GHRELIN ROLE IN HYPOTHALAMUS-PITUITARY-OVARIAN AXIS
INTRODUCTION
Ghrelin is a peptide composed of 28 amino acids which was originally isolated from human and rat stomach as an endogenous ligand to the growth hormone secretagogue receptor (GHSR) (1). Ghrelin peptide exists in two major molecular forms: an acylated peptide at serine 3 form and an unacylated one. Acylation, catalyzed by ghrelin O-acyltransferase, is indispensable for ghrelin to bind to GHS-R1a (2, 3). N-octanoylated serine 3 residue is essential for stimulation of GH release (1). Non-acylated ghrelin was found to be present in human serum in higher levels as compared to acylated ghrelin. The former seemed to be devoid of any endocrine action. However, it was able to exert cardiovascular and anti-proliferative effect, probably by binding to different GHSR subtypes (4, 5). Des-Gln14-ghrelin is another endogenous ligand for the GHS-R1a resulting in alternative splicing of ghrelin gene acylated at serine 3 (1).
The effects of ghrelin are mediated via a seven-transmembrane G protein-coupled receptor -GHSR. Two subtypes of this receptor have been identified so far: 1) functionally active, high affinity GHS-R1a, signal transduction form of the receptor and 2) the biologically inactive GHS-R1b, lacking transmembrane domains 6 and 7 and thus being unable to bind a ligand or transduce a signal (6, 7). Published data indicated that both GHSRs were widely distributed, e.g. in hypothalamus, pituitary, stomach, heart, lung, pancreas, kidney, adipose tissue and immune system which suggested that ghrelin exposed both peripheral and central effects (8, 9). However, ghrelin may act through an additional, not yet examined receptor. Data of Baldanzi et al. (10) showed that in cardiomyocytes, ghrelin and des-acylated ghrelin exhibit an anti-apoptotic effect through binding to a novel, unidentified receptor that is distinct from GHSR-1a. They suggested that ghrelin activity is not mediated by GHSR-1a because no expression of GHSR-1a was detected in cardiomyocytes. Moreover, both ghrelin and des-acylated ghrelin recognize a common high binding site, although only ghrelin but not des-acylated ghrelin binds to GHSR-1a receptor (1). The putative novel receptor is expected to be highly similar to GHSR-1a, since it differs only as its lack of ability to distinguish between esterified and non-esterified ghrelin peptide. Whether such a receptor is encoded by alternative splicing of GHSR-1a gene or by a distinct gene still remains to be determined (10).
Ghrelin is a multifaceted hormone playing an important role in the regulation of GH secretion, food intake, and energy balance in vertebrates (11, 12). Ghrelin also stimulates insulin release and regulates GH, adrenocorticotropic hormone (ACTH), and prolactin (PRL) secretion. In addition, ghrelin affects many physiological functions like sleep, gastric motility, cardiovascular function and behavior, as well as cell proliferation, production of pro-inflammatory cytokines and glucose (9, 13, 14) (Fig. 1). Several studies suggest that ghrelin has also an important role in regulating female reproduction by affecting the synthesis and secretion of reproductive hormones from hypothalamus or pituitary, and by regulating ovarian functions (15, 16). Recent study by Sirotkin et al. (17) indicates the involvement of both hypothalamic and ovarian ghrelin/GHS-R1 systems in mediating the effects of nutritional status and ghrelin on reproductive processes. Notwithstanding, only limited research has addressed the physiology and role of ghrelin in the hypothalamic-pituitary-ovarian axis in different species. This mini-review summarizes the results of a series of new studies on the effect of ghrelin on the hypothalamus, pituitary and ovarian functions. Moreover, interaction between leptin and kisspeptin has also been described.
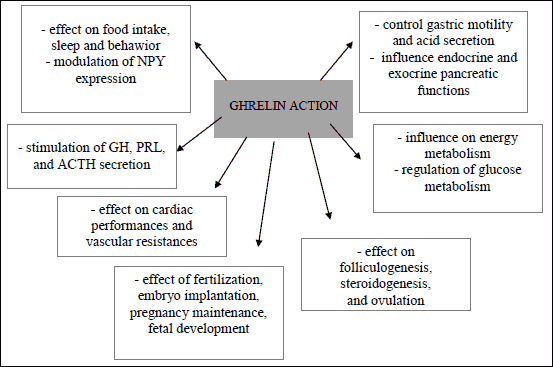 |
Fig. 1. Multidirectional effect of ghrelin. |
GHRELIN AND THE HYPOTHALAMUS – PITUITARY AXIS
Ghrelin and the hypothalamus
The central nervous system, especially in the hypothalamus and pituitary, contains primary sites of ghrelin action. Expression of ghrelin in the hypothalamic arcuate nucleus (ARC) appears to be an important region controlling appetite (18). Injection of ghrelin into the rat cerebral ventricle III stimulates food intake (19). Ghrelin containing-neurons (hypothalamic neurons adjacent to the third ventricle between the dorsal, ventral, para-ventricular, and ARC) project efferent fibers to neurons that contain neuropeptide Y (NPY) and agouti-related protein (AgRP) which might stimulate the release of these orexigenic peptides. Peripheral administration of ghrelin increases c-fos expression in the ARC NPY/AgRP neurons (20) and ablation of both AgRP and NPY neurons completely abolishes the orexigenic effect of ghrelin (21). GHS-R expression has been localized to NPY-expressing cells with 90% of ARC neurons co-expressing NPY and GHS-R (22, 23). GHS-R is found to be expressed in the vagus nerve also. Furthermore, blockade of gastric vagal afferents in rats abolishes ghrelin induced feeding and prevents the ghrelin-induced rise in c-fos expression within the ARC (24). Opposite effects to orexigenic action of ghrelin appear in the form of leptin which exerts its anorectic effect via the ARC, where both NPY/AgRP and pro-opiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons express leptin receptors (25). Leptin inhibits NPY/AgRP neurons and activates POMC/CART neurons (26, 27), resulting in reduced food intake (26) and increased energy expenditure (28). Electrophysiological studies have shown that leptin inhibits a subpopulation of GHS-responsive neurons (29) and that ghrelin acts directly on leptin-responsive cells in the ARC (30). Thus, ghrelin and leptin act on NPY and AgRP-co-expressing cells in the ARC in opposition to one another (31, 32). Both ghrelin and leptin are involved in the regulation of GHS-R in the ARC but not in the ventromedial nuclei, with ghrelin increasing GHS-R expression but leptin decreasing GHS-R mRNA (33). The relative sensitivity of the hypothalamus to these orexigenic and anorectic signals therefore is a key in the delicate balance of body weight regulation (33). Moreover, interaction between leptin and ghrelin has influence on the reproductive and food intake axes, which includes interactions with neuropeptides that are also involved in reproduction (34). It is well known fact that reproductive function is highly dependent on food intake, body status or metabolic disorders. For example, obesity can exert effects upon the hypothalamic-pituitary-ovarian axis and as such disturb menstrual cyclicity and ovulation. A large questionnaire study of 3 638 women demonstrated that menstrual cycle irregularity and an ovulation were correlated with being overweight or obese (35). Indeed, the grossly obese women had a rate of menstrual disturbance 3.1 times that of women with normal weight (36). Furthermore, obesity affects ovulation, oocyte maturation, endometrial development, uterine receptivity, implantation and miscarriage.
