MECHANISM OF ACTION OF TROLOX ON DUODENAL CONTRACTILITY
INTRODUCTION
Vitamin E is the term used for eight naturally occurring essential fat-soluble nutrients called tocopherols (1-4). Vitamin E is an essential nutrient in the human body that must be provided by foods and its absorption from the intestine is a selective process (3-4). Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) is a hydrophilic analogue of vitamin E with a chromane structure similar to α-tocopherol. The role of vitamin E in human nutrition, health, and disease has broadened and changed over the past two decades. α-Tocopherol is a phenolic antioxidant, the main lipid soluble antioxidant in the body of lipoproteins and biomembranes (2). Although mainly acting as an antioxidant, vitamin E can also be a pro-oxidant. It can even have nonantioxidant functions: as a signalling molecule, as a regulator of gene expression, and, possibly, in the prevention of cancer and atherosclerosis (4-5).
α-Tocopherol protects the bladder smooth muscle from the hydrogen peroxide-induced peroxidation (6) and duodenal mucosae from ethanol-induced injury (7). It also attenuates oxidative stress and collagen deposition during the development of experimental chronic pancreatitis (8) as well as nuclear factor kappaB (NF-κB) activation and pro-inflammatory cytokine production induced by lipopolysaccharide (9). Trolox reduces hepatocellular damage (10), protects from ischaemia/reperfusion damage (11), ameliorates the effects of ethanol on acetylcholine-induced response and oxidative stress in isolated rabbit duodenum (12), ameliorates duodenal lipopolysaccharides (LPS)-induced disturbances (13) and abrogates storage-related oxidative stress in small bowel (14).
Some of the cellular actions of α-tocopherol are independent of its antioxidant ability (15). α-Tocopherol, but not β-tocopherol, inhibits thrombin-induced protein kinase C activation and endothelin secretion in endothelial cells. α-Tocopherol has the biological effect of inhibiting the release of proinflammatory cytokines, via inhibition of the 5-lipoxygenase pathway (16-17). The antioxidant effects of vitamin E have been described but the non-antioxidant effects are not well known. In the present work, we propose to study the mechanism of action of Trolox (non-antioxidant effect) on rabbit duodenal motility and contractility.
MATERIALS AND METHODS
Animals
Male New Zealand rabbits, weighing 2–2.5 kg, were fed with standard rabbit food and given free access to water. The rabbits were humanely handled and put down in accordance with the Spanish Policy for Animal Protection RD1201/2005 and the European Union Directive 2010/63/EU.
Chemicals
Acetylcholine (ACh), Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), Bay K8644 (a L-type Ca2+ channel activator), apamin (a blocker of small-conductance Ca2+-activated K+ channels, SKCa), charybdotoxin (a selective blocker of intermediate- and large-conductance Ca2+-activated K+ channels, IKCa and BKCa), glibenclamide (a blocker of ATP sensitive K+ channels), quinine (a blocker of voltage-sensitive K+ channels), tetraetylammonium chloride (TEA, a non-specific K+ channels blocker), 2,5-dideoxiadenosine (DOA, an adenylyl cyclase inhibitor) and nimesulide (a cyclooxygenase-2 (COX-2) inhibitor), were obtained from Sigma (Madrid, Spain). 1H-[1,2,4]oxadiazolo [4, 3-a]quinoxalin-1-one (ODQ, a guanylyl cyclase inhibitor) was purchased from Tocris (Madrid, Spain). All chemicals were analytical grade. Trolox was dissolved in Krebs solution. Bay K8644 was dissolved in ethanol. Glibenclamide, DOA and ODQ were prepared in dimethylsulfoxide. Apamin was diluted in acetic acid. All other chemicals were dissolved in distilled water.
Preparation of duodenal segments and experimental protocols
Segments of rabbit duodenum were removed. Isometric recordings of the longitudinal and circular smooth muscle of the duodenum were performed as described previously (13, 18). Whole thickness segments were vertically suspended in a thermostatically controlled organ bath containing Krebs solution (in mM: NaCl 120, KCl 4.70, CaCl2 2.40, MgSO4 1.20, NaHCO3 24.50, KH2PO4 1.00 and glucose 5.60) at 37°C to achieve pH 7.4 and continuously gassed with 95% O2 and 5% CO2. Each segment was connected to an isometric force transducer (Pioden UF1, Graham Bell House, Canterbury, UK). The segments were stretched passively to an initial tension of 20 mN. The mechanical activity was amplified (The MacLab Bridge Amp, AD Instruments Inc, Milford MA, USA) with a range of 2 mV and recorded for further analysis using the MacLab Systems software. The segments were allowed to equilibrate in Krebs solution for 45 min before use.
