THE EMERING ROLE OF HELMINTHS IN TREATMENT
OF THE INFLAMMATORY BOWEL DISORDERS
INTRODUCTION
The nematodes have accompanied mankind for centuries and have a significant impact on the occupied tissue niches, as well as the general health of their hosts. The development of the defence mechanisms of these parasites resulted in a strategy of modulating the host immune responses (1). Based on recent observations, including clinical trials, it has been suggested that the host-parasite biocenotic relationship between humans and nematodes of the gastrointestinal tract can be considered as a mutualism, rather than a typical parasitism. This relationship became evident when a high level of personal hygiene, pasteurisation of food and access to a wide range of medicines resulted in a significant reduction of helminth infections in populations of countries with “very high human development” according to the Human Development Index. It has been proven that progressive environmental sterilisation is usually accompanied by an increased incidence of immune-mediated diseases, including inflammatory bowel diseases (IBDs), which is particularly pronounced in North America and Western Europe (2, 3) (Fig. 1).
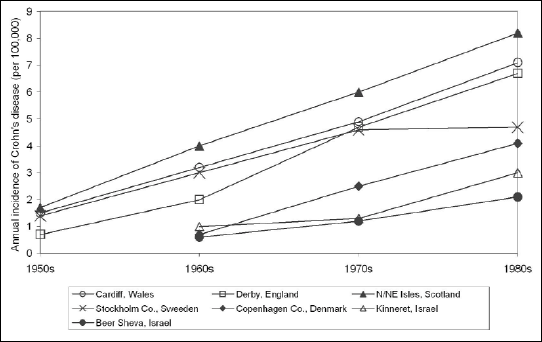 |
Fig. 1. Trends in the average annual incidence (new cases) of Crohn’s disease in chosen locales. Based on: Elliott DE, FASEB J 2000; 14: 1848-1855. |
In developing countries of Africa, South America and Asia, where the level of personal and environmental hygiene is relatively low, incidence of autoimmune diseases is also significantly lower. Limitations in exposure to common bacterial and parasitic pathogens in populations of highly and very highly developed countries have probably contributed to depletion of immunological memory and the development of hypersensitivity mechanisms. Currently, autoimmune diseases are a major problem for the healthcare system in the United States of America as well as Western European countries, ranking just behind cardiovascular diseases, cancer and metabolic disorders (3). Analysing the causes of the increased incidence of autoimmune diseases, as explained by The Hygiene Hypothesis, the preventive role of intestinal nematodes was highlighted in the pathogenesis of inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis (4).
THE HYGIENE HYPOTHESIS
In his analysis of the progressive incidence of allergic rhinitis, which is described in the category of a post-industrial revolution epidemic, Strachan (5) drew attention to the significant negative correlation between the occurrence of this disease, as well as atopic dermatitis, in children and the number of children in the family, which he explained by the lower hypersensitivity in children who are more often exposed to microorganisms as a result of constant contact with their siblings. Thus the observed increase in the incidence of atopic syndromes in recent years can be linked to the improvement of personal hygiene, progress in childhood infectious diseases prevention and the declining participation of large families in society, which is particularly evident in populations of highly developed countries. Strachan’s concept, described as “the hygiene hypothesis”, has over time been extended to other diseases, including autoimmune diseases, encephalitis, atherosclerosis and some types of cancer (6-8).
Diminution of susceptibility to allergic diseases was initially linked mainly to early exposure to microorganisms involved in the aetiology of childhood infectious diseases, but with time, non-pathogenic microorganisms, especially the intestinal microflora, short-chain fatty acids released by the intestinal bacteria from food and finally parasites, were shown to be able to modulate atopy by reducing the amount of immunoglobulin E produced by the host (9). The hygiene hypothesis has been documented quite well with the results of epidemiological studies, although they are not unequivocal (10, 11). It does not offer an adequate explanation for the observed incidence of asthma, which is currently decreasing or remains in the plateau phase in societies of developed countries (12-16), while remaining high in Brazil (17) and with a growing trend in the USA in urban populations of African Americans with relatively low economic status (18). It has been postulated that, in the case of atopic asthma, the prevalence depends not so much on microbial or parasitic exposure in early childhood and the prenatal period, but rather on constant exposure throughout life (9). Children’s exposure to protective factors in the development of autoimmune diseases can be modified by the number of siblings in the family, socio-economic status, gender and age of the siblings (birth order), and health status of the parents (atopy), especially the mother, e.g. congenital infections and humoral immunological response in the foetus is determined by the diet and drug intake during pregnancy (19, 20). Particularly controversial in Strachan’s hypothesis was the relationship between decreasing exposure to protective factors in the development of autoimmune diseases and the improvement of household amenities, as well as higher standards of personal cleanliness. This relationship was not confirmed by extensive epidemiological studies; houses are continuously swarmed with pathogenic, commensal, free-living microorganisms that are introduced by people, animals, with food, water, and sometimes air, where they essentially maintain their qualitative structure of multi-species complexes regardless of the hygienic rigours, constituting a permanent source of exposure (19). Therefore, in order to avoid simplifications in interpretations of Strachan’s concept, it has been proposed to call it “the microbial deprivation hypothesis” (21). A strong argument in favour of Strachan’s hypothesis is its biological credibility. In connection with the humoral basis of allergic conditions with quantitative TH2 dominance, the protective role of microorganisms can be explained by activating PRR signalling pathways (PRRs, pattern recognition receptors), which modulate immunological polarisation, promoting the TH1 lymphocyte differentiation. However, many parasitic invasions promote TH2 T-cell differentiation and thus can relieve symptoms of autoimmune diseases associated with TH1 T-cell overproduction (22). Immunological polarisation, based on mutual antagonism of TH1 and TH2 T-cells, can be regulated by the quality of the invasive agent as well as its dose (9). However, currently, the “direct microorganism effect” concept on mutual TH1/TH2 balance is interpreted in terms of a far-reaching simplification. It is suggested that these factors work rather as a positive stimulus required for proper regulatory T-cell (TREG) development, and therefore, in the absence of such stimuli, the suppression abilities of the TH1/TH2 effector cells are impaired, predisposing to autoimmune or allergic disorders. This concept has been developed in the “old friends” hypothesis (23-25). This concept involves a decrease in preventive factors, contributing to an autoimmune response to non-pathogenic or weakly pathogenic microorganisms that have accompanied mammals for a long time in the evolutionary history. In this context, saprophytic Mycobacterium spp., Lactobacillus spp. and some helminths are taken into consideration. They are recognised by innate immune response mechanisms as relatively harmless and used as a natural adjuvant in TREG activation.