Ghrelin and gonadotropins secretion
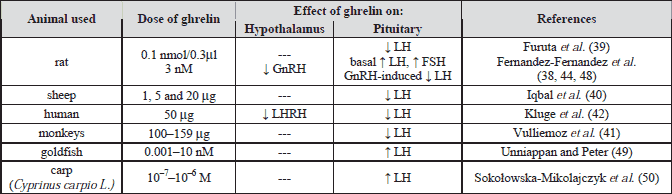
Recenty it was propose that ghrelin is a possible signal of energy deficiency for reproductive system (37). In ovariectomized rats, ghrelin inhibits gonadotropin-releasing hormone (GnRH) by hypothalamic fragments (38). Increasing evidence supports an inhibitory effect of ghrelin in the regulation of gonadotropin secretion (Table 1). Ghrelin was found to significantly decrease the frequency of LH pulses in ovarectomized rats (39), sheep (40), monkeys (41) and human (42, 43). In addition, Fernandez-Fernandez et al. (44) showed that ghrelin inhibited LH secretion in gonadectomized rats, whereas follicle-stimulating hormone (FSH) levels remained unaffected. Moreover, ghrelin decreased LH responsiveness to GnRH in vitro (45). Systematically administrated human ghrelin attenuates the GnRH-induced preovulatory surge of the gonadotropins in sheep (40). In ovariectomized rats, Ogata et al. (46) demonstrated the suppressive effect of intracerebroventricular injection of ghrelin on pulsative LH secretion was mediated by beta-endorphin. Moreover, preclinical studies have reported the inhibitory actions of ghrelin on LH secretion in vivo and ghrelin’s ability to delay onset of puberty (47). On the opposite side, other in vitro experiments observed that ghrelin dose dependently stimulated basal LH and FSH secretion by pituitary tissue of adult female rats and this process required the presence of nitric oxide and was modulated by ovarian signals (48). Likewise, ghrelin stimulated LH secretion from cultured pituitary cells from goldfish (49). In another study, Sokolowska-Mikolajczyk et al. (50) demonstrated that ghrelin increased LH secretion from cultured pituitary cells obtained from sexually mature female carp (Cyprinuscarpio L.). In contrast, in women during the menstrual cycle, administration of ghrelin did not affect either basal or GnRH-induced LH and FSH secretion (51). Additionally, ghrelin activity was strongly dependent on experimental models. For example, Fernandez-Fernandez et al. (38) in in vivo experiments demonstrated inhibitory action of ghrelin on FSH and LH release in rats, however, ghrelin dose dependently stimulated basal LH and FSH release by pituitary tissue in vitro. This stimulation was not present at estrous or after ovariectomy. These data demonstrate a complex mode of action of ghrelin with inhibitory effects at central level and direct stimulatory action on basal gonadotropin secretion.
Some evidences suggest that ghrelin indirectly decreased gonadotropin secretion by actions on central NPY, AgRP or orexin expression (52, 53), which exhibit inhibitory effect on LH secretion (15). Third-ventricular infusion of NPY suppressed LH secretion (54) and estradiol enhances the effect of NPY on LH-releasing hormone release in ovariectomized rhesus (55). On the other side, Forbes et al. (56) demonstrated that ghrelin administration significantly reduced LH pulsatility and suppression of kisspeptin mRNA expression in ovariectomized rats and suggested that down-regulation of kisspeptin expression may play a critical role in the transduction of ghrelin-induced suppression of reproduction function often observed during calorific restriction. However, Kim et al. (57) demonstrated that kisspeptin did not alter AgRP, ghrelin, Kiss1r mRNA expression in the hypothalamic NPY-secreting cell lines (mHypoE-42 and mHypoE-38 cells).
Ghrelin and prolactin secretion
Most studies have shown that ghrelin increased prolactin (PRL) release through both central and peripheral actions. For example, in normally cycling women, bromocriptine (suppresses basal PRL secretion) blocked the stimulating effect of ghrelin on PRL release (58). In human, ghrelin stimulated lactotrophs to PRL secretion (59) and this effect is not additive with that of dopamine receptor blocker, metoclopramide (60) and with that of thyrotropin-releasing hormone (61). GHSR antagonism prevented cortistatin-induced PRL secretion in vitro, and ghrelin or GHSR agonists stimulated PRL release in humans (62, 63). Further, Yang et al. (64) demonstrated that GHSR -/- mice have reduced pituitary PRL mRNA and lactotroph cell number supporting the above studies that ghrelin has stimulatory effect on PRL secretion. On the other hand, in pre-pubertal rodents, ghrelin administered centrally inhibited secretion of PRL (65). Iqbal et al. (40) demonstrated that neither the infusion of ovine ghrelin into the third cerebral ventricle nor bolus injections of various doses of human DAP-octanoyl3 ghrelin had any effect on plasma PRL levels in sheep. This suggested that ghrelin does not act via central mechanisms to regulate PRL secretion in this species, although a direct effect on the pituitary lactotropes cannot be ruled out.