After the adaptation period, the spontaneous contractions of the duodenum and the ACh (0.1 mM) responses were recorded in Krebs solution and considered as the control responses. The inhibitors were added to the bath 15 min before the addition of Trolox for 90 min and then a second ACh (0.1 mM) response was evoked. This last response to ACh was compared with the first response to ACh and expressed as percentage. Each experimental protocol was systematically performed on 4 longitudinal and 4 circular muscle segments taken from the same rabbit and repeated in three or four animals. Segments that did not show spontaneous activity were discarded and each preparation served as its own control.
Analysis of data
The amplitude (in mN) and the frequency (contractions per minute, cpm) of spontaneous contractions, and the integrated mechanical activity per second (mN s–1), were calculated as previously described (18). Data are presented as mean percentage with respect to control ± S.E.M. Data sets were compared using one-way variance analysis (ANOVA) tests and P-values were determined using the Scheffe F test. Differences with P-values <0.05 were considered statistically significant.
RESULTS
Spontaneous contractions
The spontaneous contractions of longitudinal and circular smooth muscle of rabbit duodenum were rhythmic and phasic with an amplitude of 20.1 ± 2.4 mN, and 3.6 ± 0.8 mN and a frequency of 14.2 ± 0.5 cpm and 13.2 ± 0.6 cpm (n=24) respectively. At a concentration of 12 mM, Trolox reduced the amplitude to 1.2 ± 0.4 mN and 1.0 ± 0.2 mN and the frequency to 0.9 ± 0.4 cpm and 3.0 ± 0.7 cpm (n=24) of longitudinal and circular smooth muscle of spontaneous contractions respectively (Fig. 1).
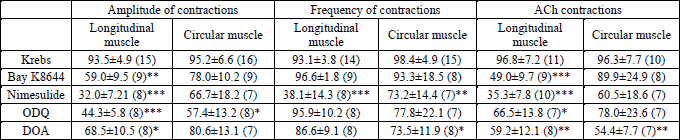
We studied the effect per se of the inhibitors used in this study in longitudinal muscle, Bay K8644 (0.01 µM), nimesulide (1 µM), ODQ (1 µM) and DOA (10 µM) reduced the amplitude of spontaneous contractions and ACh-contractions, while nimesulide reduced only the frequency of contractions. In circular muscle, ODQ reduced the amplitude and nimesulide reduced the frequency of spontaneous contractions; DOA reduced the ACh-contractions in duodenum (Table 1). The effects per se of the K+-channel inhibitors used in this study have been described previously in rabbit duodenum (19, 20).
Effect of Trolox
Trolox 12 mM induced a reduction on the amplitude and frequency of spontaneous contractions and on the ACh-contractions, that were reverted by quinine (10 µM) in longitudinal and circular muscles (Figs. 1 and 2).
The Trolox effect on the amplitude of spontaneous contractions was reverted by charibdotoxin (0.01 µM) and glibenclamide (0.1 µM) only in circular muscle. Furthermore, Bay K8644 (0.01 µM), apamin (0.1 µM), tetraetylammonium chloride (TEA, 5 mM) and 2,5-dideoxiadenosine (DOA, 10 µM) decreased the Trolox effect on amplitude of contractions in circular muscle, and Bay K8644, charibdotoxin and TEA in longitudinal muscle (Fig. 1).