Among the alternative hypotheses to the hygiene hypothesis and its modifications, explaining the rise of autoimmune disease incidence in highly developed countries, the predisposing role of physico-chemical components of environmental pollution, exposition to allergens, diet, obesity and lifestyle is emphasised (6, 26-28). An interesting variation of the “old friends” hypothesis is the hypothesis of early immune challenge (6), which highlights the role of helminths that are able to modify their host immunological processes and can form a tolerant environment for themselves, in which inflammation is suppressed. In this context, the effects of parasites are associated with modulation of TREG activity, which may explain the suppression of inflammation induced by non-pathogenic environmental factors or self-antigens of the host.
INFLAMMATORY BOWEL DISEASES
Inflammatory bowel diseases are a heterogeneous group of idiopathic, chronic inflammatory diseases, characterised by various genotype disturbances leading to an aggressive immune response, including CD4+ T cells, against antigens of intestinal bacteria. The beginning and the reactivation of the condition is associated with environmental factors that are capable of destabilising the intestinal mucosal barrier, stimulating the immune response and disrupting the balance between commensal and pathogenic intestinal bacteria (29-31).
The most common forms of IBDs are ulcerative colitis, caused by a diffuse, non-specific inflammatory process of rectal and colonic mucosa, leading in some cases to ulcers, and Crohn’s disease, a transmural, mainly granulomatous inflammation, which can affect any part of the gastrointestinal tract. In the latter IBD, the inflammatory process begins in the mucosa, but then gradually involves all layers of the gastrointestinal wall, leading to its destruction and fibrosis, which results in fistulas and intestinal stenosis (32).
Chronic inflammation is caused by continuous exposure to toxic components of intestinal microflora and genetic host susceptibility (30). Currently, 163 gene loci are known to influence the manifestation of IBD symptoms, increasing the risk of these diseases or determining the clinical phenotype, as well as the location of the lesions (33). An especially significant role in IBD pathogenesis is played by the genes encoding the proteins involved in signal transduction and pathogen identification, (NOD2/CARD15, NOD1/CARD4, TLR4, CD14), determining the intestinal mucosal barrier (OCTN1/SLC22A4, OCTN2/SLC22A5, DLG5), mediators of immune responses (cytokines, adhesion molecules, determining connections between cells, as well as cells with extracellular matrix) or are involved in antigen presentation to T lymphocytes (HLA) (29). The genetic background of IBDs may involve candidate-gene mutations and gene polymorphisms, as well as the possibility of epigenetic control of their expression by environmental factors that are able to promote methylation in promoter regions of candidate genes and influence the chromatin arrangement, as well as DNA availability for transcription through histone protein modifications (29, 34, 35).
The mucosal immune system of the intestine is prepared to respond to pathogen antigens, maintaining the tolerance for commensal antigens of intestinal bacteria. Incorrect functioning of the immune system in patients with IBDs leads to induction of local inflammation in the intestinal mucosa by the intestinal microflora, resulting in leakage of the intestinal barrier and its disintegration (30, 36, 37). In the pathogenesis of IBDs the fundamental importance of dendritic cells should not be forgotten (38). Their role varies depending on the degree of their differentiation and they play an important role in maintaining a proper immune balance in the intestine (38, 39).
Immature dendritic cells maintain an appropriate level of immune tolerance towards antigens present in the intestine, including those from the gut microflora (39). Fully mature dendritic cells show higher capacity for antigen presentation and initiation of the immune response, leading to a loss of immune tolerance, which promotes the development of inflammation in the intestine. It has been shown that the lipopolysaccharides derived from E. coli significantly accelerate the maturation of dendritic cells. This disturbs the immunological balance of the intestine and reduces the level of immune tolerance, which leads to an increased synthesis of pro-inflammatory cytokines and exacerbation of the inflammation in the intestine (38). This suggests that there is a strong relationship between the composition of the intestinal microflora and the immune response in the intestine in patients with IBDs.
CD4+ T lymphocytes that are stimulated in regions of inflammation produce large amounts of cytokines, which determine the further course of the immune response. In patients with Crohn’s disease, the humoral response including TH1 cells is dominant, and is supported by TH17 cells, although a change of immune polarisation is possible towards TH2 cells with the progress of inflammation. Activation of TH1 and TH17 lymphocytes is observed in the course of Crohn’s disease, and is associated with an increase in the activity of cytokines produced by these cells, i.e. IFN-γ and IL-17. TH1 cells are stimulated to synthesise IFN-γ by IL-12, originating from antigen-presenting cells. Production of IL-17 in TH17 cells is induced by IL-6, TGFβ, and IL-23, secreted by antigen-presenting cells (mainly dendritic cells), as well as macrophages and neutrophils, which are recruited to inflamed tissue. Probably the IL-12 - IFN-γ and IL-23 - IL-17 signalling pathways are activated independently, since IFN-γ and IL-17 demonstrate mutual suppression. In addition, IL-12 induces production of IL-21, both participating in the activation of transcription factor T-bet, which plays a key role in differentiation and activation of TH1 lymphocytes. Resistance of activated TH1 cells to apoptosis is associated with the role of IL-6 in positive regulation of anti-apoptotic gene expression (BCL2 and BCLL1). In patients with ulcerative colitis, an increased response involving TH2 lymphocytes is observed. TH2 cells are activated by NK with IL-13. This results in an increased synthesis and release of cytokines responsible for humoral immune response. NK cells are activated to produce IL-13 by dendritic cells, through expression of atypical MHC molecule- CD1d- capable of presenting bacterial lipid antigens. The main pathogenic role in IBDs is assigned to the pro-inflammatory cytokines, which are predominant in relation to cytokines that are antagonistic to their activity. These are produced by TH1 and TH17 lymphocytes, selectively activated in Crohn’s disease, as well as monocytes and granulocytes, which are recruited to the site of inflammation (Table 1). In response to bacterial antigens/adjuvants in these cells, the NFκB pathway is activated via PRRs, which are associated with the production of proteins that induce and modulate inflammation (30).

