THYMOQUINONE AND CURCUMIN PREVENT GENTAMICIN-INDUCED
LIVER INJURY BY ATTENUATING OXIDATIVE STRESS, INFLAMMATION
AND APOPTOSIS
2Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
INTRODUCTION
Aminoglycoside antibiotics have long been used in antibacterial therapy. Gentamicin is an aminoglycoside antibiotic derived from Micromonospora purpurea. It is effective against most of the life threatening gram negative bacterial infections (1, 2). Gentamicin is an important therapeutic agent used in poultry and animals to treat different diseases (e.g., collibacillosis, salmonellosis) (3).
The liver is a target of the metabolism and biotransformation of drugs and xenobiotics. Although many publications reported the nephrotoxicity and ototoxicity of gentamicin, few studies revealed its hepatotoxicity (4). Of these studies, Al-Kenanny et al. (5) demonstrated that the intraperitoneal administration of gentamicin for 8 days induced marked liver injury indicated by profound elevation of serum AST and ALT activities.
Plants remain to be the mainstay in the treatment of many diseases. They have fewer side effects than other conventional drugs. Plants used as food and in traditional medicine are more likely to yield antioxidants and pharmacologically active compounds (6-9).
Curcumin is a major yellow pigment in turmeric ground rhizome of Curcuma longa Linn., which is used widely as a spice and colouring agent in several foods such as curry, mustard and potato chips as well as in cosmetics and drugs (10, 11). Curcumin has several pharmacological effects including anti-bacterial, anti-inflammatory, anti-oxidant, anti-cancer, anti-hyperlipidemic and anti-diabetic potentials (12-14).
Thymoquinone (TQ), the active ingredient of Nigella sativa, was found to exert anti-inflammatory (15) and antioxidative (16) functions. In addition, a number of studies indicated the hepatoprotective effect of TQ against tert-butyl hydroperoxide toxicity in isolated rat hepatocytes (17), and carbon tetrachloride-induced hepatotoxicity in mice (18), as well as in experimental models of epilepsy in mice (19) and ethanol-induced hepatotoxicity in rats (20).
Based on these literatures, this study was designed to assess the preventive, antioxidant and antiapoptotic effects of curcumin and thymoquinone on gentamicin-induced liver injury in albino rats.
MATERIALS AND METHODS
Chemicals
Gentamicin was purchased from Memphis Company for Pharmaceutical and Chemical Industries (Cairo, Egypt). Curcumin and thymoquinone were supplied from Sigma Chemicals Co. (USA). They were stored at 2–4°C and protected from sunlight. All other chemicals were of analytical grade and were obtained from standard commercial supplies.
Experimental animals
All animal procedures were in accordance with the recommendations of the Canadian Committee for Care and Use of Animals (21). All efforts were done to reduce the number and suffering of animals.
Male albino rats (11–13 weeks old) weighing about 130–150 g were used. They were obtained from the animal house of the National Research Center, El-Giza, Egypt. They were kept under observation for about 2 weeks before the onset of the experiment to exclude any intercurrent infection. The chosen animals were housed in plastic well-aerated cages at normal atmospheric temperature (25 ± 5°C) and normal 12-hour light/dark cycle. Moreover, they had free access to water and were supplied daily with standard diet ad libitum. Composition of the diet, as a percent of total is 19% protein, 7.5% fat, 60% carbohydrate and 13.5% vitamins and minerals.
Experimental design and animal grouping
Gentamicin was intreperitoneally administered at dose of 100 mg/kg body weight (b.w.) (22, 23), every other day, for 21 days. Both curcumin and thymoquinone were dissolved in 1% carboxymethylcellulose (CMC) and were administered by oral gavage. The experimental animals were divided into four groups, each group comprising six rats designated as follows:
Group 1 (normal rats): the rats of this group were administered the equivalent volumes of saline (0.9%) and CMC (1%), every other day for 21 days.
Group 2 (gentamicin): the rats of this group were administered gentamicin and the equivalent volume of CMC (1%), every other day for 21 days.