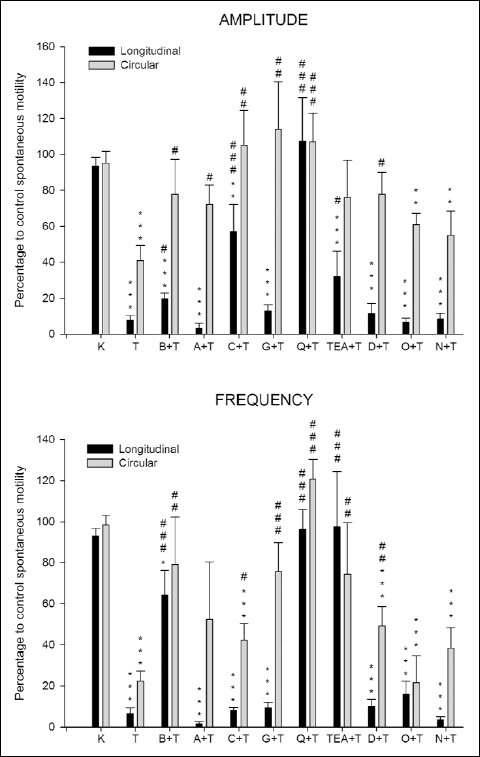 |
Fig. 1. Effect of the incubation for 90 min with Krebs (K, control) or Trolox (T, 12 mM) on amplitude and frequency of spontaneous contractions of the longitudinal and circular smooth muscle from rabbit duodenum. The effect of Bay K8644 (B, 0.01 µM), apamin (A, 0.1 µM), charibdotoxin (C, 0.01 µM), glibenclamide (G, 0.1 µM), quinine (Q, 10 µM), tetraetylammonium (TEA, 5 mM), 2,5-dideoxiadenosine (DOA, 10 µM), ODQ (0.1 µM), nimesulide (N, 1 µM) added 15 min before Trolox (12 mM) on amplitude and frequency spontaneous contractions. Columns are mean percentage values with respect to spontaneous contractions in Krebs (control), and vertical bars indicate SEM. **P<0.01, *** P<0.001 vs. Krebs. #P<0.05, ## P<0.01, ### P<0.001 vs. Trolox. |
The effect of Trolox on the frequency of spontaneous contractions was reverted by TEA (5 mM) only in longitudinal muscle. Moreover, Bay K8644 (0.01 µM), charibdotoxin (0.01 µM), glibenclamide (0.1 µM), TEA (5 mM) and 2,5-dideoxiadenosine (DOA, 10 µM) decreased the Trolox effect in circular muscle and Bay K8644 in longitudinal muscle (Fig. 1).
The effect of Trolox on ACh-induced contractions was reverted by Bay K8644 (0.01 µM), ODQ (1 µM) or nimesulide (1 µM) only in circular muscle (Fig. 2). Furthermore, apamin (0.1 µM), charibdotoxin (0.01 µM), glibenclamide (0.1 µM), TEA (5 mM), and 2,5-dideoxiadenosine (DOA, 10 µM) reduced the ACh-induced contractions in circular muscle, and Bay K8644, apamin, charibdotoxin, glibenclamide, DOA, ODQ or nimesulide in longitudinal muscle (Fig. 2).
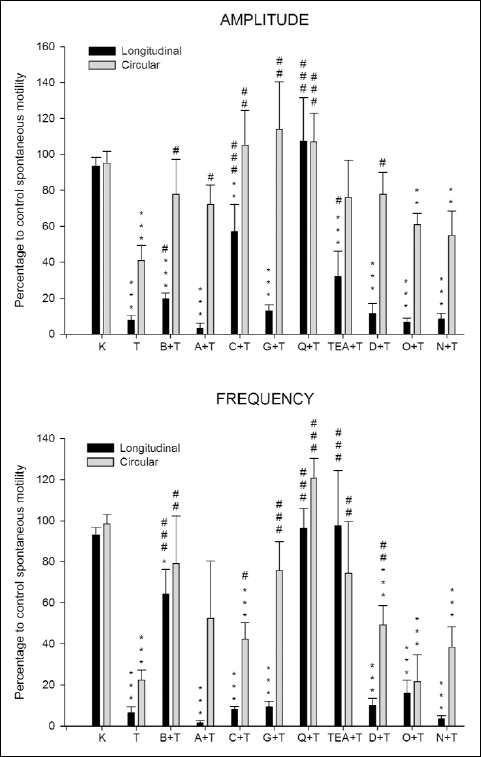 |
Fig. 2. Effect of the incubation for 90 min with Krebs (K, control) or Trolox (T, 12 mM) on ACh-induced contractions in longitudinal and circular smooth muscle from rabbit duodenum. The effect of Bay K8644 (B, 0.01 µM), apamin (A, 0.1 µM), charibdotoxin (C, 0.01 µM), glibenclamide (G, 0.1 µM), quinine (Q, 10 µM), tetraetylammonium (TEA, 5 mM), 2,5-dideoxiadenosine (D, 10 µM), ODQ (O, 1 µM,) and nimesulide (N, 1 µM) added 15 min before Trolox (T, 12 mM) on ACh-induced contractions. Columns are mean percentage values of integrated mechanical activity to control (ACh-induced contractions), and vertical bars indicate SEM. *P<0.05, **P<0.01, *** P<0.001 vs. Krebs. #P<0.05, ## P<0.01, ### P<0.001 vs. Trolox. |
DISCUSSION
The antioxidant properties of vitamin E or α-tocopherol have been extensively studied in various processes (6-14). In addition, vitamin E has other non-antioxidant actions (21). Vitamin E influences the activity of several enzymes (e.g. protein kinase C (PKC), protein phosphatase 2A (PP2A), COX-2, 5-lipooxygenase, nitric oxide synthase, nicotinamide adenine dinucleotide phosphate-oxidase, superoxide dismutase, phopholipase A2 and modulates the expression of genes that are involved in atherosclerosis (22).