THE ROLE OF HELMINTHS IN MODULATION OF THE HOST IMMUNE RESPONSE
Modulation of Toll-like receptor expression
Interference of Toll-like receptor (TLR)-dependent signalling pathways, which play a crucial role in regulation of inflammation and pathogen recognition, can contribute to chronic inflammation and disturbance of the homeostasis of intestinal microflora and gastrointestinal tract mucosa, resulting in various clinical IBD phenotypes (40). Currently, 13 mammalian TLRs are known (41), all of which are transmembrane receptors that are present in the cell membranes as a cell surface receptor or in intracellular organelles. TLRs are expressed throughout the whole gastrointestinal tract, in enterocytes, myofibroblasts, neuroendocrine cells and cells within the lamina propria, including T cells and dendritic cells (40). TLR activation results in adaptor protein recruitment, including the TIR domain - containing adapter protein 1 (TIRAM1), TIR domain - containing adaptor protein inducing interferon (TRIF), myeloid differentiation primary - response protein 88 (MyD88), TRIF-related adaptor molecule (TRAM), which eventually leads to activation of NFκB, JNK, p38, ERKs and interferon regulatory factors (IRFs), resulting in the production of pro-inflammatory cytokines (30, 42-46). Toll-like receptors in dendritic cells activate signalling pathways and stimulate the mobilisation of other subpopulation of immune cells, including CD8+ T cells, B cells and macrophages (47).
Helminths can both activate and limit the TLR expression (40). Proteases of Trematoda specifically degrade TLR3 in endosomes in macrophages, which restricts the activation effectiveness of these cells (48). Lyso-phosphatidylserine, isolated from Schistosoma mansoni, contains a specific acyl chain, which, through a TLR2-dependent pathway, promotes dendritic cell differentiation, which induces regulatory T cells to produce anti-inflammatory IL-10 (49). Excretory-secretory products (ESP) of helminth parasites can also modulate the signal transmission in canonical pathway of NFκB activation, involving MyD88 (50), as well as the alternative TRIF-dependent signalling pathway (46), which also regulates type 1 interferon production. Rodents infected with intestinal nematodes showed increased expression of the MUC2 gene, associated with activation of a TLR2-dependent signalling pathway, which results in mucus overproduction and therefore improves mucosal membrane barrier integrity (51). Increased TLR2 and MUC2 gene expression was observed in vitro in human enterocytes exposed to proteins of intestinal trematode, Gymnophalloides seoi (52). The protective importance of the inner mucous membrane, lining the intestinal epithelium and consisting mainly of MUC2 intestinal mucin, was confirmed in an animal model of ulcerative colitis (53, 54).
Induction of proliferation and activation of TH2 lymphocytes
The concept of the preventive role of helminths in autoimmune diseases development is essentially based on TH1-TH2 interaction, as well as on the profile of cytokines produced by these cells. Stimulation of proliferation and activity of TH2 cells by helminths is a mechanism of the specific immune response, limiting the range of expansion of these parasites by increasing the humoral immune response that leads to the production of specific antibodies against its antigens (55). Anti-inflammatory cytokines production by TH2 cells, with simultaneous inhibition of the TH1 lymphocyte differentiation and activation, explains the negative correlation between the prevalence of Crohn’s disease associated with aggressive TH1 humoral immune response in some of the studied populations and helminthiases (56-59). The significance of helminth invasion in limiting the development and duration of chemically induced colitis with TH1 overproduction was confirmed under experimental conditions in animal models.
Induction of proliferation of regulatory T cells
TREG cells are a subpopulation of immunosuppressant T helper cells. Activation of these cells leads to increased cytokine activation, limiting the differentiation of effector CD4+ T cells, i.e. TH1, TH2 and TH17 cells (37). These play an important role in formation of an immunological tolerance environment in the gastrointestinal tract, which on the one hand simplifies the parasite survival in the intestine, and on the other hand protects against the development of hypersensitivity in the intestinal wall (60). Among the CD4+ TREG cells, three separate cell types can be distinguished: CD4+CD25+ TREG cells, which inhibit the effector cells by direct contact or by production of immunosuppressant cytokines; TR1 cells, which secrete large amounts of IL-10; and TH3 cells, which primarily produce TGFβ (37).
Both effector and regulatory lymphocytes have an effect on antigen-presenting cells through cytokine secretion and subsequent feedback loop - IFN-γ, a product of TH1 cells up-regulates IL-12p40 and MHC class II molecules expression, while TREG cells, that secrete IL-10 or TGFβ, inhibit their activity (30). A separate group of TREG cells involved in the control of the intestinal mucosa homeostasis through pro-inflammatory CD4+ T cell suppression consists of CD8+ T cells, i.e. CD8+CD28- (61), CD8+CD122+ (62), CD8+CD11c+ (63) and CD8+ Qa-1-restricted cells (64).
Helminths are able to cause TH1 lymphocyte suppression both by provoking an immune response involving TH2 cells, as well as by inducing the differentiation of TREG lymphocytes (65).
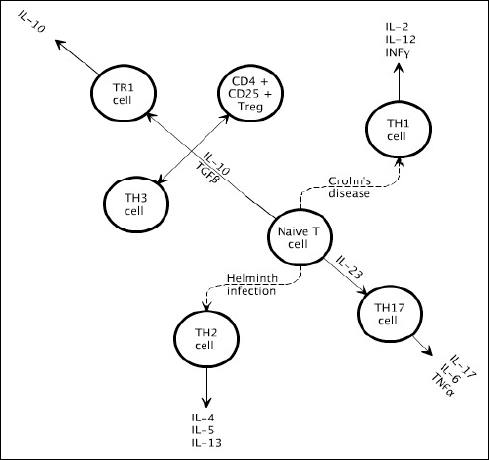 |
Fig. 2. T-cell subsets. Naive CD4+ T cells are stimulated by antigen-presenting cells and the cytokine environment to proliferate into a certain subset: TH1, TH2 and TH17. CD+4 regulatory T cells can be subdivided into CD4+CD25+TREG, TR1 and TH3 cells. Crohn’s disease is characterised by TH1 and TH17 inflammation, whereas helminths induce TH2 and regulatory T cells. |
Cystatins production
Cystatins are a superfamily of proteins that inhibit cysteine proteinases (66). Their substrates are endogenous helminth serine proteases, particularly asparaginyl endopeptidase (AEP), as well as cathepsin L and S, and to a lesser extent cathepsin B, along with, after secretion, host enzymes, which is associated with their role in immunomodulation. They interfere with antigen presentation to T-helper cells by inhibition of serine proteases in dendritic cells and/or macrophages (67). The influence of nematodal cystatins on CD4+ T cells is complex, including additionally selective modulation of cytokine synthesis in monocytes, especially stimulation of IL-10 production (68). This effect involves inhibition of expression of some co-stimulatory molecules (CD40, CD80) and MHC class II (HLA-DR).