Group 3 (gentamicin + thymoquinone): the rats of this group were administered gentamicin and thymoquinone at dose level 20 mg/kg b.w. (24), every other day for 21 days.
Group 4 (gentamicin + curcumin): The rats of this group were administered gentamicin and curcumin at dose level 20 mg/kg b.w. (25), every other day for 21 days.
At the end of the experiment, rats were fasted overnight and blood samples were obtained from jugular vein under mild diethyl ether anesthesia. The obtained blood samples were left to clot, and then they were centrifuged at 3000 rpm for 15 minutes. The clear non-hemolyzed sera were aspirated into three Eppendorf tubes for each rat. The sera were kept in deep freezer at –20°C pending biochemical analysis.
After blood collection, the rats were decapitated and rapidly dissected. Pieces of liver from each rat were fixed in neutral buffered formalin pending histological and immunohistochemical investigations. Liver (0.5 G) from each rat was homogenized in 5 ml sterile saline (0.9% NaCl). The homogenates were centrifuged at 3000 rpm for 5 minutes and the supernatants were separated pending determination of markers of oxidative stress and antioxidant defense system.
Biochemical investigations
Serum total protein and albumin levels were assayed by colorimetric methods using reagent kits obtained from Biodiagnostic Company (Egypt) according to manufacturer’s instructions. Serum globulin level was calculated by subtracting serum albumin level value from that of serum total protein content for each rat. Albumin/globulin ratio was also calculated.
Liver lipid peroxidation, assayed as malondialdehyde (MDA), and reduced glutathione (GSH) content were measured according to the methods of Preuss et al. (26) and Beutler et al. (27) respectively. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were also determined according to the methods of Marklund and Marklund (28) and Kar and Mishra (29), respectively.
Histopathological examination
After decapitation and dissection, liver from each rat was rapidly excised and then perfused in saline solution. Liver samples from four groups were fixed in 10% neutral buffered formalin. The fixed liver samples were transferred to National Cancer Institute (NCI), Cairo University (Egypt) for further processing. These formalin-fixed tissues were embedded in paraffin, sectioned (5 µm), stained with hematoxylin and eosin (H&E) (30), and examined under light microscope for histopathological assessment.
Immunohistochemical investigation
Liver samples taken for immunohistochemical studies were fixed in 10% neutral buffered formalin and then after dehydration and embedding in paraffin, cut into 5 µm sections and mounted on positive slides. To identify caspase 3, Bax and Bcl-2 proteins, preparations from all groups were used. For each preparation, a negative control was performed (a slide without primary antibody).
Immunolocalization technique for caspase 3, Bax and Bcl-2 was prepared on 5 µm thickness liver sections according to Pedrycz and Czerny (31) and Hussein and Ahmed (32). In brief, anti-caspase, anti-Bax and anti-Bcl-2 (diluted 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), were incubated with sections for 60 min. Primary antibodies were diluted in Tris-buffered saline 1% bovine serum albumin. Thereafter, a biotinylated secondary antibody directed against mouse immunoglobulin (DakoCytomation Kit) was added and incubated for 15 min, followed by horseradish peroxidase conjugated with streptavidin (DakoCytomation Kit) for further 15 min incubation. At the sites of immunolocalization of the primary antibodies, a reddish to brown color appeared after adding 3-amino-9-ethylcarbasole (DakoCytomation Kit) for 15 min. The specimens were counterstained with hematoxylin for 1 min and mounted using the Aquatex fluid (Merk KGaA, Germany). All liver sections were incubated under the same conditions with the same concentration of antibodies and at the same time, so the immunostaining was comparable among the different experimental groups.
Statistical analysis
Statistical analysis was performed using SPSS v.16. Results were articulated as mean ± standard errors (S.E.) and all statistical comparisons were made by means of one-way ANOVA test followed by Duncan’s multiple range test post hoc analysis. P value <0.05 was considered significant.
