mentioned, that the recent study by Han et al. (37), showed that among persons with the WAGR syndrome (Wilms' tumor, aniridia, genitourinary anomalies and mental retardation), BDNF haploinsufficiency is associated with lower levels of serum BDNF and with childhood-onset obesity. However, surprisingly, it was recently shown that [BDNF]s positively correlated with risk factors for metabolic syndrome and type 2 diabetes mellitus in middle age group of subjects (38). Moreover, a significantly elevated [BDNF]s in newly diagnosed female patients with type 2 diabetes mellitus was recently shown by Suwa et al. (39). According to some authors (38-40) elevated levels of plasma / serum BDNF concentrations may be an early marker of pathological metabolic changes in the body.
In view of the above presented contradictory results regarding the relationship between plasma/serum BDNF concentrations and the risk of metabolic syndrome in humans (36, 38-40), in the present study we aimed to investigate the bimodal effect of moderate intensity endurance training i.e. on the basal and on the exercise induced plasma BDNF concentrations in young healthy men, also in relation to the changes in the insulin resistance. We have hypothesized, that this kind of training, considered as an intervention beneficial to the health status (for review see e.g. (20)) should result in an increase in the basal ("chronic") as well as in the exercise induced ("acute") increase in [BDNF]p without disturbances in insulin resistance in young healthy man.
Subjects
Thirteen young, healthy and physically active men (means ± S.E: age 22.7 ± 0.5 yr, body height 180.2 ± 1.7 cm, body weight 77.0 ± 2.5 kg and BMI 23.7 ± 0.6 kg . m-2) participated in five week endurance training. The maximum oxygen uptake (
All subjects were aware of the aims of the study and gave informed written consent. The study protocol was approved by the Local Ethical Committee and was performed in accordance with the Declaration of Helsinki.
Exercise protocol
The incremental exercise test was performed on the cycloergometer Ergo-Line GmbH & Co KG 800s (Bitz, Germany). Before the test, a 6-min resting period was allowed to determine the resting stage of the cardio-respiratory parameters. The exercise test was performed at pedalling rate of 60 rev . min-1 started at power output 30 W, followed by a gradual increase of power output by 30 W every 3 minutes and it was continued until exhaustion (41). The exercise incremental test was performed twice: three days before and three days after completing a 5-week endurance training.
Gas exchange variables
Gas exchange variables were measured continuously breath by breath using the Oxycon Champion, Mijnhardt BV (Bunnik, The Netherlands), starting from the 6th minute prior to exercise until the test was stopped. Before and after each test, gas analysers were calibrated with certificated calibration gases as previously described by Zoladz et al. (42).
Blood sampling
Blood samples for measurements of plasma insulin, glucose and BDNF concentrations were taken at rest in the morning hours 7:30 - 8:00 a.m. in fasting state, twice: before and after five weeks of endurance training. The blood samples were taken before and during the incremental exercise test using the Abbot Int-Catheter, Ireland (18G/1.2 ´ 45 mm) inserted (about 15 minutes prior to the onset of the incremental exercise test) into the antecubital vein. The catheter was connected to an extension set using a "T" Adapter SL Abbot, Ireland (the tube 10 cm in length). Immediately before each blood sampling, 1 mL of blood volume was drawn in order to eliminate blood from the catheter and the T-set. Blood samples for plasma lactate measurements were taken prior to the exercise test, at the end of each step of the incremental exercise (the last 15 seconds before increase of power output) and at the moment of ending the exercise protocol.
Plasma lactate measurements
The samples (0.5 mL each) for measurements of plasma lactate concentration ([La-]p) were placed in 1.8 mL Eppendorf tubes, containing 1 mg ammonium oxalate and 5 mg sodium fluoride and mixed for about 20 seconds and then centrifuged. The blood plasma (about 200 µL) was stored at -40°C for further analysis of [La-]p using an automatic analyser Vitros 250 Dry Chemistry System, Kodak (Rochester, NY, USA). Lactate threshold (LT) in this study was defined as the highest power output above which plasma lactate concentration [La-]p showed a sustained increase of more than 0.5 mmol . L-1 . step-1 (see (42)).