It seems that cystatins produced by intestinal nematodes have a similar immunomodulatory effect, which is understood to be a manifestation of a universal defence strategy of these and other internal parasites. Increased IL-10 synthesis was observed in murine macrophages and enterocytes after administration of recombinant cystatin of the liver fluke Clonorchis sinensis, which was associated with the remission of chemically induced colitis (69). Recombinant cystatin of the intestinal nematode Nippostrongylus brasiliensis is able to interfere with the endocytic pathway of antigen processing and presentation, inhibiting the lysosomal cathepsin L, and to a lesser extent cathepsin B. On the other hand, cystatins of the free-living nematode Caenorhabditis elegans promote TH1 cell differentiation with subsequent inflammation because of relatively strong affinity to cathepsin B along with a simultaneous stimulation of IL-12 production (67).
Glycoprotein ES-62 production
ES-62 is an oligomeric glycoprotein that is expected to have aminopeptidase activity. During post-translational modification, a phosphorylcholine moiety is added to the ES-62 molecule, which to a large degree determines its immunomodulatory properties. Phosphatidylcholine is one of the pathogen-associated molecular patterns (PAMPs), which enable the microorganism or parasite identification by antibodies or C-reactive protein (CRP), but at the same time increases the possibility of its survival by modulating the host immune system functions. ES-62 acts on effector cells most likely via its TLR2 receptors causing: 1) maturation of dendritic cells, which activate the TH2 cell differentiation; 2) interference with production of pro-inflammatory cytokines in induced macrophages; 3) inhibition of cell division activity in conventional B lymphocytes, while stimulating the proliferation of the B1 lymphocyte subpopulation, able to bind the phosphorylcholine moiety via B-cell antigen receptor (BCR), with simultaneous IL-10 production in these cells (70, 71).
Alternative activation of macrophages
One of the immunomodulatory functions of helminths is their impact on the alternative pathway of macrophage activation, which might have an anti-inflammatory and immunosuppressive effect (72). Schistosomae mansoni proteins are potent inducers of TH2 cell proliferation, whose activation leads to the synthesis of anti-inflammatory cytokines, such as IL-4 and IL-10 (73). Consequently, there is an imbalance in the TH1/TH2 ratio in the favour of the TH2 cells, resulting in an increase in TREG cells, which are characterised by their immunosuppressive effect (74, 75), as well as an increase in expression of alternative pathway of macrophage activation markers, such as arginase I, which has an anti-inflammatory potential (75, 76).
PARASITIC NEMATODES
The roundworms of the nematodes class are mostly free-living species, found in fresh or salt water, in the mud or the soil surface, and they are rarely parasites of plants or animals, especially vertebrates. A noted health hazard due to high pathogenicity is caused by Ascaris lumbricoides, larvae of Toxocara spp., hookworms (Ancylostoma duodenale, Necator americanus), Trichinella spiralis and some species of the Filarioidea superfamily, especially Wuchereria bancrofti, Brugia malayi and Onchocerca volvulus (71) (Fig. 3).
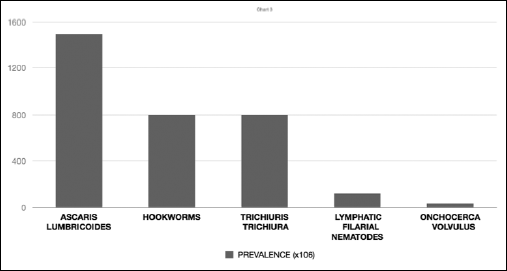 |
Fig. 3. Estimated prevalence of the major medically important human nematode parasites; there are two species of hookworms that parasitize humans: the Old World hookworm, Ancylostoma duodenale, and the New World Necator americanus. The most important filarial nematodes of the lymphatic system are Wuchereria bancrofti and Brugia malayi. Based on: Harnett, Expert Rev Mol Med 2008; 10: e18. |
Some of the nematodes are human-specific parasites (Enterobius vermicularis), while others use a wide range of mammalian hosts (Trichinella spiralis), and others can be accidental paratenic hosts of humans (Toxocara) (77). Ontogeny of nematodes is characterized by five developmental stages, separated by four moultings. The most numerous are oviparous nematodes. Depending on the species, the eggs are laid by females in various developmental stages - before cleavage (Ascaris), at the end of cleavage (Enterobius) or after larvae development inside the egg (Strongyloides). There are also ovoviviparous nematodes, whose larva in the first stage hatches from the egg in the uterus of the parent system (Trichinella) or causes the conversion of the eggshell into an elastic sheath surrounding the active larva (Wuchereria). An invasive form for humans is usually the third larval stage (L3), formed after the second moulting. The second larval stage (L2) can be exceptionally invasive, which takes place in the development cycle of Ascaris; however, the larva migrates in a complex way in the host organism, which is considered a phylogenetic reminiscence of an intermediate host, which was eliminated during evolution, establishing the monoxenous type of development (78). Among the nematodes, a large variety of life-cycles are distinguished. Some of them engage in temporary parasitism, when the parasitic lifecycle is led by the larvae or adult forms. Moreover, an alternation of generations can occur in free-living and parasitic Strongyloides stercoralis.
Since the transition to parasitism, nematodes have been forced to develop adaptation mechanisms that enable them to survive in the immunologically unfavourable environment of the host. Among the defence strategies of these parasites, their ability to modulate the immune system of the host was described through their influence on immunological polarisation, the profile of produced cytokines and the efficiency of signalling pathways, as well as activity inhibition of certain enzymes. This effect is associated with secretion of a number of immunomodulators, such as cytokine homologues, which are able to interfere with the effector mechanisms of the host immune system (68, 79, 80).
PROSPECTIVE USE OF HELMINTHS IN INFLAMMATORY BOWEL DISEASE TREATMENT
Intestinal nematodes are able to modulate, and even inhibit, the immune responses of the host via their excretory-secretory products. Thus, there are attempts to use controlled invasion of these parasites in therapy of autoimmune diseases, including type 1 diabetes, multiple sclerosis, autoimmune liver diseases and IBD (8). In an experimental model of Crohn’s disease in rodents, after the induction of colitis through exposure to trinitrobenzene sulfonic acid and infection with Heligmosomoides polygyrus, inflammation remission was observed (Table 2).