RESULTS
Biochemical effects
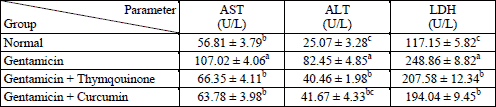
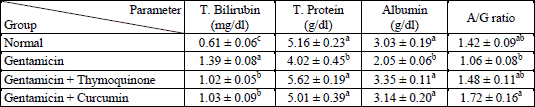
The administration of gentamicin for 3 weeks produced a significant elevation of AST, ALT and LDH activities. The treatment of gentamicin-administered rats with thymoquinone and curcumin significantly decreased these elevated values (Table 1). Serum bilirubin concentration was significantly increased as a result of gentamicin administration. The treatment of gentamicin-administered rats with both thymoquinone and curcumin significantly reduced the elevated bilirubin level (Table 2). In contrast, the total protein and albumin concentrations were decreased as result of gentamicin administration. The concurrent oral supplementation of gentamicin-administered rats with thymoquinone and curcumin significantly increased the declined total protein and albumin levels (Table 2).
Serum TNF-α concentration was profoundly increased in gentamicin-administred rats. The treatment with thymoquinone and curcumin significantly decreased this elevation towards the normal level (Table 3).
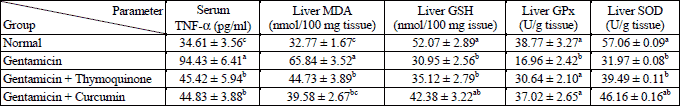
With regards the oxidative stress, the liver lipid peroxidation was significantly increased while the glutathione content, glutathione peroxidase and superoxide dismutase activities were increased as result of gentamicin administration. The treatment of gentamicin-administered rats with both thymoquinone and curcumin significantly decreased the elevated lipid peroxidation whereas they detectably increased the glutathione content, glutathione peroxidase and superoxide dismutase activities (Table 3).
By comparing the gentamicin- administered rats treated with thymoquinone with those treated with curcumin, the differences between these two groups for all tested biochemical parameters were not significant. Thus, the effects of thymoquinone and curcumin are more or less similar.
Histopathological effects
Liver of animals in the normal control group 1 showed normal hepatic architecture, where the hepatocytes are arranged around the central vein and alternate with blood sinusoids. Each hepatic cell possesses a limiting membrane centrally placed large nucleus and prominent nucleoli (Fig. 1). Liver sections of rats administered gentamicin revealed vacuolar degeneration characterized by vacuoles of different sizes present in the cytoplasm of hepatocytes. These vacuoles were clear in appearance, round in shape and had sharp boundaries suggesting fatty changes (Fig. 2a). Dilated sinusoids and hydropic-degenerated hepatocytes (Fig. 2b), dilated congested portal vein and fatty changes (Fig. 2c), dilated congested portal vein and newly formed bile ductules (Fig. 2d), inflammatory cells infiltration in the portal area, dilated congested portal vein and bile duct (Fig. 2e), and hydropic-degenerated hepatocytes, fatty changes, inflammatory cell infiltration and congested portal vein (Fig. 2f) were also noticed in liver sections of gentamicin-administered rats. Liver sections of the rats administered gentamicin and thymoquinone revealed nearly normal structure of hepatocytes but they still showed dilated sinusoids and hydropic-degenerated hepatocytes (Fig. 3a). The liver sections from rats administered gentamicin and curcumin also revealed nearly normal structure of hepatocytes but they still exhibited dilated central vein and mild lymphocytes infiltration (Fig. 3b).