GHRELIN EXPRESSION IN THE OVARY
Expression of GHS-R1a in the ovary
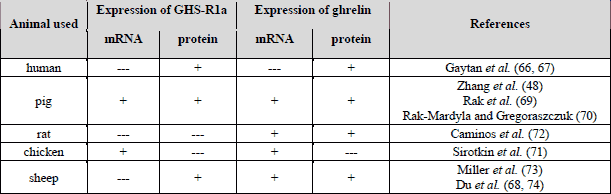
The fully functional GHS-R1a was found in human (66, 67), sheep (68), pig (69, 70), and chicken (71) ovaries. In cyclic human ovary, expression of GHS-R1a showed a wider pattern of tissue distribution, with detectable specific signal in oocytes as well as in somatic, follicular, and luteal cells from early, mature, and regressing corpus luteum (CL). Of particular note was the observation that expression of follicular GHS-R1a paralleled follicle development with stronger immunostaining in granulosa and theca layers of healthy antral follicles (66). Expression of GHS-R1a was also detected in the cells of ovarian surface epithelium (67). Interestingly, GHS-R1a was expressed in both mRNA and protein levels in sheep oocytes and in pre-implantation blastulas obtained from oocytes fertilized in vitro (68). In the oocytes, the levels of GHS-R1a mRNA decreased from the stage of germinal vesicle to meiotic metaphase II, then increased immediately at the 2-cell stage formation and remained stable until the blastocyst was ready for implantation. GHS-R1a protein was detected in most abundant levels in the plasma membrane. In pig ovary, mRNA expression of GHSR and protein expression of GHS-R1a were reported (70). In this study, both GHSR and GHS-R1a expression were significantly higher in ovarian follicles collected from normally cycling animals than in those from pre-pubertal pigs and suggest ghrelin sensitivity during porcine sexual maturation. In fragments of chicken ovarian follicular wall, including theca and granulosa cells, mRNA expression of GHS-R1a was detected by Sirotkin et al. (71). Moreover, the following three splice variants of GHS-R1a were expressed in chicken ovaries: first full-length type 1a transcript (cGHS-R1a), a second variant termed cGHS-R1av (with deletion of a 48-bp fragment of cGHS-R1a cDNA in the 5’ region of exon 2), and a third GHS-R1a isoform termed cGHSRtv, 432-bp amplicon resulting from premature splicing of exon 1, retention of a 126-bp fragment of intron I and premature initiation of exon 2 (71).
Expression of ghrelin in the ovary
Strong ghrelin immunostaining was demonstrated in ovarian hilus interstitial cells. In immuno-positive cells, cytoplasm was uniformly stained in granular form, whereas cell nuclei were immuno-negative (66). Otherwise, interstitial cells derived from the human theca interna of atretic follicles failed to show specific ghrelin immunostaining (66). Similarly, ghrelin signal was not detected in human ovarian follicles at any developmental stage. However, specific ghrelin immuno-reactivity was observed in young (d 15-19) and mature (d 20-24) human CL, whereas expression of the hormone disappeared from regressing luteal tissue (64). However, in the rat, expression of ghrelin mRNA was observed in the ovary throughout the whole estrous cycle, although relative mRNA levels varied dependently on the stage of the cycle, with lowest levels in proestruos and maximum values in diestrous (72). Ghrelin protein expression was predominantly located in the luteal compartment of the rat ovary; especially intense in steroidogenic luteal cells of the current cycle. Cyclic expression of ovarian ghrelin mRNA was disrupted by inhibition of the preovulatory gonadotropin surge and subsequent ovulation by administration of a potent GnRH antagonist (72). In adult ovine ovary, ghrelin was immuno-localized in granulosa cells of ovarian follicles at all developmental stages (primordial, primary, secondary, pre-antral and antral) and in luteal cells of sheep corpus luteum (73). In some sections, positive ghrelin and GHS-R1a immuno-reaction in the oocyte was observed (74). Du et al. (74) also pointed out that ghrelin gene and protein expression was seen in sheep ovary with a detectable specific signal in oocytes and in somatic follicular cells. In situ hybridization for ghrelin mRNA showed a wide pattern of hybridization within the ovarian follicles along with an observation that ghrelin mRNA was clearly visible in oocytes, cumulus cells, granulosa and theca cells, as well as in cells of the ovarian surface epithelium. The relative ghrelin mRNA levels varied dependently on the stage of the cycle in sheep, with the highest expression during the development of corpora lutea and lowest during their regressing phase (74). In pig ovary, ghrelin mRNA expression depended on the stage of the estrous cycle, with lowest expression in proestrous and maximum in estrous and diestrous (75). Likewise expression of ghrelin mRNA and protein in ovarian follicles collected from pig during pre-pubertal time and estrous normal cycle was observed (70). Additionally, during a short, 24 h organ culture of porcine ovarian follicles collected from pre-pubertal animals, ghrelin was secreted into the culture medium in amounts 4.67 pg/ml (76). These results were further confirmed by immunohistochemical analysis showing a strong ghrelin immuno-reactivity in steroidogenic luteal cells and granulosa cells. Moreover, increase in ghrelin expression during porcine CL development up to maximum levels in the late luteal phase was observed. Immuno-analysis showed that along with CL development ghrelin protein localization was seen in the cytoplasm of large luteal cells only. Intensity of immuno-reaction increased with CL development (77).
The presence of ghrelin/GHS-R1a (Table 2) in various ovarian cells suggests a potential role of ghrelin in the control of several aspects of ovarian cell function such as: steroid hormone secretion, cell proliferation or apoptosis.