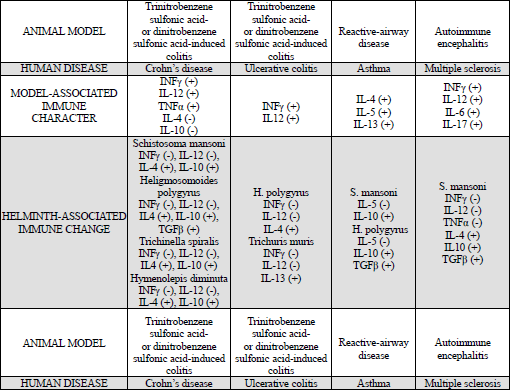
In transgenic IL-10–/– mice, in which acute colitis occurs as a result of intense TH1 immune response to natural intestinal microflora, progression may be inhibited due to Heligmosomoides polygyrus or Trichuris muris infection. The former is linked to local reduction of INF-γ and subunit p40 of IL-12 production, as well as increased expression of the transcription factor Fox3P in TREG cells (81). In an experimental model, the possibility of therapeutic use of nematodes in alleviating the symptoms of ulcerative colitis was confirmed. In this model using mice, colitis was induced by acetic acid, which damages the intestinal mucosa. This leads to penetration of bacteria and their products from the colon lumen into the lamina propria, as well as cytokine secretion by activated enterocytes, and consequently to recruitment of neutrophils that are capable of damaging the intestinal epithelium. In pathogenesis of ulcerative colitis both cytokines of TH2 lymphocytes and to a lesser extent TH1 and TH17 lymphocytes are involved. Helminth invasions stimulate the production of IL-10 and TGFβ, which induce the differentiation of FoxP3+ TREG cells. These play a key role in maintaining the immune homeostasis, since they control the intensity of both TH1 and TH2 responses. FoxP3 protein can also inhibit the cell differentiation of TH17 lymphocytes by blocking the transcription factor RORγt, which is required in this process. In mice with acetic acid-induced colitis, after infection with Trichinella spiralis, FoxP3+ TREG cells recruitment in inflammatory foci was observed (82). Reservations regarding the practical use of helminthic therapy, resulting from the research on animal models of IBDs, concern the possible adverse interactions with pathogens of the gastrointestinal tract. One example is the increased mortality of mice infected at the same time with Heligmosomoides polygyrus and Citrobacter rodentium, a bacterial pathogen, which under experimental conditions is a model equivalent of enteropathogenic strains of Escherichia coli. Furthermore, considering, that the research on animals is conducted in a period of time predicted by the procedure of the experiment, it is difficult to assess the late consequences of parasitic invasion, which may have positive outcomes for the host (prolonged protective effect), as well as negative outcomes (induction of fibrosis) (83).
In general, worm invasions in rodents cause immunosuppression and modulation of the immune response associated with the increased production of TREG cells, B cells and macrophages, as well as with the control of immunological polarisation, as a result of an altered phenotype of the antigen-presenting cells. The effectiveness of pro-inflammatory cytokines in remission of colitis can be determined by the kinetics of their production under conditions activated by the parasite immunological process, since the use of recombinant cytokines administered in therapy of IBDs does not bring the expected results. This applies especially to induced synthesis of IL-10 and TGFβ, although the effectiveness of the latter cytokine is limited, both in experimental models of IBDs as well as in patients with Crohn’s disease and ulcerative colitis, by a functional defect of its receptor in target cells. Under normal conditions the TGFβ1 binding to its membrane receptor type II (TGF-βRII) leads to activation of TGF-β receptor type I (TGF-βRI), which is involved, through phosphorylation, in activation of signalling proteins Smad2 and Smad3. After phosphorylation, both proteins build a trimeric complex with Smad4 (co-Smad), which after translocating to the nucleus binds to DNA, participating in the regulation of gene transcription. The TGFβ signalling pathway is not activated in IBDs, as a result of overproduction of Smad7 in the intestinal mucosa, a protein that blocks the Smad3 phosphorylation through TGF-βRI (Fig. 4).
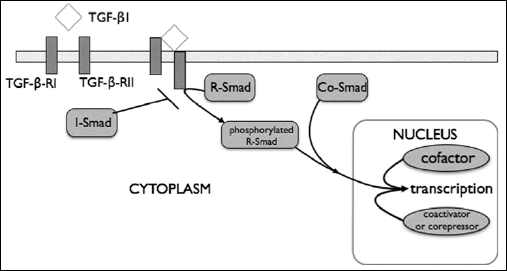 |
Fig. 4. TGFβ signalling pathway: R-Smad = receptor-regulated Smads (Smad2, Smad3); Co-Smad = common-mediated Smad (Smad 4); I-Smad = inhibitory Smad (Smad7); details in text. |
A possible cause of the Smad7 overproduction in IBDs is the hyperacetylation of lysine moieties of this protein, which blocks its ubiquitination with subsequent proteasomal degradation that is observed under normal conditions. Therefore, to improve the efficiency of helminthic therapy in IBDs, it is reasonable to unblock the TGFβ signalling pathway in enterocytes and lamina propria cells, which can be achieved by gene therapy techniques. In mice with chemically induced colitis, it was possible to alleviate the symptoms after oral administration of Smad7 antisense oligonucleotide, which led to increased Smad3 phosphorylation by activated TGF-βRI in colonic mucosa cells. In perspective, it is also postulated to silence the expression of transcriptional coactivator p300, which is involved in the acetylation of Smad7. However, such a procedure includes a proviso, i.e. TGFβ is a pleiotropic cytokine and its effects depend on the type of the cell, and in some types of cells undesirable side-effects, such as production of collagen leading to fibrosis, may occur (84). Currently, research is being conducted to evaluate the use of Smad7 antisense oligonucleotide (GED0301) in the treatment of Crohn’s disease. These studies confirmed good drug tolerability in patients (85) as well as the fact that GED0301 does not raise the risk of small intestine lesions caused by undesirable induction of fibrosis (86).
The selection of the parasite species for testing its therapeutic potential in clinical trials is determined by the degree of pathogenicity and susceptibility to anti-helminthic drugs or other commonly used medicines, as well as standard procedures for obtaining the parasite under sterile conditions.
