EFFECT OF PIPERINE, A MAJOR COMPONENT OF BLACK PEPPER,
ON THE PHARMACOKINETICS OF DOMPERIDONE IN RATS
INTRODUCTION
Certain herb and dietary supplements may produce potentially dangerous food-drug interaction as they can alter the pharmacokinetics and/or pharmacodynamics of certain medications (1, 2). Among dietary products, black pepper which is the most common culinary spice and considered an essential constituent of soups world-wide, particularly in South Asia. In traditional medicine, black pepper has been used as an analgesic and anti-inflammatory agent and in the treatment of epilepsy and snake venom poisoning (3-5). The beneficial effects of black pepper in the management of pain and epilepsy may be attributed to its active constituent, piperine(1-peperoyl peperidine). Piperine, is the main pungent alkaloid present in the fruits of black pepper (Piper nigrum) and long pepper; Piper longum (6). Piperine has been reported to have variety of pharmacological properties such as antipyretic, analgesic and anti-inflammatory (4,7), cytoprotective, antioxidant and anticonvulsant effects (4, 6, 7). Piperine also has been reported to exhibit an antidepressant and memory enhancing effects in animal models (8, 9). Numerous reports have shown that piperine inhibits the intestinal efflux transporter P-glycoprotein (P-gp) which plays an important role in drug absorption and disposition and the major drug metabolizing enzyme CYP3A4 (10-13).
Domperidone, a potent dopamine D2-receptor antagonist, is an orally active anti-emetic agent with long safety record (14). The main route of metabolism of domperidone is hydroxylation and oxidative N-dealkylation by CYP3A4. Studies in vitro suggest that other CYP isoforms contribute little (15,16). Domperidone is also a substrate of P-glycoprotein (17-18).
There seem to be no studies on the possible interaction between domperidone and piperine. Therefore, the present work was undertaken to investigate the possible pharmacokinetic interaction following the concurrent administration of domperidone and piperine to rats.
MATERIALS AND METHODS
Materials
Domperidone and piperine were purchased from Sigma Chemical Co.(St. Louis, MO, USA). Acetonitrile and methanol were purchased from Merck Co.(Darmstadt, Germany). All other chemicals were of analytical grade and all the solvents used were of high-performance liquid chromatography (HPLC) grade. Domperidone was suspended in 0.5% methylcellulose. Piperine was suspended by using 5% tween 80 in methylcellulose solution.
Methods
Male Wistar rats (300–350 g) were obtained from the Animal Care Center, College of Medicine, King Saud University, Riyadh, Saudi Arabia. All the experimental protocols were reviewed and approved by the board of Council of Medical Research, College of Medicine, King Saud University, Riyadh and complied with the National Institutes of Health guidelines for the care and use of laboratory animals. The animals were housed under standard laboratory conditions with 12 h light: dark cycle with free access to food and water ad libitum.
Animals were then divided into five separate treatment groups, each group comprising 6 rats. Animals in group 1 received a daily dose of domperidone (20 mg/kg, p.o.) for 6 days. Rats in groups 2 and 3 received the same oral dose of domperidone for 6 days but they were given a single dose of piperine 30 mg/kg, p.o. and 60 mg/kg, p.o., respectively on day 6. Animals in group 4 were injected intraperitoneally with domperidone (20 mg/kg) for 6 days. Rats in group 5 received the same intraperitoneal dose of domperidone for 6 days but they were given a single oral dose of piperine (30 mg/kg) on day 6. The doses of piperine were selected based on previous study (7).
The rats were then anaesthetized with ether, the right femoral artery was surgically exposed and was cannulated using a fine flexible polyethylene tube. The other end of the tube was then drawn under the skin to an incision in the back region and was connected to a syringe containing heparinized saline. Rats were given 1000 IU/kg heparin through the cannula prior to drug treatment.