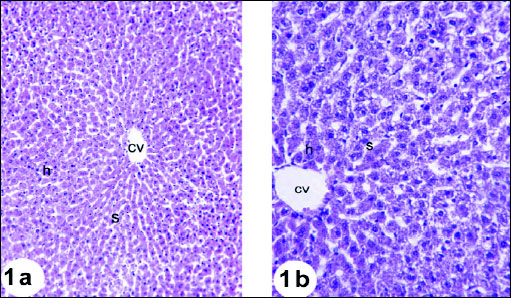 |
Fig. 1. Photomicrographs of the liver of normal rats showing the characteristic histological structures, central vein (cv), hepatocytes (h) and sinusoids (s). Fig. 1a (H&E; × 100); Fig. 1b (H&E; × 400) |
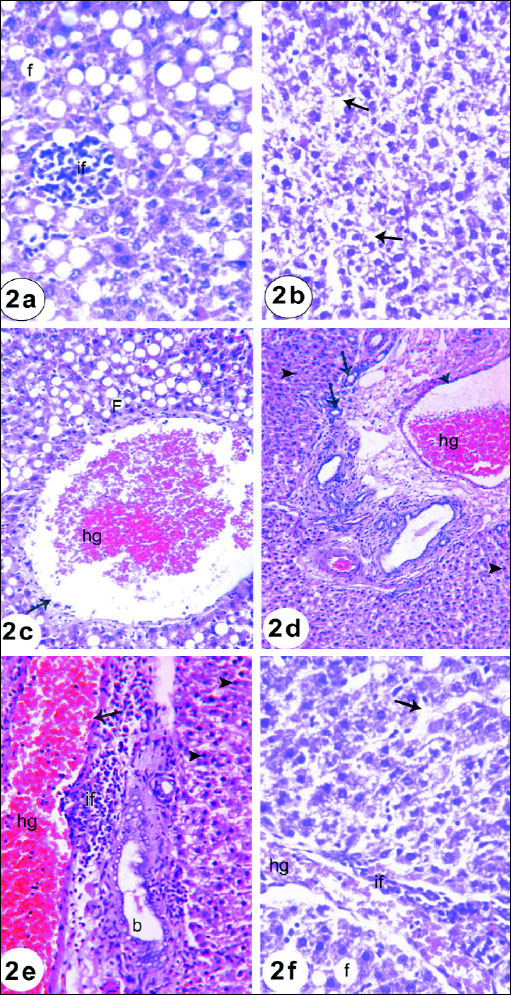 |
Fig. 2a. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing focal inflammatory cells infiltration (if) and diffuse fatty changes (f). (H&E; × 400). Fig. 2b. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing dilated sinusoids and hydropic-degenerated hepatocytes (arrow). (H&E; × 400). Fig. 2c. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing dilated congested (hg) portal vein (arrow) and fatty changes (F). (H&E; × 400). Fig. 2d. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing dilated congested (hg) portal vein, newly formed bile ductules (arrow) and hepatocytes with pyknoyic nuclei (arrow head) (H&E; × 400). Fig. 2e. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing inflammatory cells infiltration (if) in the portal area, dilated congested (hg) portal vein (arrow), dilated bile ductules (b) and hepatocytes with pyknotic nulei (arrow head). (H&E; × 400). Fig. 2f. A photomicrograph of the liver of rat given gentamicin for 3 weeks showing hydropic-degenerated hepatocytes (arrow), fatty changes (f), inflammatory cell infiltration (if) and congested (hg) portal vein. (H&E; × 400). |
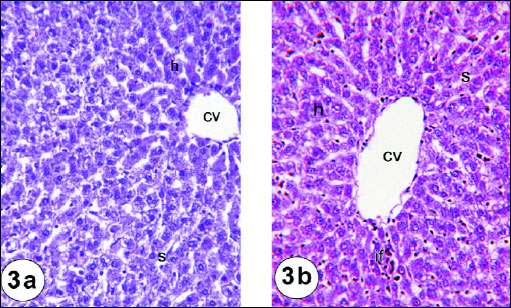 |
Fig. 3a. A photomicrograph of the liver of rat treated with thymoquinone and gentamicin for 3 weeks depicting nearly normal structure of hepatic cells(c), central vein (cv) and sinusoids (s). (H&E; × 400). Fig. 3b. A photomicrograph of the liver of rat treated with curcumin and gentamicin for 3 weeks showing mild normal structure of hepatic cell but there is still inflammatory cell infiltration (if). (H&E; × 400). |
Effects on caspase 3, Bax and Bcl-2
The immunohistochemistry staining was used to detect the expression of activated caspase 3 in the liver tissues of the rats in the present study. The expression of caspase 3 in cytoplasm and nucleus was remarkably increased in the liver of rats treated with gentamicin (Fig. 5) as compared with that in normal rats (Fig. 4). The administration of thymoquinone (Fig. 6) and curcumin (Fig. 7) to gentamicin-treated rats reduced the elevated caspase 3 in both cytoplasm and nucleus more or less to normal level.