DIRECT ACTION OF GHRELIN ON THE OVARIAN FUNCTION
Ghrelin and steroid hormone secretion
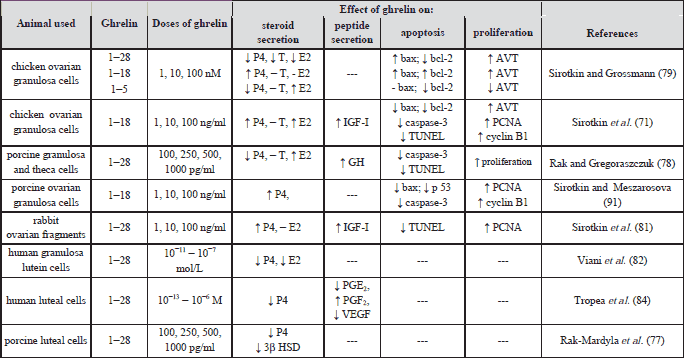
Data concerning the role of ghrelin in ovarian hormone secretion are different and depend on experimental model, animal species as well as on their reproductive and/or endocrine status (Table 3). Also, in co-culture of granulosa and theca cells collected from pre-pubertal porcine ovarian follicles, exogenous ghrelin induced estradiol (E2) secretion into culture medium (79). Our not published study demonstrated opposite effect in cultured whole ovarian follicles collected from estrous cycle pigs, where ghrelin had inhibitory effect on estradiol secretion by reduced CYP19 expression. However, in chicken ovarian granulosa cells culture ghrelin (fragments1-18) at 1-100 ng/ml stimulated secretion of progesterone (P4), E2, insulin-like growth factor type I (IGF-I) and arginine vasotocin (AVT) (79). In order to localize the active core of the 28-amino acid molecule responsible for hormonal activity of ghrelin, two fragments 1-5; 1-18 and the whole molecule were added separately each to the culture medium. Dependently on the dose applied, the effect of ghrelin action on rabbit granulosa cells culture stimulated secretion of P4 (1 ng/ml), IGF-I (100 ng/ml) while decreased P4 at 10 ng/ml, IGF-I at 10 ng/ml and testosterone (T) at 1–10 ng/ml (81). Ghrelin pre-treatment of animals resulted in suppression or even reversal of subsequent LH and IGF-I effects on P4, E2 and IGF-I secretion by cultured rabbit granulosa cells (82). On the other hand, in cultured human luteinizing granulosa cells collected from women with infertility due to unilateral or bilateral tubal disorder, ghrelin had an inhibitory effect on P4 and E2 secretion (82). Similarly, injection of ghrelin during the estrous cycle in rats reduced the serum concentration of E2 and P4 and the expression of receptors ER (β) and PR (A+B) in the ovary (83). In porcine cultured CL, ghrelin at 100 up to1000 pg/ml doses decreased P4 secretion via inhibition of 3β-HSD activity and protein expression (77). Similarly, in human mid-luteal cells, ghrelin reduced basal and hCG-stimulated P4 secretion, decreased luteotropic prostaglandin PGE2 release and increased luteolytic PGF2α levels (85). This suggests that the imbalance between luteotropic and luteolytic factors could be a mechanism by which ghrelin negatively influenced luteal function into luteolysis (84). Data of Romani et al. (85) showed that in cultured human luteal cells culture P4 and VEGF release were significantly reduced by unacylated ghrelin. They suggested that similar to ghrelin, unacylated ghrelin might play a role in regulating the luteal cell function that affected both luteal steroidogenesis and luteotrophic/luteolytic imbalance. Most of these studies suggest inhibitory action of ghrelin on ovarian steroidogenesis. A similar inhibitory effect of ghrelin was observed in pre-implantation mouse embryos development (86, 87).
Ghrelin and ovarian apoptosis
In the ovary, massive cell death occurs during neonatal and postnatal life as an integral part of the normal ovarian development. According to evidence from animal studies, ghrelin has an anti-apoptotic effect in the ovary. In porcine ovarian cells collected from pre-pubertal animals, ghrelin inhibited cell apoptosis by decreasing caspase-3 activity and DNA fragmentation (78). In chicken ovarian granulosa cells, ghrelin decreased expression of caspase-3, bax, bcl-2 and TUNEL-positive cells (71). Granulosa cells isolated from ovaries of ghrelin-treated rabbits showed lower expression of TdT (terminal deoxynucleotidyltransferase), than control animals (81). Moreover, the rates of DNA fragmentation, as estimated by detection of bromodeoxyuridine labeled DNA fragments and TUNEL assay were reduced in the presence of ghrelin in porcine ovarian cells (78). Additionally, antioxidant properties of ghrelin in the rat ovary were observed by Kheradmand et al. (88), who demonstrated that antioxidant enzyme activity assays as well as measurement of glutathione content and thiobarbituric acid reactive substances level reduced significantly in the ghrelin-exposed animals. Furthermore, Kheradmand et al. (89) demonstrated that the number of CL was significantly lower and the number of ovarian follicles was higher in the ghrelin treated group than that in the control. Electron microscopic analysis also indicated some intracellular changes associated with apoptosis and cell death such as presence of secondary lysosome, apoptotic bodies, nuclear chromatin condensation as well as margination, nuclear segmentation and vacuolization of cytoplasm of granulosa and theca cells (89). These studies indicate that ghrelin acts as a survival factor by regulating anti-apoptotic effects in ovarian cells. Ovarian follicular atresia is induced by activation of both the extrinsic (death receptor) and intrinsic (mitochondrial) pathways in ovary (90). However, mechanism(s) of anti-apoptotic action of ghrelin need further investigation.