After surgery, each animal was housed individually in a specially made wooden cage (22 × 10 × 7 cm) with a metal grid top and kept in a temperature-controlled room (21±1°C). As soon as the animals were recovered from the anaesthesia, group 1 was orally challenged with domperidone (20 mg/kg) alone, while those in groups 2 and 3 were administered a combination of domperidone (20 mg/kg, p.o.) and piperine (30 mg/kg,p.o.) or domperidone (20 mg/kg, p.o.) and piperine (60 mg/kg, p.o.), respectively. Rats in group 4 were injected intraperitoneally with domperidone (20 mg/kg) alone, while those in group 5 were given a combination of domperidone (20 mg/kg) with piperine (30 mg/kg, p.o.). Blood samples (0.3 ml) were withdrawn via the cannula and collected into heparinized Eppendorff tubes at 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0,10.0 and 12 hours. The cannula was flushed with an equal volume of heparinized saline after each sample withdrawal.
Determination of plasma domperidone concentrations
The plasma concentrations of domperidone were determined by modification of the method described (19). Briefly, blood samples from rats which had received domperidone alone or a combination of domperidone together with piperine were centrifuged at 6000 RPM for 10 min on a Gallenhamp Angle Head Centrifuge. An aliquot (100 µl) of the plasma was added to a 100 µl of chromatography grade acetonitrile solution. The suspension was thoroughly shaken and then centrifuged at 8000 RPM for 5 min on a select-a-fuge 24 Biodynamics. A100 µl of supernatant was taken and dried by nitrogen gas and then reconstituted with 50 µl prefiltered and degassed mobile phase consisting of 40% acetonitrile HPLC grade and 60% of 0.1 M phosphate buffer pH 3.9 dissolved in HPLC grade water. Aliquots (20 µl) of acetonitrile supernatant were injected into a HPLC system consisting of Schimadzu (Japan) SPD 10 AVP detector and LC 10 AVP pump, system controller SCL 10 AVP, wave length was 280 nm and waters reverse phase (NOVA-PAK C-18, 3.9 × 15 mm) column. The domperidone retention time was
4 min. This procedure provided a detection limit of 0.02 µg/ml.
Statistical analysis
The pharmacokinetic parameters of domperidone were determined using WinNonlinpro2.1 (Pharsight, Mountain view, CA, U.S.A). The area under the plasma concentration-time curve (AUC) was calculated by the linear trapezoidal method. The maximum plasma concentration (Cmax) and the time to reach the maximum plasma concentration (Tmax) were observed values from the experimental data. The elimination rate constant (Kcl) was estimated by regression analysis from the slope of the line of best fit and the half- life (t1/2) of the drug was obtained by 0.693/Kcl. The differences between any two respective treatment groups were analyzed for significance using the Student’s t-test. P values equal to or less than 0.05 were considered significant.
RESULTS
The mean plasma concentration-time profiles of an oral dose of domperidone (20 mg/kg, p.o.) administered alone or in combination with piperine (30 and 60 mg/kg, p.o.) is shown in Fig. 1. The pharmacokinetic parameters derived from these data are summarized in Table 1. The absorption of domperidone from the gastrointestinal tract (GIT) appears to be fairly rapid and was completed with a mean Tmax of 2.0 ± 0.0 h for domperidone alone and domperidone together with piperine. Treatment of rats with piperine significantly increased the mean area under the curve (AUC) for domperidone from 3.80 ± 0.03 µg/ml h (domperidone alone) to 6.54 ± 0.04 µg/ml h (domperidone together with 30 mg/kg piperine) and to 7.80 ± 0.04 µg/ml h (domperidone together with piperine, 60 mg/kg). Similarly, the maximum plasma concentration (Cmax) and the elimination half-life (t1/2) of domperidone were significantly higher than those obtained in animals that were given domperidone alone(P<0.05, Table 1). The time needed to reach Cmax (Tmax), however, was not changed.