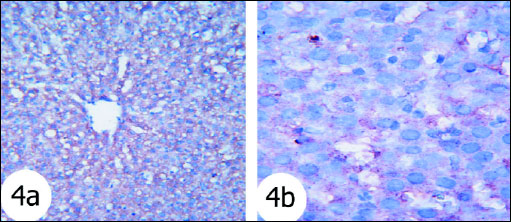 |
Fig. 4. Immunohistochemical localization of activated caspase-3 antigen in the liver tissue of rats. Fig. 4a and Fig. 4b Photomicrographs depicted normal liver stained for cleaved caspase-3. The hepatocytes exhibited weak nuclear staining. (immunohistochemical stain; × 100 & × 400). |
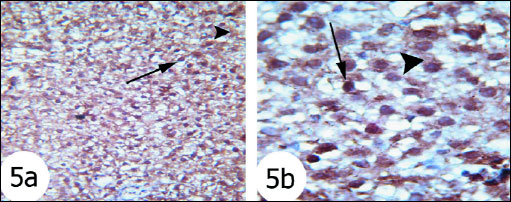 |
Fig. 5. Immunohistochemical localization of activated caspase-3 antigen in the liver tissue of rats. Fig. 5a and Fig. 5b Photomicrographs showed that activated caspase 3 expression was intense cytoplasmic and nuclear staining (arrows) in gentamicin-treated rats. (immunohistochemical stain; × 100 & × 400). |
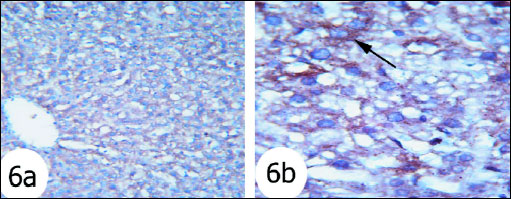 |
Fig. 6. Immunohistochemical localization of activated caspase-3 antigen in the liver tissue of rats. Fig. 6a and Fig. 6b Photomicrographs showed that caspase 3 expression was moderate reaction in the gentamicin and thymoquinone-treated rats. (Immunohistochemical stain; × 100 & × 400). |
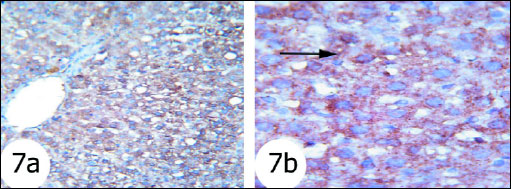 |
Fig. 7. Immunohistochemical localization of activated caspase-3 antigen in the liver tissue of rats. Fig. 7a and Fig. 7b Photomicrographs showed less reaction to caspase 3 antibodies mainly in the cytoplasm of hepatocytes in gentamicin and curcumin-treated rats. (immunohisto-chemical stain; × 100 & × 400). |
Bax proapoptotic protein was increasingly expressed to great extent in nucleus and cytoplasm of hepatocytes of gentamicin-treated rats (Fig. 9) as compared with normal rats (Fig. 8) which exhibited mild expression. The gentamicin-treated rats administered thymoquinone (Fig. 10) and curcumin (Fig. 11) exhibited moderate expression of cytoplasmic Bax.