Clinical trials of IBDs treatment typically use Trichuris suis, a swine parasite that is phylogenetically close to Trichuris trichiura and is able to transiently colonise the human colon. Trichuris suis is a geohelminth, which eliminates the possibility of infection thorough direct contact. Fertilised eggs of this nematode require several weeks of incubation in moist soil before reaching invasiveness. This parasite can be obtained in large amounts from the colon of farmed pigs in an environment free of pathogens and cultured in vitro prior to laying by the fertilised females eggs, which after the necessary incubation can be administered to a patient (81). After oral administration of invasive eggs of this parasite, in a single dose of 2500 eggs, relief of symptoms was achieved in a small group of patients with Crohn’s disease and ulcerative colitis (4 and 3 patients respectively) (87). In the case of Crohn’s disease, similar results were observed in the majority of patients of a larger group (29 patients) when invasive parasite eggs at the same dose were administered several times over a longer period of time (every 3 weeks for 24 weeks) (88). In both of these studies, patients were informed about the methods of treatment. The effectiveness of controlled Trichuris suis invasion in relieving the clinical picture of ulcerative colitis was confirmed in a randomised, placebo-controlled, double-blind, clinical trial (2500 invasive parasite eggs per dose, repeated every 2 weeks for 12 weeks). Relief of ulcerative colitis symptoms was significantly higher in the patients taking the eggs than those receiving placebo (43.3% and 16.7% of all respondents, in a group consisting of 54 patients) (89).
There have also been attempts to use Trichuris suis in the treatment of other immune-mediated diseases, such as allergic rhinitis, peanut allergy, multiple sclerosis and autism (90).
Attempts to use other intestinal nematodes that are specific human parasites with dose-controlled pathogenicity in the treatment of IBDs have also been conducted. In a single case in a patient with a non-specific ulcerative colitis resistant to pharmacological treatment, remission of symptoms and alleviation of inflammation in the colon was observed after ingestion of invasive Trichuris trichiura eggs (91). Trials using Necator americanus attempted to use this hookworm in the treatment of asthma and celiac disease (90). Parasite administration to a patient, in the case of Necator americanus, is associated with the active skin penetration by invasive larvae and then their obligatory passage into the bloodstream. Larvae move to the lungs, from where, after penetration from the vessels into the alveoli, through bronchi and trachea, they reach the throat where, after swallowing, they get into gastrointestinal tract (92). In a group of 9 patients with Crohn’s disease after a single administration of 50 invasive Necator americanus larvae, symptoms relief was observed in 2 of them whose symptoms were most severe, while in the remaining patients with mild symptoms, no improvement was noted (93).
CONCLUSIONS
The therapeutic effects of a controlled parasitic nematode infection in the course of IBDs have been known for many years in animal models (94) and recently also in humans (93).
Helminthic therapy, as an unconventional method, can be emotionally unacceptable, and thus it is difficult to expect its full acceptance. Perhaps the solution to this problem is the further research on a deeper understanding of the molecular mechanism of the host immune response modulation by intestinal parasites in animal models of IBDs as well as other diseases with similar aetiology, so that it will be possible to use recombinant helminth immunomodulators as traditional pharmacological treatments (83). Last year’s reports on the use of recombinant proteins of parasites indicate the reduction of the colonic mucosa inflammation. It was observed that the nematode protein asparaginyl-tRNA synthetase limits the inflammation of the colonic mucosa in mice by blocking the IL-8 receptors (95). It can be expected that in the near future, recombinant parasitic proteins will play an important role in the treatment of patients with IBDs. In the search for new alternative therapies for IBDs the use of new pharmacological substances that will help to achieve or maintain the state of remission should also be mentioned. Currently there is ongoing research on the use of substances such as melatonin (96) and glitazones (97). It has been shown that melatonin is a peptide hormone physiologically produced by the pineal gland, exerts anti-inflammatory and immunomodulatory effects and thus contributes to the beneficial therapeutic outcome in patients with ulcerative colitis (96). Experimental studies in rodents have shown that the administration of melatonin reduces inflammation in the large intestine through inhibition of NO production and expression of COX-2 (96, 98), inhibition of NF-kappa activation (96, 99) as well as modulation of macrophage activity (100). The results suggest that melatonin may be used as adjuvant therapy in order to sustain the state of remission (96).
Other studies relate to the anti-inflammatory potential of PPAR-gamma agonists (glitazones) in the treatment of IBDs. Many previous studies have confirmed the positive effects of rosiglitazone and troglitazone in maintaining the remission of intestinal inflammation in IBDs (97). Experimental studies have proven that agonists of PPAR-gamma receptor significantly enhance the expression of anti-inflammatory cytokines such as IL-4 and IL-10 and reduce the expression of pro-inflammatory cytokines important in the pathogenesis of IBDs, such as IL-2 or INF (97, 101).
Noteworthy are the studies focusing on finding new prognostic markers of inflammation in patients with IBDs, which would be helpful both in assessment of exacerbation as well as the course of the disease. Correlation between the serum leptin concentration and intestinal inflammation in patients with IBDs as well as the possible involvement of leptin in the pathogenesis of IBDs was studied (102). It has been shown that colonic inflammation is closely associated with an overexpression of leptin mRNA in the colon mucosa (102), while increased levels of leptin correlate with the increased amount of pro-inflammatory cytokines in serum such as IL-1SS, IL-6 and TNF-α.
However, it was noted that the serum concentrations of leptin did not correlate with the extent of inflammatory lesions, as well as the severity of colon inflammation in patients with ulcerative colitis. Nevertheless, due to many conflicting findings concerning the role of leptin in IBDs (102-104), we will have to wait with its use as a prognostic marker of inflammation in IBDs.
Conflict of interests: None declared.
REFERENCES
- Weinstock JV. Helminths and mucosal immune modulation. Ann NY Acad Sci 2006; 1072: 356-364.
- Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol 2006; 28: 515-523.
- Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 2010; 10: 3-12.
- Elliott DE, Weinstock JV. Where are we on worms? Curr Opin Gastroenterol 2012; 28: 551-556.
- Strachan DP. Hay fever, hygiene, and household size. Br Med J 1989; 299: 1259-1260.
- Kramer A, Bekeschus S, Broker BM, Schleibinger H, Razavi B, Assadian O. Maintaining health by balancing microbial exposure and prevention of infection: the hygiene hypothesis versus the hypothesis of early immune challenge. J Hosp Infect 2013; 83 (Suppl. 1): S29-S34.
- Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res 2013; 19: 2834-2841.
- Ben Ami Shor D, Harel M, Eliakim R, Shoenfeld Y. The hygienic theory harnessing helminth and their ova to treat autoimmunity. Clin Rev Allergy Immunol 2013; 45: 211-216.
- Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol 2013; 13: 70-77.
- Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 2000; 55, (Suppl. 1): S5-S10.