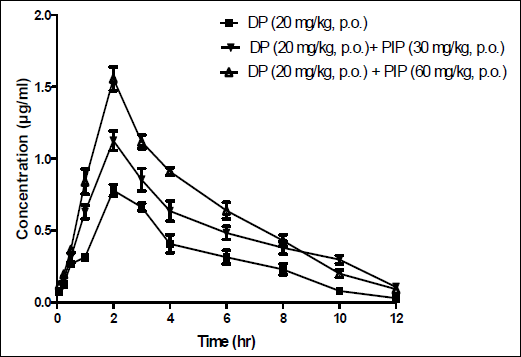 |
Fig. 1. Mean plasma concentration/time profiles of domeperidone (DP) after an oral administration of DP (20 mg/kg) to rats alone or when given concurrently with piperine (PIP; 30 mg/kg, p.o.; 60 mg/kg). Each point represents the mean ± S.E.M of 6 observations. |
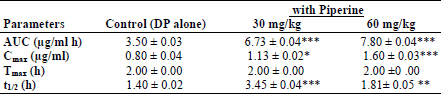
(* P<0.05, ** P<0.01 and *** P<0.001).
The intraperitoneal pharmacokinetics of domperidone (20 mg/kg) in the presence and absence of piperine (30 mg/kg, p.o.) were also evaluated in rats. Fig. 2 shows the mean plasma concentration- time profiles following the administration of domperidone (20 mg/kg, i.p.) or in combination with piperine (30 mg/kg, p.o.). Table 2 summarises the various pharmacokinetic parameters. Treatment of rats with piperine significantly increased the AUC, Cmax and t1/2 of domperidone when compared to those obtained in animals that were injected with domperidone alone (P<0.05, Table 2). The pharmacokinetic parameters obtained when domperidone was given intraperitoneally, although less but was not significant, when compared to those of oral domperidone (Table 1 and 2).
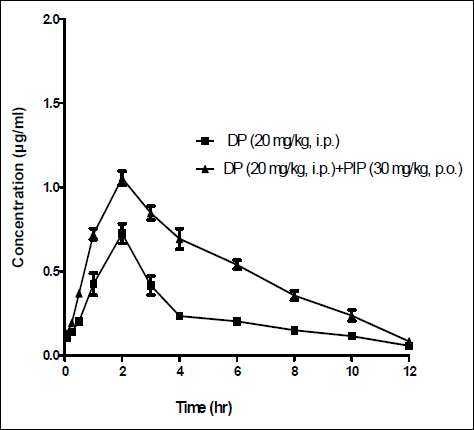 |
Fig. 2. Mean plasma concentration/time profiles of domeperidone (DP) after an i.p administration of DP (20 mg/kg) to rats alone or when given concurrently with piperine (PIP; 30 mg/kg, p.o.). Each point represents the mean ± S.E.M of 6 observations. |

(* P<0.05; *** P<0.001).
DISCUSSION
The results of the present study show that the concurrent administration of piperine with domperidone significantly increases the area under the plasma-concentration curve (AUC), the maximum plasma concentration (Cmax) and the elimination half- life (t1/2) of the latter drug in rats.
It has been shown that domperidone undergoes rapid and extensive hepatic metabolism by hydroxylation and oxidative N-dealkylation (20, 21). In vitro studies have revealed that CYP3A4 is the major form of cytochrome P-450 involved in the N-dealkylation of domperidone, whereas other CYP isoforms have little contribution in this pathway (15, 16). Numerous studies have shown that piperine inhibits hepatic CYP3A4 enzymatic activity, consequently decreases the metabolism of other drugs when administered concurrently (12, 22). For instance, piperine has been shown to increase plasma concentrations of theophylline, phenytoin and rifampin, likely by inhibition of metabolic pathways (11, 23, 24). It is thus possible that the increased levels of domperidone observed in this study following its concurrent administration with piperine may result, at least in part, from inhibition of hepatic CYP3A4 enzymes by piperine.