Plasma BDNF measurements
Plasma BDNF concentrations [BDNF]p were analyzed by enzyme immunoassay using ELISA kit EK-033-22, by Phoenixes Pharmaceuticals, Inc, CA, USA with a detection range from 7.8 - 500 pg . ml-1 and with < 3% cross-reactivity with others neurothrophines. The intra-assay and inter-assay variations were < 10% and < 12%, respectively.
Plasma insulin and glucose measurements
Plasma insulin was measured by IRMA using the INS-IRMA kit (BioSource, Belgium). Analytical sensitivity for this measurement was 1 µIU/ml and intra- and interassay CV were < 2.4% and 6.8%. For IRMA method the radioactivity of the samples were measured by using gamma scintillation counter (Wallac, Finland).
Plasma glucose was measured by enzymatic method (dry chemistry) by using Vitros 950 (Johnson and Johnson, USA).
Insulin resistance
Based on the fasting plasma concentrations of glucose and insulin, the level of insulin resistance was calculated, using the homeostatic model assessment version 2 (HOMA2-IR) (see (43)).
Endurance training programme
The subjects underwent a 5-week endurance training programme (for details see (44)). Training was performed on cycloergometers Monark 874 E at pedaling rates amounting to 60 rev . min-1. The programme included four training sessions per week. Two various training protocols were applied: (a) continuous endurance cycling - performed at the power output (PO) corresponding to 90% of oxygen consumption measured at previously determined lactate threshold (90% Vo2 at LT) lasting 40 minutes and (b) intermittent endurance cycling composed of 6 minute cycling without resistance (unloaded cycling) followed by 3 minute cycling at the power output corresponding to 50%
The athletes
For the comparison of the basal [BDNF]p in untrained and in the trained subjects, in the present study we have also involved a group of 16 athletes (means ± S.E: age 22.8 ± 0.7 yr, body height 182.3 ± 1.7 cm, body weight 74.7 ± 2.8 kg, BMI 22.5 ± 0.7 kg . m2), specialized in various kinds of athletic events (for 8 ± 1 years), (including sprinters, jumpers and runners). Blood samples for measurements of plasma BDNF, glucose and insulin concentrations were taken at rest at 7:30 - 8:00 a.m. in fasting state. The relevant measurements were performed as described above.
Statistics
The results are expressed as mean and standard deviation (x ± SE). Statistical significance was tested using Wilcoxon-signed-rank test (for paired samples) and Wilcoxon-Mann-Whitney test (for two independent samples). Non-asymptotic, exact, two-sided P-values are presented (see the Results section). Correlation between two variables was tested with Spearman's correlation analysis. The statistics was done using the statistical packet StatXact 6.1 and STATISTICA 7.1.
Body mass, body mass index (BMI)
No significant changes in the body mass (77.0 ± 2.5 kg and 76.5 ± 2.4 kg, respectively, for pre-training and post-training values) and in BMI (23.7 ± 0.6 kg . m-2 and 23.5 ± 0.6 kg . m-2, respectively, for pre-training and post-training values), was found after 5 weeks of the endurance training.
Maximal oxygen uptake (Vo2max)
Vo2max increased from 3472 ± 94 ml . min-1 to 3585 ± 95 ml . min-1 (P = 0.057) in response to training. Significant increase in Vo2max expressed per kg of body mass was observed (from 45.29 ± 0.93 ml . kg-1 . min-1 to 47.14 ± 1.12 ml . kg-1 . min-1, P = 0.03).
Power output reached at Vo2max (POmax)
POmax after training was also significantly higher (277 ± 7 W vs. 255 ± 7 W, P = 0.0005) after the endurance training.