Previously, we have shown that Trolox antagonizes the reduction evoked by ethanol or LPS on the duodenal contractility of rabbit (12, 13). These studies suggest that Trolox acts as an antioxidant agent.
The pattern of intestinal motility is attributed to rhythmic oscillations in the membrane potential of slow waves, and the activity of slow waves is generated in the interstitial cells of Cajal, the pacemaker cells of the intestine (23). The Ca2+ and K+ ions participate in the amplitude and frequency of spontaneous contractions of smooth muscle of small intestine (19, 24, 25). The effects per se of quinine (a blocker of voltage-sensitive K+ channels), apamin (a blocker small-conductance Ca2+-activated K+ channels), charibdotoxin (a blocker of intermediate- and large-conductance Ca2+-activated K+ channels), glibenclamide (a blocker of ATP sensitive K+ channels) and quinine (a blocker of voltage-sensitive K+ channels) have been described previously (19, 20). Bay K8644 (a L-type Ca2+ channel activator), nimesulide (a COX-2 inhibitor), ODQ (a guanylyl cyclase inhibitor) and DOA (a adenylyl cyclase inhibitor) reduced the amplitude of spontaneous contractions of longitudinal muscle and nimesulide decreased the frequency in longitudinal and circular smooth muscle (Table 1).
We studied the possible participation of Ca2+ and K+-channels and enzymes as adenylyl cyclase, guanylyl cyclase and COX-2 on the effect of Trolox in spontaneous contractions and on ACh-induced contractions of longitudinal and circular muscle from duodenum. Our results show that Trolox-induced reduction on the amplitude and frequency of spontaneous contractions was reverted by quinine in longitudinal and circular muscle. Furthermore, charibdotoxin, glibenclamide, apamin, TEA, Bay K8644 or DOA reverted or reduced the amplitude or frequency of spontaneous contractions in longitudinal or circular muscle of duodenum. These results suggest that Trolox reduces the spontaneous contractions by the activation of K+ channels (voltage-sensitive, small-, intermediate- and large-conductance Ca2+-activated, and ATP sensitive), L-type Ca2+ channels and the enzyme adenylyl cyclase.
In normoxia, the addition of D-α-tocopherol produces no discernible effect on the frequency or magnitude of spontaneous mechanical activity of colonic muscles. However, in hypoxic tissue it elicits contractile activity and restores the levels of frequency and force of spontaneous contractions in normoxia. Moreover, Trolox and vitamin K3 do not have agonist activity in the hypoxic colon (26). The inhibitors of K+ channels cause different effects on tissues. Charibdotoxin reduces Ca2+-dependent K+ currents in human esophaghus (27). The large-conductance Ca2+-activated K+ channels are constitutively activated for modulations of spontaneous activity of guinea pig ileum longitudinal muscle, but not ATP-regulated K+ channels (28). The spontaneous contractions of rat ileum decrease after quinine administration (29). The pretreatment of rabbit duodenum with charibdotoxin, apamin, glibenclamide or ODQ do not affect the terpinen-4-ol induces relaxation (30). Our results of Trolox, in part, are in accordance with studies in which the acute administration of vitamin E reduces infarct size and maintains the beneficial effect of ischemic preconditioning via mitochondrial KATP channels and cGMP (31). In myocytes of the guinea pig antrum, S(-)-Bay K8644 enhances the peak amplitude of Ba2+ currents, but on the contrary, R(+)-Bay K8644 inhibits Ba2+ currents (32). These results agree, in part, with our results of Bay K8644 because it reduced the effect of Trolox on the amplitude and frequency of spontaneous contractions in rabbit duodenum.