The drug export pump, P-glycoprotein is important for the absorption, distribution and excretion of drugs. Inhibition or induction of P-glycoprotein efflux function is a well established mechanism of drug-drug interactions (25, 26). Domperidone is also a substrate of P-glycoprotein (17, 18). Several studies have shown that piperine inhibits P-glycoprotein-mediated drug efflux during intestinal absorption (11, 12, 27). Hence, it is conceivable that the use of P-glycoprotein inhibitor such as piperine together with domperidone could increase the absorption of the latter drug from gastrointestinal tract resulting in elevated domperidone plasma concentrations. However, the role of the intestinal P-glycoprotein in the pharmacokinetic interaction between domperidone and piperine is unlikely since in this study the pharmacokinetic parameters of domperidone were not significantly affected whether given orally or intraperitoneally. Relevant to this is the study which showed that grapefruit juice, an inhibitor of P-glycoprotein (28) instead of increasing plasma levels of fexofenadine, a P-glycoprotein substrate, it decreased its plasma levels (29). On the other hand, it appears that there is a discrepancy between the finding of the present study and a previous report which had showed that pretreatment of rats with grapefruit juice extract 2 h prior to the administration of domperidone significantly increased bioavailability of domperidone (30). This contradiction may be due to the different experimental protocols followed, since in this study the animals were pretreated with piperine only 10 min prior to domperidone administration. It is tempted to speculate that a longer time may be needed for intestinal P-glycoprotein to be inhibited by piperine. Therefore, it would be of interest to determine the effect of chronically administered oral piperine on the blood levels of domperidone.
In conclusion, piperine significantly increased the plasma concentrations of domperidone in rats. Our results suggest that the increase in plasma concentration of domperidone may be due to the inhibition of cytochrome P450 3A4 enzymes by piperine. However, further studies are needed to determine the possible mechanism(s) involved in this pharmacokinetic interaction. Although our results may not be directly extrapolated to humans, caution should be exercised when piperine or piperine-containing diet are administered together with domperidone.
Acknowledgments: We are grateful to Mr J. Balla Elhardllo from the Department of Pharmacology, College of Medicine, King Saud University for valuable technical assistance.
Conflict of interests: None declared.
REFERENCES
- Wilkinson GR. The effects of diet, aging, and disease- states on presystemic elimination and drug bioavailability in humans. Adv Drug Deliv Rev 1997; 27: 129-159.
- Evans AM. Influence of dietary components on the gastrointestinal metabolism and transport of drugs. Ther Drug Monit 2000; 22: 131-136.
- Pei YQ. A review of pharmacology and clinical use of piperine and its derivatives and uses. Epilepsia 1983; 24: 177-181.
- Gupta SK, Bansal P, Bhardwaj RK, Velpandian T. Comparative antinociceptive, anti-infiammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacol Res 2000; 41: 657-662.
- Fu M, Sun ZH, Zuo HC. Neuroprotective effect of piperine on primary cultured hippocampal neurons. Biol Pharm Bull 2010; 33: 598-603.
- Kanaki N, Dave M, Padh H, Ragjani MA. Rapid method for isolation of piperine from the fruits of Piper nigrum Linn. J Nat Med 2008; 62: 281-283.
- Bukhari IA, Pivac N, Alhumayyd MS, Mahesar AL, Gilani AH. The analgesic and anticonvulsant effects of piperine in mice. J Physiol Pharmacol 2013; 64: 789-794.
- Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci 2007; 80: 1373-1381.
- Wattanathorn J, Chonpathompikunlert P, Muchimapura S, Priprem A, Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem Toxicol 2008; 46: 3106-3110.
- Atal CK, Dubey RK, Singh J. Biochemical bases of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther 1985; 232: 258-262.
- Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P- glycoprotein and CYP3A4. J Pharmacol Exp Ther 2002; 302: 645-650.