Plasma BDNF concentration [BDNF]p
Before training [BDNF]p at rest amounted to 10.3 ± 1.4 pg . ml-1. Its concentration at the end of exercise (i.e. at the Vo2max) amounting to 10.9 ± 2.3 pg . ml-1 was not significantly different (P = 0.74) from its value at rest (see Fig. 1). After 5 weeks of the endurance training [BDNF]p at rest amounted to 16.8 ± 2.1 pg . ml-1, while at the end of exercise its concentration amounting to 68.4 ± 16.0 pg . ml-1 was significantly elevated (P = 0.0002). [BDNF]p at rest after training was significantly higher than before training (P = 0.01).
 |
Fig. 1. Plasma BDNF concentrations in young healthy men (n = 13), measured at rest (Rest) and at the Vo2max (Max) during maximal incremental cycling exercise test, determined before (left panel) and after 5 weeks of the endurance training of moderate intensity (right panel). |
Moreover, the magnitude of the exercise-induced increase in [BDNF]p, defined as the difference between the end-exercise minus pre-exercise plasma concentration of BDNF (
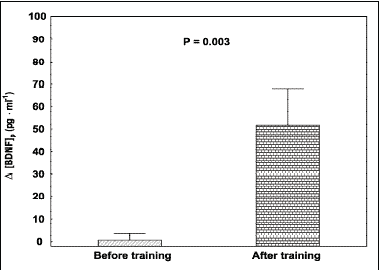 |
Fig. 2. Plasma BDNF concentrations
([BDNF]p) in young healthy men (n =13),
expressed as the difference between the end-exercise minus pre-exercise
plasma concentration of BDNF ( |
Insulin resistance
The insulin resistance, calculated using the (HOMA2-IR), was not significantly affected by the endurance training, although it tended to be lower after training. It amounted to 1.40 ± 0.13 before and 1.15 ± 0.13 after the training (P = 0.25).
Plasma BDNF concentration at rest in well trained athletes vs. untrained subjects
Additionally, we have compared the basal [BDNF]p in 16 athletes vs. 13 untrained subjects. The basal plasma BDNF concentration [BDNF]p in athletes amounting to 29.5 ± 9.5 pg . ml-1 was significantly (P = 0.013) higher (3-folds) than in the untrained subjects participating in this study (10.3 ± 1.4 pg . ml-1).
In the present study no "acute" effect of a single bout of maximal incremental exercise up to Vo2max on the end exercise plasma BDNF concentration [BDNF]p was observed before training (P = 0.74). The main and original finding of this study was that a moderate intensity training lasting 5 weeks, resulted in a significant (P = 0.01) "chronic" increase in the basal (see Fig. 1) as well as in the exercise induced "acute" increase in [BDNF]p (P = 0.0002) in young healthy men (see Fig. 2). Moreover, significantly higher basal [BDNF]p was found in well trained athletes, when compared to the untrained young healthy man (P = 0.013).
The values of the [BDNF]p found in our subjects were substantially lower than in other studies (36) but they were close to that reported by other researches (46-48). It should be mentioned however, that a substantial variations in plasma / serum BDFN concentrations were reported in the literature even in healthy subject of similar age, which are most like related to various kits and laboratory procedures applied in these studies (for discussion of this point see e.g. (29, 49)). Therefore, the changes in the [BDNF]p induced by varied interventions for an appropriate comparisons should also be expressed in relative units (for example in % of changes).
Brain-derived neurotrophic factor (BDNF) is a key mediator of neuronal synaptic plasticity in adults (50). Reduced BDNF levels in the human brain are associated with cognitive function deficit, impaired memory performance and depression (51, 52). Already more that one decade ago Neeper et al. (22) have shown that physical exercise can increase BDNF gene expression in specific brain region of rat. Moreover, the authors postulated that physical exercise induced up-regulation of BDNF could help increase the brain's resistance to damage and degeneration through BDNF's support of neuronal growth, function and survival. Since that time, the original findings by Neeper et al. (22) has been confirmed by this author (23) and by others (24, 25, 53). It was also recently reported that skeletal muscle expresses BDNF (54, 55), however the time kinetics of the BDNF expressions after single bout of exercise indicates, that the acute exercise-induced increase in [BDNF]s has its origin not in the skeletal muscle. However the training induced "chronic" increase in plasma BDNF concentration may be partly related to the up-regulation of the muscle BDNF expression.