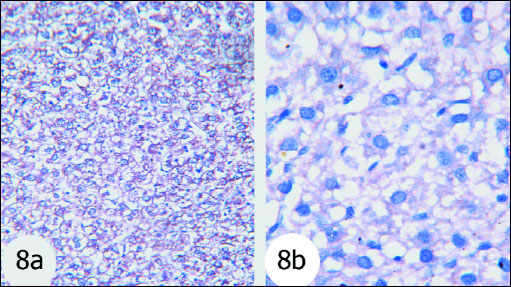 |
Fig. 8. The immunohisto-chemistry localization of Bax in the liver tissues of the rats. Fig. 8a and Fig. 8a Photomicrographs depicted that Bax expression was very low in the normal group. (immunohisto-chemical stain; × 100 & × 400). |
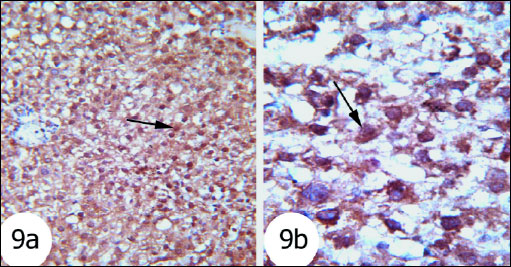 |
Fig. 9. The immunohisto-chemistry localization of Bax in the liver tissues of the rats. Fig. 9a and Fig. 9b Photomicrographs showed that Bax expression was high in the gentamicin-administered rats. (immunohistochemical stain; × 100 & × 400). |
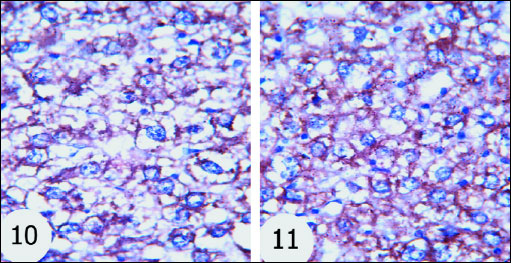 |
Fig. 10 and 11. The immuno-histochemistry localization of Bax in the liver tissues of the rats. Bax expression was decreased in gentamicin-administered rats treated with thymoquinone and curcumin respectively. (immuno-histochemical stain; × 100 & × 400). |
Antiapoptotic protein Bcl-2 expression in normal rats was shown in Fig. 12. The gentamicin-treated rats showed mild cytoplasmic reaction to Bcl-2 antibodies mainly in the cytoplasm of hepatocytes (Fig. 13) than that in normal rats. Sections of liver of gentamicin and thymoquinone treated rats (Fig. 14) as well as gentamicin and curcumin-treated rats (Fig. 15) exhibited moderate expression and reaction to Bcl-2 antibodies mainly in the cytoplasm of hepatocytes. However, thymoquinone is more effective in increasing Bcl-2 cytoplasmic content than curcumin.
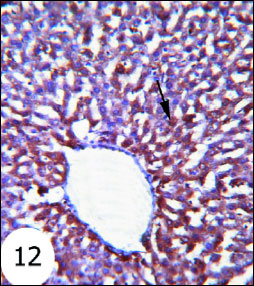 |
Fig. 12. Immunohistochemical localization of Bcl-2 in the liver tissues of rats. It showed strong cytoplasmic reaction to Bcl-2 antibodies in the hepatocytes. (immunohistochemical stain; × 100). |
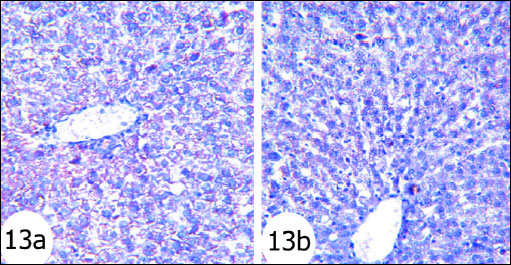 |
Fig. 13. Immunohistochemical localization of Bcl-2 in the liver tissues of rats. Fig. 13a and Fig. 13b Photomicrographs depicted weak reaction to Bcl-2 antibodies mainly in the cytoplasm of hepatocytes in gentamicin-administered rats. antibodies mainly in the cytoplasm of hepatocytes of gentamicin and curcumin-treated rats (immunohistochemical stain; × 100). |
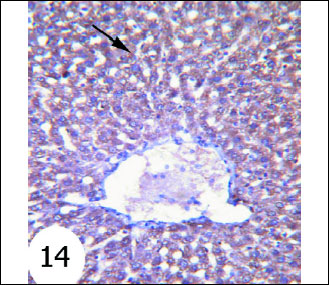 |
Fig. 14. Immunohistochemical localization of Bcl-2 in the liver tissues of rats. It showed strong staining of Bcl-2 reaction in the cytoplasm of hepatocytes of gentamicin and thymoquinone-treated rats. (immunohistochemical stain; × 100). |
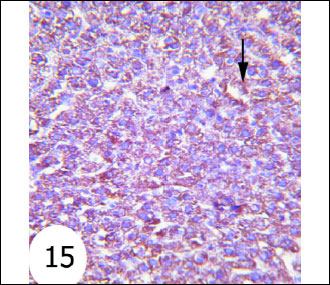 |
Fig. 15. Immunohistochemical localization of Bcl-2 in the liver tissues of rats. It depicted moderate reaction to Bcl-2 antibodies mainly in the cytoplasm of hepatocytes of gentamicin and curcumin-treated rats (immunohistochemical stain; × 100). |
DISCUSSION
Although many publications reported the nephrotoxicity and ototoxicity of gentamicin, few studies revealed its hepatotoxicity (4, 33). Thus, this study is directed to assess the effect of gentamicin on liver function and histology, oxidative stress and apoptosis and extends to evaluate the effects of concurrent administration of two anti-oxidant plant constituents thymoquinone or curcumin with gentamicin.