- Sheikh A, Strachan DP. The hygiene theory: fact or fiction? Curr Opin Otolaryngol Head Neck Surg 2004; 12: 232-236.
- Fleming DM, Sunderland R, Cross KW, Ross AM. Declining incidence of episodes of asthma: a study of trends in new episodes presenting to general practitioners in the period 1989-98. Thorax 2000; 55: 657-661.
- Ronchetti R, Villa MP, Barreto M, et al. Is the increase in childhood asthma coming to the end? Findings from three surveys of schoolchildren in Rome, Italy. Eur Respir J 2001; 17: 881-886.
- Zollner IK, Weiland SK, Piechotowski I, et al. No increase in the prevalence of asthma, allergies, and atopic sensitization among children in Germany 1992-2001. Thorax 2005; 60: 545-548.
- Asher MI, Monteford S, Bjorksten B, et al. ISAAC Phase Three Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISSAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733-743.
- Pearce N, Ait-Khailed N, Beasley R, et al. ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms plase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007; 62: 758-766.
- Pearce N, Douwes JT. The Latin American exception: why is childhood asthma so prevalent in Brasil? J Paediatr (Rio J) 2006; 82: 319-321.
- Crater DD, Heise S, Perzanowski M, et al. Asthma hospitalization trends in Charleston, South Carolina, 1956 to 1997: twenty-fold increase among black children during a 30-year period. Paediatrics 2001; 108: E97.
- Bloomfield SF, Stanwell-Smith R, Crevel RWR, Pickup J. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin Exp Allergy 2006; 36: 402-425.
- Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol 2008; 14: 165-173.
- Bjorksten B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol 2004; 25: 257-270.
- Elliott DE, Summers RW, Weinstock JV. Helminth and the modulation of mucosal inflammation. Curr Opin Gastroenerol 2005; 21: 51-58.
- Rook GA, Adams V, Hunt J, Palmer J, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol 2004; 25: 237-255.
- Rook GA, Brunet R. Old friends for breakfast. Clin Exp Allergy 2005; 35: 841-842.
- Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol 2012; 42: 5-15.
- Bernard A, Carbonnelle S, Michel O, et al. Lung hyperpermeability and asthma prevalence in schoolchildren: unexpected associations with the attendance at indoor chlorinated swimming pools. Occup Environ Med 2003; 60: 385-394.
- Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev 2004; 3: 394-400.
- Platts-Mills TA, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy 2005; 60 (Suppl. 79): 25-31.
- Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease - crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J Gastroenterol 2006; 12: 1829-1841.
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and colitis ulcerosa. Nat Clin Pract Gastroenterol Hepatol 2006; 3: 390-407.
- Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep 2007; 9: 497-507.
- Bartnik W. Choroby ukladu pokarmowego. In: Choroby wewnetrzne. Stan wiedzy na rok 2011, A. Szczeklik (ed). Krakow, Medycyna Praktyczna, 2011, pp. 775-1083.
- Ananthakrishnan AN, Xavier RJ. How does genotype influence disease phenotype in inflammatory bowel disease? Inflamm Bowel Dis 2013; 19: 2021-2030.
- Lees Cw, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut 2013; 60: 1739-1753.
- Hasler R, Feng Z, Backdahl L, et al. A functional methylome map of colitis ulcerosa. Genome Res 2013; 22: 2130-2137.
- Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol Clin North Am 1995; 24: 475-507.
- Ruyssers NE, De Winter BY, De Man JG, et al. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol 2008; 2008: 567314.
- Radwan P, Radwan-Kwiatek K, Tabarkiewicz J, Radej S, Rolinski J: Enhanced phenotypic and functional maturation of monocyte-derived dendritic cells from patients with active Crohn’s disease and ulcerative colitis. J Physiol Pharmacol 2010; 61: 695-703.
- Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut 2003; 52: 1522-1529.
- Sun S, Wang X, Wu X, et al. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors 2011; 4: 186.
- Shi Z, Cai Z, Sanchez A, et al. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem 2011; 286: 4517-4524.
- Kravchenko VV, Mathison JC, Schwamborn K, Mercurio F, Ulevitch RJ. IKKi/IKKepsilon plays a key role in integrating signals induced by pro-inflammatory stimuli. J Biol Chem 2003; 278: 26612-26619.
- Brown SL, Riehl TE, Walker MR, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 2007; 117: 258-269.
- Park S, Zhao D, Hatanpaa KJ, et al. RIP1 activates PI3K-Akt via a dual mechanism involving NF-kappaB mediated inhibition of the TOR-S6K-IRS1 negative feedback loop and downregulation of PTEN. Cancer Res 2009; 69: 4107-4111.
- Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis 2011; 12: 327-332.
- Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta 2011; 412: 513-520.
- Golab J, Jakobisiak M, Lasek W, Stoklosa T. Immunologia. Warszawa, PWN, 2009.
- Donnelly S, O’Neill SM, Stack CM, et al. Helminth cysteine proteases inhibit TRIF-dependent activation of macrophages via degradation of TLR3. J Biol Chem 2010; 285: 3383-3392.
- Van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem 2002; 277: 48122-48129.
- Falcon C, Carranza F, Martinez FF, et al. Excretory-secretory products (ESP) from Fasciola hepatica induce tolerogenic properties in myeloid dendritic cells. Vet Immunol Immunopathol 2010; 137: 36-46.
- Moncada DM, Kammanadiminti SJ, Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol 2003; 19: 305-311.
- Lee KD, Guk SM, Chai JY. Toll-like receptor 2 and Muc2 expression on human intestinal epithelial cells by Gymnophalloides seoi adult antigen. J Parasitol 2010; 96: 58-66.
- Petersson J, Schreiber O, Hansson GC, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 2011; 300: G327-G333.
- Kawashima H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol Pharm Bull 2012; 35: 1637-1641.
- Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, van Marck EA. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 2004; 53: 99-107.
- Elliott DE, Urban JF, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J 2000; 14: 1848-1855.
- Fiasse R, Latinne D. Intestinal helminths: a clue explaining the low incidence of inflammatory bowel diseases in Subsaharan Africa? Potential benefits and hazards of helminth therapy. Acta Gastroenterol Belg 2006; 69: 418-422.
- Kabeeerdoss J, Pugazhendhi S, Subramaniam V, Binder HJ, Ramakrishna BS. Exposure to hookworms in patients with Crohn’s disease: a case-control study. Aliment Pharmacol Ther 2011; 34: 923-930.
- Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol 2002; 169: 6112-6119.