Ghrelin and ovarian proliferation
Studies have reported that ghrelin has a direct effect on ovarian cell proliferation; for example, in pig (78), chicken (71), and rabbit ovaries (81), ghrelin increased cell proliferation. In both granulosa cells and lysates of whole ovarian chicken follicular walls, ghrelin treatment induced markers of cell proliferation: PCNA (proliferating cell nuclear antigen), a marker of the S/phase of the cell cycle, and cyclin B1, a marker of the G2/phase (71, 91). Moreover, granulosa cells from ghrelin-treated rabbits had higher expression of PCNA than those from control animals (81). This observation is consistent with previous reports indicating that in co-culture of porcine granulosa and theca cells, exogenous ghrelin significantly increased cell proliferation (78). The above observations indicate that ghrelin stimulated ovarian cell proliferation, which is an important process in ovarian function, since the release of oocytes and production of hormones are required for female reproduction.
Ghrelin and activation of GHS-R1a
In cultured porcine ovarian follicles, some ghrelin effects on ovarian function were mediated by GHS-R1a (69). Recently, it was demonstrated that ghrelin receptor antagonist, (D-Lys-3)-GHRP-6 prevents the stimulatory effect of ghrelin on aromatase activity, estradiol secretion and cell proliferation (59). In contrast, the inhibitory action of ghrelin on ovarian apoptosis was not affected by (D-Lys-3)-GHRP-6, suggesting that ghrelin is able to control ovarian apoptosis independent of GHS-R1a, since caspase-3 activity was not reversed by a selective antagonist of GHS-R1a (69). Moreover, Sirotkin et al. (92) demonstrated, that (D-Lys3)-GHRP-6 added alone at 1, 10 and 100 ng/ml to the porcine granulosa cells culture promotes all markers of cell proliferation (PCNA, cyclin B1 and MAPK/ERK1,2), inhibits all markers of apoptosis (bax, p53 and caspase-3) and stimulates the release of all three steroid hormones: progesterone, testosterone and estradiol. They suggested that similar effects of (D-Lys3)-GHRP-6 (inhibitor of GHS-R1a) and ghrelin 1-18 (its stimulator) on porcine ovaries are not mediated by GHS-R1a but by sites other than GHS-R1a (92). Some studies suggest that, which are released into the duodenal lumen in response to food ingestion, could stimulate pancreatic enzyme secretion through activation of entero-pancreatic reflex via cholecystokinin release (93). Also, motor and secretory activity, as well as the rhythm of cell proliferation in the gastrointestinal tract and liver, are subject to many circadian rhythms, mediated by autonomic cells and ghrelin (94).
Intracellular mechanism of ghrelin action
Mitogen activated protein kinases (MAPKs) and triphosphoinositol (PI-3) kinase are serine/threonine kinases mainly involved in the activation of nuclear transcription factors that control cell proliferation, cell differentiation and apoptosis. There is increasing evidence that PI-3 kinase is widely involved in the survival of cells and activates an intracellular serine kinase Akt/PKB, acting via inhibition of caspases activity (95, 96). Both PI-3 kinase and MAPK not only regulate cell apoptosis and cell survival, but also regulate ovarian hormone secretion (97). In porcine ovarian cells, ghrelin significantly increased phospho-ERK 1/2 (extracellular signal-regulated kinases) and PI-3 kinase activity and protein expression in a dose and time dependent manner, where the maximum effect was observed after 15 min of cell incubation (98). Moreover, ovarian cells treated with ghrelin together with selective inhibitors of ERK 1/2 (PD098059) and PI-3 kinase (wortmannin), cell proliferation and apoptosis returned to control levels, suggesting participation of the ERK 1,2 and PI-3 kinase pathways in ghrelin-mediated cell proliferation and apoptosis (98). Next, Popelkova et al. (99) reported that ghrelin increased the level of phosphorylated MAPK expression in bovine oocytes in vitro. Immunocytochemical analysis showed that in chicken ovarian cells, ghrelin increased MAPK/ERK 1,2 levels (100). Additionally, in hamster ovarian cells, ghrelin stimulated the phosphorylation of ERK 1,2 in a time and dose responsive manner with maximum effect observed after 5 and 10 min of cell incubation (101). In chicken ovarian cells, Sirotkin et al. (100) suggested that MAPK, tyrosine kinases and cyclic-dependent protein kinases could also be regulators of avian ovarian secretion and intracellular mediators of ghrelin action in the ovary. However, in the next study Sirotkin et al. (102) suggested that MAPK is probably not a mediator of ghrelin and obestatin effect on porcine oocyte nuclear maturation. On the other hand, Bai et al. (103) observed that ERK1,2 and p90 rsk pathway was associated with maturation of ovis aries oocyte in vitro. Ghrelin is a hormone that activates ERK and PI-3 kinase survival signaling pathways in ovarian follicular cells. Most recently, it has been shown that ERK controlled the first step in synthesis of nucleotides for production of DNA and RNA, and consequently affected cell survival and proliferation. Additionally, PI-3 kinase is a general mediator of cell survival and has been shown to regulate the activity of transcription factors and modulate protein members of the Bcl2 family, thus preventing the pro-apoptotic action of caspases. The ghrelin-mediated activation of both ERKs and PI-3 kinase resulted in stimulation of cell proliferation and inhibition of apoptosis in ovarian follicular cells. Next intracellular mechanism of ghrelin action was observed by Chrenek et al. (104), who examined whether cyclic adenosine monophosphate (cAMP) regulated ghrelin effects on ovarian cell steroidogenesis. It was observed, that administration of a cAMP analog (dbcAMP) inverted the inhibitory effect of ghrelin on progesterone secretion, but not of estradiol release by isolated ovarian fragments, suggesting the involvement of cAMP-dependent intracellular mechanisms in down-regulation of rabbit ovarian steroidogenesis and in modification, but not in mediating effect of ghrelin on ovarian steroid hormones release (104).