In the present study, the administration of gentamicin for 3 weeks produced a significant elevation of serum AST, ALT and LDH activities as well as total bilirubin concentration while it produced a significant decrease in serum total proteins and albumin levels. These results are in accordance with those obtained by other investigators (2, 34). The increase of the intracellular enzymes AST, ALT and LDH in serum in association with the elevation of serum bilirubin and a depletion of albumin, the major plasma protein synthesized by the liver, reflects the damage of hepatocytes and liver injury. This suggestion is supported in the present study by the liver histopathological changes which include vacuolar degeneration of hepatocytes, hydropic-degenerated hepatocytes, hepatocytes pyknotic nuclei, fatty changes and inflammatory cells infiltration in the portal area in gentamicin-administered rats. The obtained liver histological results are in agreement with those reported by Khan et al. (23) and Al-Kenanny et al. (5).
Oxidative stress is one of the key mechanisms responsible for liver damage and disease progression. Antioxidants, on the other hand, try to combat the oxidative stress and minimize its deteriorated effects (35). In conductance with this previous elucidation, the present study revealed that liver GSH content and the activities of antioxidant enzymes, GPx and SOD, were declined significantly in the gentamicin-administered group as compared with the normal control one. The lipid peroxidation, on the other hand, was profoundly elevated in gentamicin-administered rats. These data are in concurrence with those reported by Khan et al. (23), Al-Kennany et al. (5) and Ademiluyi et al. (34). Based on these findings, it can be elucidated that gentamicin induces an increase in the oxidative stress and production of free radicals and suppresses the antioxidant defense system in liver. The exacerbated increase of lipid peroxidation by gentamicin impairs membrane lipids and causes hepatocytes necrosis and damage. The suppressive effect of gentamicin on the nonenzymatic and enzymatic antioxidants results in an excess production of reactive oxygen species which not only deleteriously affects membrane lipids but also deteriorates proteins and nucleic acids. This in turn leads to liver toxicity, dysfunction and damage.
The concurrent treatment with thymoquinone or curcumin prevented the gentamicin-induced elevations in serum AST, ALT and LDH activities and total bilirubin concentration. The lowered serum total proteins and albumin levels and A/G ratio in gentamicin-administered rats were successfully alleviated as a result of treatment with thymoquinone and curcumin. This improvement in liver function parameters is associated with amendment of gentamicin-induced liver histological perturbations and enhancement of antioxidant defense system. Both thymoquinone and curcumin decreased the gentamicin-induced elevation in liver lipid peroxidation and enhanced the antioxidant defense system by increasing liver glutathione content and liver glutathione peroxidase and superoxide dismutase activities. These findings are in accordance with Lebda et al. (36) who reported that thymoquinone protected the liver enzyme leakage and prevented lipid peroxidation induced by D-galactosamine, indicating that the membrane stabilizing effect of thymoquinone might be ascribed to its ability to scavenge the free radicals produced by galactosamine and therefore protects the liver cell against oxidative damage. The data presented in this study are also in agreement with many other investigators who reported that curcumin has protective effects against chemicals-induced liver injury. Samuhasaneeto et al. (37) elucidated that curcumin supplementation resulted in improving liver steatosis (fatty changes) and inflammatory cells’ infiltration, decreasing the elevation of hepatic lipid peroxidation in ethanol treated rats. Fu et al. (38) and Soetikno et al. (39) revealed that curcumin significantly protects the liver from injury as indicated by reducing the serum AST, ALT, and ALP activities, and by amending the histological architecture of the liver in carbon tetrachloride and streptozotocin treated rats. The present study are in concurrence with that of Garcia-Nino and Pedraza-Chaverri (40) who reported that curcumin reduces the hepatotoxicity induced by arsenic, cadmium, chromium, copper, lead and mercury, prevents histological injury, lipid peroxidation and glutathione depletion, maintains the liver antioxidant enzyme status and protects against mitochondrial dysfunction.