In the present study, the diminution of ACh-induced contractions evoked by Trolox was reverted by quinine in the longitudinal and circular muscle and by Bay K8644, ODQ, or nimesulide in circular muscle, being quinine the most potent blocker. Moreover, the effect of Trolox on ACh-induced contrations was decreased in the presence of apamin, charibdotoxin, glibenclamide, TEA or DOA in longitudinal or circular muscle of duodenum. Our results suggest that the in the Trolox effect on ACh-induced contractions participates K+ and Ca2+ channels, as well as the enzymes guanylyl cyclase, adenylyl cyclase and COX-2. In fact, these Ca2+ or K+ channels and enzymes are involved in intestinal contractions. Quinine does not modify the ACh-contractions in smooth muscle of rabbit duodenum (20). Ca2+-free solutions diminish the ACh-contractions in longitudinal and circular muscle of small intestine (25). Activation of Ca2+-activated K+ channels is involved in duodenal dysmotility induced by ethanol (18).
2, 2´-azobis (2-amidinopropane) dihydrochloride reduces the amplitude of the spontaneous contractions of rabbit duodenal muscle by inward rectifier and intermediate and large-conductance Ca2+-activated K+ channels (33). Diclofenac, a nonsteroidal anti-inflammatory drug, diminishes the delayed-rectifier K+ current in NSC-34 neuronal cells and dorsal root ganglion neurons by the activation of M-type K+ current (34). Dopamine responses were reduced by adenylyl cyclase inhibition on mouse ileum contractility (35). Nimesulide decreases isoprenaline-induced inhibition of postoperative ileus in rat circular jejunal muscle (36). These results agree with our results with nimesulide in duodenum.
In conclusion, our results suggest that the Trolox-induced reduction on the contractility of rabbit duodenum are mediated by K+ channels, L-type Ca2+ channels, adenylyl cyclase, guanylyl cyclase and COX-2.
Acknowledgements: This work was funded by a grant from Gobierno de Aragon, Spain (B61) and Fondo Social Europeo.
Conflict of interests: None declared.
REFERENCES
- Fritsma GA. Vitamin E and autoxidation. Am J Med Technol 1983; 49: 453-456.
- Bjorneboe A, Bjorneboe GE, Drevon A. Absorption, transport and distribution of vitamin E. J Nutr 1990; 120: 233-242.
- Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem 2001; 57: 43-56.
- Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res 2005; 49: 7-30.
- Tafazoli S, Wright JS, O’Brien J. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem Res Toxicol 2005; 18: 1567-1574.
- Matsumoto S, Leggett RE, Levin RM. The effect of vitamin E on the response of rabbit bladder smooth muscle to hydrogen peroxide. Mol Cell Biochem 2003; 254: 347-351.
- Koyuturk M, Bolkent S, Ozdil S, Arbak S, Yanardag R. The protective effect of vitamin C, vitamin E and selenium combination therapy on ethanol-induced duodenal mucosal injury. Hum Exp Toxicol 2004; 23: 391-398.
- Gomez JA, Molero X, Vaquero E, Alonso A, Salas A, Malagelada JR. Vitamin E attenuates biochemical and morphological features associated with development of chronic pancreatitis. Am J Physiol 2004; 287: G162-G169.
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alphα-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol 2005; 169: 97-105.
- Park SW, Lee SM. The beneficial effect of Trolox on sepsis-induced hepatic drug metabolizing dysfunction. Arch Pharm Res 2004; 27: 232-238.
- Sagach VF, Scrosati M, Fielding J, Rossoni G, Galli C, Visioli F. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol Res 2002; 45: 435-439.
- Fagundes DS, Gonzalo S, Grasa L, et al. Trolox reduces the effect of ethanol on ACh-induced contractions and oxidative stress in the duodenum. Rev Esp Enferm Dig 2011; 103: 396-401.
- Fagundes DS, Gonzalo S, Arruebo MP, Plaza MA, Murillo MD. Melatonin and Trolox ameliorate duodenal LPS-induced disturbances and oxidative stress. Dig Liver Dis 2010; 40: 40-44.
- Salehi P, Walker J, Madsen K, Churchill TA. Control of oxidative stress in small bowel: relevance to organ preservation. Surgery 2006; 139: 317-323.
- Azzi A, Breyer I, Feher M, et al. Specific cellular responses to alphα-tocopherol. J Nutr 2000; 130: 1649-1652.
- Martin-Nizard F, Boullier A, Fruchart JC, Duriez P. Alphα-tocopherol but not betα-tocopherol inhibits thrombin-induced PKC activation and endothelin secretion in endothelial cells. J Cardiovasc Risk 1998; 5: 339-345.