SUMMARY
This mini-review presents selected aspects of ghrelin role in hypothalamus, pituitary and ovarian functions. Although ghrelin is a peptide hormone secreted from the stomach, the fact that its functional receptor, GHS-R1a, is also expressed in the hypothalamic-pituitary-gonadal axis and that ghrelin is synthesized in the ovary in many species suggests its role as local, autocrine and/or paracrine regulators in several aspects of reproduction. Increasing data show that ghrelin has inhibitory effect on gonadotropins and PRL secretions. Some studies suggest an indirect action of ghrelin, since ghrelin acts as a central orexigenic signal by NPY, AgRP or orexin, which in turn plays an inhibitory role in central hypothalamus-pituitary-gonads axis control. Interaction between leptin and ghrelin has influence on the reproductive and food intake axes, which includes interactions with neuropeptides that are also involved in the reproduction. Down-regulation of kisspeptin expression may play a critical role in the transduction of ghrelin-induced suppression of reproduction function often observed during calorific restriction. Available data show that ghrelin directly regulates ovarian functions, such as steroid synthesis, cell apoptosis or proliferation. Reproductive effects are highly dependent on the body energy status. Finally, the study suggests that ghrelin, acting at central and peripheral level, could be one of the signal mechanisms linking nutritional balance and hypothalamus-pituitary-ovarian axis.
Abbreviations: ACTH, adrenocorticotropic hormone; AgRP, aqouti-related protein, ARC, arcuate nucleus; AVT, arginine vasotocin; CART, cocaine- and amphetamine-regulated transcript; CL, corpus luteum; E2, estradiol; ERK 1/2, extracellular signal-regulated kinases; FSH, follicle-stimulating hormone; GH, growth hormone; GHSR, growth hormone secretagogue receptor; GnRH, gonadotropin-realesing hormone; IGF-I, insulin-like growth factor type I; LH, luteinizing hormone; MAPK, mitogen activated protein kinases; NPY, neuropeptide Y; P4, progesterone; PCNA, proliferating cell nuclear antigen; PI-3, triphosphoinositol; PLC, phospholipase C; POMC, pro-opiomelanocortin; PRL, prolactin; TdT, terminal deoxynucleotidyltransferase.
Acknowledgements: The author would like to thank the Professor Stanislawa Stoklosowa for professional supervision. Advice of Professor Ewa Gregoraszczuk is greatly appreciated. This work was supported by the DS/MND/WBiNoZ/IZ/6/2011 and partly by DS/MND/WBiNoZ/8/2012, Institute of Zoology, Jagiellonian University, Cracow, Poland.
Conflict of interests: None declared.
REFERENCES
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656-660.
- Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 2008; 105: 6320-6325.
- Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA 2008; 105: 10750-10755.
- Cassoni P, Papotti M, Ghe C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001; 86: 1738-1745.
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255-4261.
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996; 273: 974-977.
- Petersenn S, Rasch AC, Penshorn M, Beil F, Schulte H. Genomic structure and transcriptional regulation of the human growth hormone secretagogue receptor. Endocrinology 2001; 14: 2649-2659.
- Gnanapavan S, Kola B, Bustin S, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002; 87: 2988-29891.
- Gualillo O, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett 2003; 552: 105-109.
- Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and desacyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/Akt. J Cell Biol 2002; 159: 1029-1037.
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25: 426-457.
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707-709.
- Korbonits M, Grossman AB. Ghrelin: update on a novel hormonal system. Eur J Endocrinol 2004; 151(Suppl.1): S67-S70.
- Wang G, Lee HM, Englander E, Greeley GH. Ghrelin - not just another stomach hormone. Regul Pept 2002; 105: 75-81.
- Muccioli G, Lorenzi T, Lorenzi M, et al. Beyond the metabolic role of ghrelin: a new player in the regulation of reproductive function. Peptides 2011; 32: 2514-2521.
- Szczepankiewicz D, Skrzypski M, Pruszynska-Oszmalek E, et al. Importance of ghrelin in hypothalamus-pituitary axis on growth hormone release during normal pregnancy in the rat. J Physiol Pharmacol 2010; 61: 443-449.
- Sirotkin AV, Pavlova S, Tena-Sempere M, et al. Food restriction, ghrelin, its antagonist and obestatin control expression of ghrelin and its receptor in chicken hypothalamus and ovary. Comp Biochem Physiol A Mol Integr Physiol 2013; 164: 141-153.
- Lu S, Guan JL, Wang QP, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett 2002; 321: 157-160.
- Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol 2003; 480: 3-11.
- Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 2002; 325: 47-51.
- Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004; 145: 2607-2612.
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999; 70: 306-316.
- Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology 1997; 138: 771-777.
- Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120-1128.
- Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 1999; 48: 828-833.
- Schwartz MW, Woods SC, Porte Jr D, Seeley JR, Baskin DG. Central nervous system control of food intake. Nature 2000; 404: 661-671.
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 2003; 24: 225-253.
- Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob. mice. Science 1995; 269: 540-543.
- Tung YC, Hewson AK, Dickson SL. Actions of leptin on growth hormone secretagogue-responsive neurones in the rat hypothalamic arcuate nucleus recorded in vitro. J Neuroendocrinol 2001; 13: 209-215.
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol 2002; 14: 580-586.
- Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540-2547.
- Tschop M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 2002; 143: 558-568.
- Nogueiras R, Tovar S, Mitchell SE, et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 2004; 53: 2552-2558.
- Evans JJ, Anderson GM. Balancing ovulation and anovulation: integration of the reproductive and energy balance axes by neuropeptides. Hum Reprod Update 2012; 18: 313-332.
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction 2010; 140: 347-364.
- Hartz AJ, Kalkhoff RK, Rimm AA, McCall RJ. A study of factors associated with the ability to maintain weight loss. Prev Med 1979; 8: 471-483.
- Barreiro ML, Tena-Sempere M. Ghrelin and reproduction: a novel signal linking energy status and fertility? Mol Cell Endocrinol 2004; 226: 1-9.
- Fernandez-Fernandez R, Tena-Sempere M, Navarro V, et al. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology 2005; 82: 245-255.
- Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun 2001; 288: 780-785.
- Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology 2006; 147: 510-519.
- Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 2004; 89: 5718-5723.
- Kluge M, Schusser P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppress secretion of luteinining hormone in humans. J Clin Endocrinol Metab 2007; 92: 3202-3205.
- Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab 2008; 93: 3633-3639.
- Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 2004; 20: 103-107.
- Tena-Sempere M. Ghrelin: novel regulator of gonadal function. J Endocrinol Invest 2005; 28: 26-29.
- Ogata R, Matsuzaki T, Iwasa T, et al. Hypothalamic ghrelin suppresses pulsatile secretion of luteinizing hormone via beta-endorphin in ovariectomized rats. Neuroendocrinology 2009; 90: 364-370.
- Martini AC, Fernandez-Fernandez R, Tovar S, et al. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 2006; 147: 2374-2382.
- Fernandez-Fernandez R, Tena-Sempere M, Roa J, et al. Direct stimulatory effect of ghrelin on pituitary release of LH through a nitric oxide-dependent mechanism that is modulated by estrogen. Reproduction 2007; 133: 1223-1232.
- Unniappan S, Peter RE. in vitro and in vivo effects of ghrelin on luteinizing hormone and growth hormone release in goldfish. Am J Physiol Regul Integr Comp Physiol 2004; 286: 1093-1101.
- Sokolowska-Mikolajczyk M, Socha M, Szczerbik P, Epler P. The effects of ghrelin on the in vitro spontaneous and sGnRH-A stimulated luteinizing hormone (LH) release from the pituitary cells of common carp (Cyprinus carpio L.). Comp Biochem Physiol A Mol Integr Physiol 2009; 153: 386-390.
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Messinis IE. Effect of ghrelin on gonadotrophin secretion in women during the menstrual cycle. Human Reprod 2009; 24: 976-981.
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 2000; 141: 4797-4800.
- Tena-Sempere M, Barreiro ML, Gonzalez LC, et al. Novel expression and functional role of ghrelin in rat testis. Endocrinology 2002; 143: 717-725.
- Kaynard AH, Pau KY, Hess DL, Spies HG. Third-ventricular infusion of neuropeptide Y suppresses luteinizing hormone secretion in ovariectomized rhesus macaques. Endocrinology 1990; 127: 2437-44.
- Woller MJ, Terasawa E. Estradiol enhances the action of neuropeptide Y on in vivo luteinizing hormone-releasing hormone release in the ovariectomized rhesus monkey. Neuroendocrinology 1992; 56: 921-925.
- Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett 2009; 460: 143-147.
- Kim GL, Dhillon SS, Belsham DD. Kisspeptin directly regulates neuropeptide Y synthesis and secretion via the ERK1/2 and p38 mitogen-activated protein kinase signaling pathways in NPY-secreting hypothalamic neurons. Endocrinology 2010; 151: 5038-5047.
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE. Blockage of ghrelin-induced prolactin secretion in women by bromocriptine. Fertil Steril 2010; 94: 1478-1481.
- Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab 2003; 88: 1537-1542.
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE. Effect of ghrelin and metoclopramide on prolactin secretion in normal women. J Endocrinol Invest 2011; 34: 276-279.
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE. Effect of ghrelin and thyrotropin-releasing hormone on prolactin secretion in normal women. Horm Metab Res 2010; 42: 204-208.
- Tassone F, Broglio F, Destefanis S, et al. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J Clin Endocrinol Metab 2003; 88: 5478-5483.
- Svensson J, Lonn L, Jansson JO, et al. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab 1998; 83: 362-369.
- Yang H, Dixit VD, Patel K, et al. Reduction in hypophyseal growth hormone and prolactin expression due to deficiency in ghrelin receptor signaling is associated with Pit-1 suppression: relevance to the immune system. Brain Behav Immun 2008; 22: 1138-1145.
- Tena-Sempere M, Aguilar E, Fernandez-Fernandez R, Pinilla L. Ghrelin inhibits prolactin secretion in prepubertal rats. Neuroendocrinology 2004; 79: 133-141.
- Gaytan F, Barreiro M, Chopin L, et al. Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J Clin Endocrinol Metab 2003; 88: 879-887.
- Gaytan F, Morales C, Barreiro M, et al. Expression of growth hormone secretagogue receptor type 1a, the functional ghrelin receptor, in human ovarian surface epithelium, mullerian duct derivatives, and ovarian tumors. J Clin Endocrinol Metab 2005; 90: 1798-1794.
- Du C, Li H, Cao G, Xilingaowa, Wang C, Li C. Expression of the orexigenic peptide ghrelin and the type 1a growth hormone secretagogue receptor in sheep oocytes and pre-implantation embryos produced in vitro. Reprod Domest Anim 2010; 45: 92-98.
- Rak A, Szczepankiewicz D, Gregoraszczuk EL. Expression of ghrelin receptor, GHSR-1a, and its functional role in the porcine ovarian follicles. Growth Horm IGF Res 2009; 19: 68-76.
- Rak-Mardyla A, Gregoraszczuk E. Expression of ghrelin and its receptor during different physiological stages in pig ovary. J Physiol Pharmacol 2012; 63: 95-99.
- Sirotkin A, Grossmann R, Maria-Peon M, Roa J, Tena-Sempere M, Klein S. Novel expression and functional role of ghrelin in chicken ovary. Mol Cell Endocrinol 2006; 26: 257-258: 15-25.
- Caminos JE, Tena-Sempere M, Gaytan F, et al. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology. 2003; 144: 1594-1602.
- Miller DW, Harrison JL, Brown YA, et al. Immunohistochemical evidence for an endocrine/paracrine role for ghrelin in the reproductive tissues of sheep. Reprod Biol Endocrinol 2005; 3: 60.
- Du C, Xilingaowa, Cao G, et al. Expression of the orexigenic peptide ghrelin in the sheep ovary. Domest Anim Endocrinol 2009; 36: 89-98.