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003; 170: 3939-3943.
- Menager-Marcq I, Pomie C, Ramagnoli P, Meerwijk JP. CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology 2006; 131: 1775-1785.
- Endharti AT, Okuno Y, Shi Z, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol 2011; 186: 41-52.
- Fujiwara D, Chen L, Wei B, Bran J. Small intestine CD11c+ CD8+ T cells suppress CD4+ T cell-induced immune colitis. Am J Physiol Gastrointest Liver Physiol 2011; 300: G939-G947.
- Yao Y, Han W, Liang J, et al. Glatiramer acetate ameliorates inflammatory bowel disease in mice through the induction of Qa-1-restricted CD8+ regulatory cells. Eur J Immunol 2013; 43: 125-136.
- Eliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann NY Acad Sci 2012; 1247: 83-96.
- Oliveira AS, Filho JX, Sales MP. Cysteine proteinases and cystatins. Braz Arch Biol Technol 2003; 48: 91-104.
- Hartmann S, Lucius R. Modulation of host immune responses by nematode cystatins. Int J Parasitol 2003; 33: 1291-1302.
- Klotz C, Ziegler T, Figueiredo AS, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLOS Pathog 2011; 7: e1001248.
- Jang SW, Cho MK, Park MK, et al. Parasitic helminth cystatin inhibits DSS-induced intestinal inflammation via IL-10+F4/80+ macrophage recruitment. Korean J Parasitol 2011; 49: 245-254.
- Harnett W, McInnes IB, Harnett MM. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol Lett 2004; 94: 27-33.
- Harnett W, Harnett MM. Therapeutic immunomodulators from nematode parasites. Expert Rev Mol Med 2008; 10: e18.
- Zaccone P, Burton OT, Gibbs S, et al. Immune modulation by schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol 2010; 2010: 795210.
- Pearce EJ, Kane CM, Sun J, Taylor JJ, McKee AS, Cervi L. TH2 response polarization during infection with die helminth parasite Schistosoma mansoni. Immunol Rev 2004; 201: 117-126.
- Filbey KJ, Grainger JR, Smith KA, et al. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol 2014; 92: 436-448.
- Jang JC, Nair MG. Alternatively activated macrophages revisited: new insights into the regulation of immunity, inflammation and metabolic function following parasite infection. Curr Immunol Rev 2013; 9: 147-156.
- Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One 2014; 9: e94188.
- Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. Philadelphia, Lea & Febiger, 1984.
- Czaplinski B. Helmintologia lekarska. In: Zarys parazytologii lekarskiej, R Kadlubowski (ed). Warszawa, PZWL, 1988, pp. 159-206.
- Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites - masters of regulation. Immunol Rev 2004; 201: 89-116.
- Whelan RA, Hartmann S, Rausch S. Nematode modulation of inflammatory bowel disease. Protoplasma 2012; 249: 871-886.
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol 2007; 37: 457-464.
- Ashour DS, Othman AA, Shareef MM, Gaballah HH, Mayah WW. Interactions between Trichinella spiralis infection and induced colitis in mice. J Helminthol 2013; 88: 210-218.
- McKay DM. The therapeutic helminth? Trends Parasitol 2009; 25: 109-114.
- Monteleone G, Boirivant M, Pallone F, McDonald TT. TGF-b1 and Smad7 in the regulation of IBD. Mucosal Immunol 2008; 1 (Suppl. 1): S50-S53.
- Monteleone G, Fantini MC, Onali S, et al. Phase I clinical trial of Smad7 knockdown using antisense oligoucleotide in patients with active Crohn’s disease. Molecular Therapy 2012; 20: 870-876.
- Zorzi F, Calabrese E, Monteleone I, et al. A phase 1 open-label trial shows that Smad7 antisense oligonucleotide (GED0301) does not increase the risk of small bowel strictures in Crohn’s disease. Aliment Pharmacol Ther 2012; 36: 850-857.
- Summers RW, Elliott DE, Quadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003; 98: 2034-2041.
- Summers RW, Elliott DE, Urban FJ, Thompson RA, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut 2005; 54: 87-90.
- Summers RW, Elliott DE, Urban FJ, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005; 128: 825-832.
- Jouvin MH, Kinet JP. Trichuris suis ova: testing a helminth-based therapy as an extension on the hygiene hypothesis. J Allergy Clin Immunol 2012; 130: 3-10.
- Croesce J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s disease patients and reservoir donors. Gut 2006; 55: 136-137.
- Catanessi C, Young ND, Nejsum P, et al. The transcriptome of Trichuris suis - first molecular insights into a parasite with curative properties for key immune diseases of humans. PLoS One 2011; 6: e23590.
- Broadhurst MJ, Leung JM, Kashyap V, et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2010; 2: 60ra88.
- Ince MN, Elliott DE, Setiawan T, et al. Role of T cell TGF-beta signaling in intestinal cytokine response and helminthic immune modulation. Eur J Immunol 2009; 39: 1870-1887.
- Kron MA, Metwali A, Vodanovic-Jankovic S, Elliott D. Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis. Clin Vaccine Immunol 2013, 20: 276-281.
- Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J, Chojnacki J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol 2011; 62: 327-334.
- Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparison of the anti-inflammatory and therapeutic actions of PPAR-gamma agonists rosiglitazone and troglitazone in experimental colitis. J Physiol Pharmacol 2012; 63: 631-640.
- Dong WG, Mei Q, Yu JP, Xu JM, Xiang L, Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat model of colitis. World J Gastroenterol 2003; 9: 1307-1311.
- Li JH, Yu JP, Yu HG, et al. Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm 2005; 2005: 185-193.
- Marquez E, Sanchez-Fidalgo S, Calvo JR, la de Lastra CA, Motilva V. Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J Pineal Res 2006; 40: 48-55.
- Desreumaux P, Dubuquoy L, Nutten S, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 2001; 193: 827-838.
- Biesiada G, Czepiel J, Ptak-Belowska A, et al. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J Physiol Pharmacol 2012: 63: 471-481.
- Zumbach MS, Boehme MW, Wahl P, Stremmel W, Ziegler R, Nawroth PP. Tumor necrosis factor increases serum leptin levels in humans. J Clin Endocrinol Metab 1997; 82: 4080-4082.
- Tuzun A, Uygun A, Yesilova Z, et al. Leptin levels in the acute stage of ulcerative colitis. Gastroenterology 2004; 19: 429-432.
A c c e p t e d : August 18, 2014