- Devaraj S, Jialal I. Alphα-tocopherol decreases interleukin-1 beta release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler Thromb Vasc Biol 1999; 19: 1125-1133.
- Fagundes DS, Grasa L, Arruebo MP, Plaza MA, Murillo MD. Ca2+-activated K+ channels involved in duodenal dismotility induced by ethanol. Alcohol Alcohol 2007; 42: 291-295.
- Lamarca V, Grasa L, Fagundes DS, Arruebo MP, Plaza MA, Murillo MD. K+ channels envolved in contractility of rabbit small intestine. J Physiol Biochem 2006; 62: 227-236.
- Barona I, Fagundes DS, Gonzalo S, et al. Role of TLR4 and MAPK in the local effect of LPS on intestinal contractility. J Pharm Pharmacol 2011; 63: 657-662.
- Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res 2000; 39: 231-255.
- Munteanu A, Zingg JM, Azzi A. Anti-atherosclerotic effects of vitamin E - myth or reality? J Cell Mol Med 2004; 8: 59-76.
- Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal tract. Annu Rev Physiol 1999; 61: 19-43.
- Murillo MD, Plaza MA, de Pedro MJ, Arruebo MP. The effect of Ca2+ antagonists on spontaneous motility from sheep duodenum. J Pharm Pharmacol 1994; 46: 138-140.
- Grasa L, Rebollar E, Arruebo MP, Plaza MA, Murillo MD. The role of Ca2+ in the contractility of rabbit small intestine in vitro. J Physiol Pharmacol 2004; 55: 639-650.
- Kelly MJ, Mathie AZ, Vallance C. A pharmacological action of vitamin E unrelated to its antioxidant capacity. Methods Find Exp Clin Pharmacol 2006; 28: 499-505.
- Wade GR, Laurier LG, Preiksaitis HG, Sims SM. Delayed rectifier and Ca2+-dependent K+ currents in human esophagus: roles in regulating muscle contraction. Am J Physiol 1999; 277: G885-G895.
- Hong SJ, Roan YF, Chang CC. Spontaneous activity of guinea pig ileum longitudinal muscle regulated by Ca2+-activated K+ channel. Am J Physiol 1997; 272: G962-G971.
- Jing F, Liu M, Yang N, Liu Y, Li X, Li J. Relaxant effect of chloroquine in rat ileum: possible involvement of nitric oxide and BKCa. J Pharm Pharmacol 2013; 65: 847-854.
- Nascimento NR, Leal-Cardoso JH, Lessa LM, Roriz-Filho JS, Cunha KM, Fonteles MC. Terpinen-4-ol: mechanisms of relaxation on rabbit duodenum. J Pharm Pharmacol 2005; 57: 467-474.
- Andreadou I, Iliodromitis EK, Tsovolas K, et al. Acute administration of vitamin E triggers preconditioning via K(ATP) channels and cyclic-GMP without inhibiting lipid peroxidation. Free Radic Biol Med 2006; 41: 1092-1099.
- Zhu HL, Teramoto N. Antagonistic actions of S(-)-Bay K 8644 on cyclic nucleotide-induced inhibition of voltage-dependent Ba2+ currents in guinea pig gastric antrum. Naunyn Schmiedebergs Arch Pharmacol 2008; 378: 609-615.
- Hernandez L, Grasa L, Fagundes DS, et al. Role of potassium channels in rabbit intestinal motility disorders induced by 2, 2’-azobis (2-amidinopropane) dihydrochloride (AAPH). J Physiol Pharmacol 2010; 61: 279-286.
- Huang CW, Hung TY, Liao YK, Hsu MC, Wu SN. Underlying mechanism of regulatory actions of diclofenac, a nonsteroidal anti-inflammatory agent, on neuronal potassium channels and firing: an experimental and theorethical study. J Physiol Pharmacol 2013; 64: 269-280.
- Zizzo MG, Mule F, Mastropaolo M, Serio R. D1 receptors play a major role in the dopamine modulation of mouse ileum contractility. Pharmacol Res 2010; 61: 371-378.
- Goetz B, Benhaqi P, Muller MH, Kreis ME, Kasparek MS. Changes in beta-adrenergic neurotransmission during postoperative ileus in rat circular jejunal muscle. Neurogastroenterol Motil 2013; 25: 154-e84.
A c c e p t e d : November 13, 2013