It was shown that serum BDNF concentration [BDNF]s in healthy humans is by about 100 - fold higher than in plasma [BDNF]p (46, 56). However, the changes in [BDNF]p are considered to reflect its changes in the brain (46). It was demonstrated that platelets bind, store and release a large amount of BDNF upon agonist stimulation (57). According to Fujimura et al. (57) this store of the BDNF can be used at the site of traumatic injury to facilitate the repair of peripheral nervous or other tissues that contain TrkB. It was show that in the adult nervous system BDNF plays a predominant role in neuronal plasticity (51). Therefore, it was postulated by Rojas Vega et al. (28), that the exercise-induced peripheral increase in BDNF may help to increase the brain's resistance to damage and neurodegradation that occurs with age.
No much is known on the acute effect of a single bout of exercise on the plasma/serum BDNF concentrations in humans (28, 58), especially that the existing data come from studies involving varied groups of subjects including patients, healthy individuals as well as athletes. Gold et al. (27) have reported that single bout of prolonged exercise (30 minutes cycling at 60 % Vo2max) resulted in a significant increase in [BDNF]s both in healthy persons as well as in multiple sclerosis patients. Rojas Vega et al. (28) have reported that single bout of maximal incremental exercise resulted in a significant increase in [BDNF]s in recreational athletes (Vo2max = 56.6 ± 8.6 ml . kg-1 . min-1), whereas 10 minutes moderate aerobic cycling was not sufficient to increase the [BDNF]s above the pre-exercise level. This finding is in accordance with the study by Ferris et al. (29) showing that the magnitude of increase in [BDNF]s during exercise is exercise intensity dependent. Recently Winter et al. (59) have reported that very high intensity short duration running (2 runs until exhaustion lasting 3 minutes each, with 2 minutes break) resulted in a significant increase in [BDNF]s in young healthy men. These authors postulated that the [BDNF]s, dopamine and epinephrine seams to be the mediators by which physical exercise improves learning in humans. As mentioned above, in the present study we have found no acute effect of single bout of maximal incremental exercise on the [BDNF]p before training (see Fig. 1). The effect of single bout of exercise on the [BDNF]p may be related to the training status of the studied subjects. In view of our results training has bimodal influence on [BDNF]p and even single bout of high intensity exercise can significantly increase [BDNF]p.
Even less is known regarding the effect of training on the plasma / serum BDNF concentration in humans. To our best knowledge only one training study involving strength training was performed. Ten week resistance training performed in that study however, did not affected the [BDNF]p in middle age subjects (38). As mentioned above, the 5 week endurance training applied in our study resulted in a significant increase both in the basal plasma ("chronic") as well as in the exercise induced ("acute") [BDNF]p (see Fig. 1).
It was recently reported that low levels of [BDNF]p in humans accompanies impaired glucose metabolism as well as it was postulated that a decreased [BDNF]p may be a pathogenetic factor involved not only in dementia and depression, but also in type 2 diabetes (36). It was reported that patients with acute coronary syndromes have reduced levels of BDNF in plasma (60). El-Gharbawy et al. (61), have recently reported that [BDNF]s is lower in extremely overweight children and adolescent than those of normal weight. Moreover, a negative correlation between [BDNF]p and body weight was found in healthy volunteers (46).
However, on the other hand, according to some authors (38, 39, 40) elevated levels of plasma/serum BDNF concentrations may be an early markers of pathological metabolic changes in the body. Suwa et al. (39), found significantly elevated [BDNF]s in newly diagnosed female patients with type 2 diabetes mellitus. This is in agreement with the recent study by Levinger et al. (38), who have found that elevated concentration of [BDNF]p positively correlated with risk factors for metabolic syndrome and type 2 diabetes mellitus in middle age group of subjects. These observations are in agreement with the so called "metabolic syndrome-neurotrophic hypothesis" suggesting that BDNF may pay a role in the development of risk factors associated with the metabolic syndrome (40). According to Suwa et al. (39) the elevated concentration of BDNF observed in the early stage of type 2 diabetes mellitus is a compensatory mechanism developed in those patients. According to Hristova et al. (40) an increase in the plasma / serum BDNF concentrations (over-secretion) at an early stage of the metabolic syndrome leads to the imbalances in the autonomic nervous system and in the interaction between the neuroimmuno-endocrine system, which in the later stage of the disease process may result in a reduction in BDNF concentration in relation to healthy subjects. This hypothesis can not be ruled out by the observations showing a lower plasma/serum BDNF concentrations in patients with long standing type 2 diabetes mellitus (see e.g. Krabbe et al. (36)). However, further longitudinal study are needed to establish the role of the changes in BDNF secretion in the development of the metabolic syndrome and type 2 diabetes mellitus.