- Han Y, Chin Tan TM, Lim LY. in vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol Appl Pharmacol 2008; 230: 283-290.
- Venkatesh S, Durga KD, Padmavathi Y, Reddy BM, Mullangi R. Influence of piperine on ibuprofen induced antinociception and its pharmacokinetics. Arzneimittelforschung 2011; 61: 506-509.
- Reynolds JC. Prokinetic agents: a key in the future of gastroenterology. Gastroenterol Clin North Am 1989; 18: 437-457.
- Meuldermans W, Hurkmans R, Swysen E, et al. On the pharmacokinetics of domperidone in animals and man III. Comparative study on the excretion and metabolism of domperidone in rats, dogs and man. Eur J Drug Metab Pharmacokinet 1981; 6: 49-60.
- Ward BA, Morocho A, Kandil A, Galinsky RE, Flockart DA, Desta Z. Characterization of human cytochrome P450 enzymes catalyzing domperidone N-dealkylation and hydroxylation in vitro. Br J Clin Pharmacol 2004; 58: 277-287.
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 1996; 97: 2517-2524.
- Tsujikawa K, Dan Y, Nogawa K, et al. Potentiation of domperidone-induced catalepsy by P-glycoprotein inhibitor, cyclosporine A. Biopharm Drug Dispos 2003; 24: 105-114.
- Sivakumar T, Manavalan R, Valliappan K. Development and validation of a reverse d-phase HPLC method for simultaneous determination of domperidone and pantoprazole in pharmaceutical dosage forms. Acta Chromatographica 2007; 18: 130-142.
- Heykants J, Knaeps A, Meuldermans W, Michiels M. On the pharmacokinetic of domperidone in animals and man. 1. Plasma levels of domperidone in rats and dogs. Age related absorption and passage through the blood brain barrier in rats. Eur J Drug Metab Pharmacokinet 1981; 6: 27-36.
- Boyce MJ, BaisleyKJ, Warrington SJ. Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: a randomized, placebo-controlled, double-blind, crossover study. Br J Clin Pharmacol 2011; 73: 411-421.
- Makhov P, Golovine K, Canter D, et al. Co-administration of piperine and docetaxel results in improved antitumor efficacy via inhibition of CYP3A4 activity. Prostate 2012; 72: 661-667.
- Zutshi RK, Singh R, Zutsi U, Johri RK, Atal CK. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J Assoc Physycians India 1985; 33: 223- 224.
- Velpandian T, Jasuja R, Bhardwaj RK, Jaiswal J, Gupta SK. Piperine in food: interference in the pharmacokinetics of phenytoin. Eur J Drug Metab Pharmacokinet 2001; 26: 241-247.
- Jaszewska E, Kosmider A, Kiss AK, Naruszewicz M. Oenothera paradoxa defatted seeds extract containing pentagalloylglucose and procyanidins potentiates the cytotoxicity of vincristine. J Physiol Pharmacol 2010; 61: 637-643.
- Toklu HZ, Kabasakal L, Imeryuz N, et al. A study comparing the efficacy of antimicrobial agents versus enzyme (P-gp) inducers in the treatment of 2,4,6 trinitrobenzenesulfonic acid-induced colitis in rats. J Physiol Pharmacol 2013; 64: 439-451.
- Jin MJ, Han HK. Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food- drug interaction. J Food Sci 2010; 75: H93-H96.
- Malhotra S, Baily DG, Paine MF, Watkins PB. Seville orange juice-felodipine interaction: comparison with grape fruit juice and involvement of furocoumarins. Clin Pharmacol Ther 2001; 69: 14-23.
- Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther 2002; 71: 11-20.
- Bamburowicz-Klimkowska M, Zywiec K, Potentas A, Szutowski M. Impact of the changes in P- glycoprotein activity on domperidone pharmacokinetics in rat plasma. Pharmacol Rep 2007; 59: 752-756.
A c c e p t e d : October 16, 2014