- Zhang W, Lei Z, Su J, Chen S. Expression of ghrelin in the porcine hypothalamo-pituitary-ovary axis during the estrous cycle. Anim Reprod Sci 2008; 109: 356-367.
- Rak A, Gregoraszczuk EL. Ghrelin levels in prepubertal pig ovarian follicles. Act Vet Hung 2009; 57: 109-113.
- Rak-Mardyla A, Gregoraszczuk EL, Karpeta A, Duda M. Expression of ghrelin and the ghrelin receptor in different stages of porcine corpus luteum development and the inhibitory effects of ghrelin on progesterone secretion, 3b-hydroxysteroid dehydrogenase (3β-HSD) activity and protein expression. Theriogenology 2012; 77: 1505-1512.
- Rak A, Gregoraszczuk EL. Modulatory effect of ghrelin in prepubertal porcine ovarian follicles. J Physiol Pharmacol 2008; 59: 781-793.
- Sirotkin AV, Grossmann R. Effects of ghrelin and its analogues on chicken ovarian granulosa cells. Domest Anim Endocrinol 2008; 34: 125-134.
- Sirotkin AV, Chrenek P, Darlak K, Valenzuela F, Kuklova Z. Some endocrine traits of transgenic rabbits. II. Changes in hormone secretion and response of isolated ovarian tissue to FSH and ghrelin. Physiol Res 2008; 57: 745-751.
- Sirotkin AV, Rafay J, Kotwica J, Darlak K, Valenzuela F. Role of ghrelin in regulating rabbit ovarian function and the response to LH and IGF-I. Domest Anim Endocrinol 2009; 36: 162-172.
- Viani I, Vottero A, Tassi F, et al. Ghrelin inhibits steroid biosynthesis by cultured granulosa-lutein cells. J Clin Endocrinol Metab 2008; 93: 1476-1481.
- Fang F, Wang L, Zhang Y, Li Y, Su S, Zhang X. Role of ghrelin on estrogen and progesterone secretion in the adult rat ovary during estrous cycle. Syst Biol Reprod Med 2012; 58: 116-119.
- Tropea A, Tiberi F, Minici F, et al. Ghrelin affects the release of luteolytic and luteotropic factors in human luteal cells. J Clin Endocrinol Metab 2007; 92: 3239-3245.
- Romani F, Lanzone A, Tropea A, et al. in vitro effect of unacylated ghrelin and obestatin on human luteal cell function. Fertil Steril 2012; 97: 991-996.
- Kawamura K, Sato N, Fukuda J, et al. Ghrelin inhibits the development of mouse preimplantation embryos in vitro. Endocrinology 2003; 144: 2623-2633.
- Li L, Ferin M, Sauer MV, Lobo RA. Serum and follicular fluid ghrelin levels negatively reflect human oocyte quality and in vitro embryo development. Fertil Steril 2011; 96: 1116-1120.
- Kheradmand A, Alirezaei M, Birjandi M. Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul Pept 2010; 162: 84-89.
- Kheradmand A, Roshangar L, Taati M, Sirotkin AV. Morphometrical and intracellular changes in rat ovaries following chronic administration of ghrelin. Tissue Cell 2009; 41: 311-317.
- Tilly JL, Kowalski KI, Johnson AL, Hsueh AJ. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991; 129: 2799-2801.
- Sirotkin AV, Meszarosova M. Comparison of effects of leptin and ghrelin on porcine ovarian granulosa cells. Domest Anim Endocrinol 2010; 39: 1-9.
- Sirotkin AV, Meszarosova M, Grossmann R, Benco A, Valenzuela F. Effect of inhibitor and activator of ghrelin receptor (GHS-R1a) on porcine ovarian granulosa cell functions. Gen Comp Endocrinol 2011; 173: 105-110.
- Jaworek J, Nawrot-Porabka K, Leja-Szpak A, Konturek SJ. Brain-gut axis in the modulation of pancreatic enzyme secretion. J Physiol Pharmacol 2010; 61: 523-531.
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011; 62: 139-150.
- Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997; 275: 628-630.
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol 1997; 17: 1595-1596.
- Choi K, Auersperg N, Leung P. Mitogen-activated protein kinases in normal and (pre)neoplastic ovarian surface epithelium. Reprod Biol Endocrinol 2003; 7: 1-71.
- Rak-Mardyla A, Gregoraszczuk E. ERK 1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin action on cell proliferation and apoptosis in the porcine ovarian follicular cells. J Physiol Pharmacol 2010; 61: 451-458.
- Popelkova M, Sirotkin AV, Bezakova A, et al. Effect of IGF-I, leptin, ghrelin and MAPK-ERK on the nuclear maturation of bovine oocytes. Bull Vet Res Inst Pulawy 2006; 50: 179-181.
- Sirotkin A, Grossmann R. The role of ghrelin and some intracellular mechanisms in controlling the secretory activity of chicken ovarian cells. Comp Biochem Physiol A Mol Integr Physiol 2007; 147: 239-246.
- Mousseaux D, Le Gallic L, Ryan J, et al. Regulation of ERK1/2 activity by ghrelin-activated growth hormone secretagogue receptor 1A involves a PLC/PKC varepsilon pathway. Br J Pharmacol 2006; 148: 350-365.
- Sirotkin AV, Bezakova A, Laurincik J, Matejovicova B. Involvement of the metabolic hormones leptin, ghrelin, obestatin, IGF-I and of MAP kinase in control of porcine oocyte maturation. Animal 2011; 5: 94-99.
- Bai R, Zhao P, Cao G, Wen S, Li Q, Meng Q. Ghrelin promotion of oocyte maturation via ERK1/2 pathway in ovis aries. Cell Mol Biol (Noisy le grand) 2012; 5: 1797-1802.
- Chrenek P, Grossmann R, Sirotkin AV. The cAMP analogue, dbcAMP affects release of steroid hormones by cultured rabbit ovarian cells and their response to FSH, IGF-I and ghrelin. Eur J Pharmacol 2010; 640: 202-205.
A c c e p t e d : November 11, 2013