As mentioned above, in the present study we have observed an increase in the basal as well as in the exercise-induced increase in the [BDNF]p after moderate intensity endurance training in young healthy men with normal insulin resistance. In our subjects, the insulin resistance calculated using the homeostatic model assessment version 2 (HOMA2-IR), amounting to 1.40 ± 0.13 before training and 1.15 ± 0.13 (P = 0.25) was by 18% lower after training. Therefore the training induced increase in the [BDNF]p, accompanied by a decrease in insulin resistance, decrease in lipid peroxidation (Majerczak et al. 2008, our unpublished observations), increase in the Vo2max, increase in the power output at Vo2max, we consider as positive effects of training. It seems to be rather unlikely, that the moderate intensity training resulting in several positive responses in the body would lead to the increase in the risk factors for the metabolic syndrome and type 2 diabetes mellitus. Therefore, we postulate that in case of young healthy subjects, opposite to middle age untrained subjects (see, (38)) as well as to the diabetic patients (see, (39)) with limited adaptive capacity in the body, the training induced increase in [BDNF]p belongs to positive adaptive responses in the body. Additionally, we have compared the basal [BDNF]p in well trained athletes of national/international level (sprinters, jumpers, runners) with its level in untrained subjects. The plasma [BDNF]p in athletes was significantly higher that in the untrained subjects (P = 0.013). Therefore, our results suggest that in case of young healthy men an elevated [BDNF]p is attributable to the trained status of humans.
The training induced increase in [BDNF]p may be beneficial to the body in several ways, including its involvement in mechanism of exercise induced improvement of mood, protection and regeneration of the peripheral and central nervous system (53, 62), as well as may play a role in training induced neoangiogenesis in the cardiac and skeletal muscles (for overview see e.g. (63, 64)). Moreover the training induced increase in BDNF may be beneficial to efficacy of pharmacological antidepresant treatment (for discussion of this piont see (65)). Additionally, it may also be involved in the process of regeneration and survival of the injured motor neurons after fatiguing exercise. Moreover, we postulate that the training induced increase in [BDNF]p via its action in the central nervous system may also enhances the motor learning ability in athletes.
We have concluded that endurance training of moderate intensity has bimodal influence on the [BDNF]p: it increases both basal as well as exercise-induced plasma BDNF concentration in young healthy men. This adaptive response was accompanied by an increase in Vo2max, power generating capability and no disturbances in the insulin resistance, as calculated using the homeostatic model assessment version 2 (HOMA2-IR). Therefore, the training induced increase in [BDNF]p in young healthy men, is accompanied by positive adaptive responses in the body and should not be considered as in the case of the patients with metabolic syndrome as an early marker of pathological metabolic changes in the body, as proposed recently (38).
Acknowledgements: This study was supported by a grant nr 220/KFiB/2008 from the University School of Physical Education Krakow, Poland.
Conflicts of interest statement: None declared.
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J 1982; 1: 549-553.
- Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcomas 37 and 180. Proc Natl Acad Sci U S A 1954; 40: 1014-1018.
- Soppet D, Escandon E, Maragos J, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991; 65: 895-903.
- Ebadi M, Bashir RM, Heidrick ML, et al. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int 1997; 30: 347-374.
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci 1994; 17: 182-190.
- Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 2004; 306: 487-491.
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron 2007; 53: 261-277.
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol 2006; 174: 289-299.
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res 1999; 58: 76-87.
- Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci 1990; 10: 3469-3478.
- Sarchielli P, Greco L, Stipa A, Floridi A, Gallai V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J Neuroimmunol 2002; 132: 180-188.
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res 1996; 36: 280-286.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998; 37: 1553-1561.
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 2002; 328: 261-264.
- Sakane T, Pardridge WM. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm Res 1997; 14: 1085-1091.
- Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx 2005; 2: 120-128.
- Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003; 12: 118-126.
- Friedenreich CM. Physical activity and cancer: lessons learned from nutritional epidemiology. Nutr Rev 2001; 59: 349-357.
- Kaaks R, Lukanova A. Effects of weight control and physical activity in cancer prevention: role of endogenous hormone metabolism. Ann N Y Acad Sci 2002; 963: 268-281.
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16 (Suppl 1): 3- 63.
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002; 25: 295-301.
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature 1995; 373: 109.
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996; 726: 49-56.
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004; 124: 71-79.
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 2004; 124: 985-992.
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res 1998; 61: 147-153.
- Gold SM, Schulz KH, Hartmann S, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol 2003; 138: 99-105.
- Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res 2006; 1121: 59-65.
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007; 39: 728-734.
- Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001; 20: 267-275.
- Yeung RR. The acute effects of exercise on mood state. J Psychosom Res 1996; 40: 123-141.
- Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001; 56: 497-504.
- Bryan A, Hutchison KE, Seals DR, Allen DL. A transdisciplinary model integrating genetic, physiological, and psychological correlates of voluntary exercise. Health Psychol 2007; 26: 30-39.
- Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther 2008; 117: 30-51.
- Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 2003; 54: 70-75.
- Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007; 50: 431-438.
- Han JC, Liu QR, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med 2008; 359: 918-927.
- Levinger I, Goodman C, Matthews V, et al. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc 2008; 40: 535-541.
- Suwa M, Kishimoto H, Nofuji Y, et al. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism 2006; 55: 852-857.
- Hristova M, Aloe L. Metabolic syndrome--neurotrophic hypothesis. Med Hypotheses 2006; 66: 545-549.
- Zoladz JA, Duda K, Majerczak J. Oxygen uptake does not increase linearly at high power outputs during incremental exercise test in humans. Eur J Appl Physiol Occup Physiol 1998; 77: 445-451.
- Zoladz JA, Rademaker AC, Sargeant AJ. Non-linear relationship between O2 uptake and power output at high intensities of exercise in humans. J Physiol 1995; 488: 211-217.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487-1495.
- Majerczak J, Karasinski J, Zoladz JA. Training induced decrease in oxygen cost of cycling is accompanied by down regulation of SERCA expression in human vastus lateralis muscle. J Physiol Pharmacol 2008; 59: 589-602.
- Barstow TJ, Jones AM, Nguzen PH, Casaburi R. Influence of the muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol 1996; 81: 1642-1650.
- Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 2005; 26: 115-123.
- Staats R, Stoll P, Zingler D, Virchow JC, Lommatzsch M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax 2005; 60: 688-692.
- Marano CM, Phatak P, Vemulapalli UR, et al. Increased plasma concentration of brain-derived neurotrophic factor with electroconvulsive therapy: a pilot study in patients with major depression. J Clin Psychiatry 2007; 68: 512-517.
- Kim YK, Lee HP, Won SD, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 78-85.
- Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 2004; 101: 10827-10832.
- Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112: 257-269.
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260-265.
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev 2002; 30: 75-79.
- Astrom M-B, Krabbe K, Larsen LF, et al. The BDNF response to exercise. Abstract 2007 Foreningen af Yngre Forskere (FYF) pa Rigshospitalet, www.fyf.rh.dk
- Cuppini R, Sartini S, Agostini D, et al. BDNF expression in rat skeletal muscle after acute and repeated exercise. Arch Ital Biol 2007; 145: 99-110.
- Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull 2007; 73: 143-149.
- Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002; 87: 728-734.
- Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008; 431: 62-65.
- Winter B, Breitenstein C, Mooren FC, et al. High impact running improves learning. Neurobiol Learn Mem 2007; 87: 597-609.
- Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol 2005; 102: 169-171.
- El-Gharbawy AH, Adler-Wailes DC, Mirch MC, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab 2006; 91: 3548-3552.
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res 2008; 1199: 148-158.
- Donovan MJ, Lin MI, Wiegn P, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000; 127: 4531-4540.
- Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med 2007; 17: 140-143.
- Rogoz Z, Skuza G, Legutko B. Repeated co-treatment with imipramine and amantadine induces hippocampal brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol 2007; 58: 219-234.