Immunohistochemical findings in the present study depicted that liver pro-apoptotic protein Bax and caspase 3 expressions were remarkably increased in gentamicin-administered rats while liver antiapoptotic protein Bcl-2 was obviously decreased suggesting an increased hepatocyte apoptosis. The treatment of gentamicin-administered rats with thymoquinone and curcumin reversed these effects. Thus, both tested plant constituents may have an attenuating effect on apoptosis. Our suggestion is supported by the evidences of Gown and Willingham (41) and Tsamandas et al. (42) who stated that Bcl-2 oncoprotein regulates apoptotis by providing a survival advantage to rapidly proliferating cells while Bax protein promotes apoptosis by enhancing cell susceptibility to apoptotic stimuli. Caspase-3, cleaved from caspase-8, caspase- 9 and caspase-10, serves as a convergence point for different signaling pathways; thus, it is well suited as a read-out in an apoptosis assay and its increased expression reflects an increase in apoptosis (43).
Oxidative stress and TNF-α are known as activators of NF-κB which has a crucial role in proinflammatory gene induction during the onset of inflammation (44). Agents acting as inhibitors to TNF-α and NF-κB exert a therapeutic effect on liver injury in rats with bile duct ligation (BDL) through anti-inflammatory and antioxidant actions (45).
In the present study, serum TNF-α concentration was remarkably increased in gentamicin-administered rats and decreased as a result of concurrent administration of thymoquinone or curcumin with gentamicin. The increase of this pro-inflammatory cytokine is concomitant with the infiltration of inflammatory cells in the liver of gentamicin-administered rats and its decrease in gentamicin-administered rats treated with thymoquinone and curcumin is in association with a potential decrease or absence of inflammatory cells as indicated in histological changes in the current study. Thus, it can be suggested that both thymoquinone and curcumin may have potent anti-inflammatory effects in gentamicin-induced injury. In conductance with the current study, Tan et al. (46) reported that curcumin modulate the cytokines involved in the inflammation in human coronary artery endothelial cells in vitro.
Taken the previous findings and suggestions together, it can be concluded that both thymoquinone and curcumin could similarly prevent gentamicin-induced liver injury and histological perturbations through enhancement antioxidant defense system, suppression of oxidative stress, and attenuation of inflammation and apoptosis.
Conflict of interests: None declared.
REFERENCES
- Kaloyanides GJ. Metabolic interactions between drugs and renal tubulo-interstitial cells: role in nephrotoxicity. Kidney Int 1991; 39: 531-540.
- Nale LP, More PR, More BK, Ghumare BC, Shendre SB, Mote CS. Protective effect of Carica papaya seed extract in gentamicin induced hepatotoxicity and nephrotoxicity in rats. J Int Pharm Bio Sci 2012; 3: 508-515.
- Giurov B. Drug sensitivity of Salmonella strains isolated from poultry in 1980-1984. Vet Med Nauki 1986; 23: 10-17.
- Lee WM. Drug induced hepatotoxicity. N Eng J Med 2003; 349: 474-485.
- Al-Kenanny ER, Al-Hayaly LK, Al-Badrany AG. Protective effect of arabic gum on liver injury experimentally induced by gentamycin in mice. J Kufa Vet Med Sci 2012; 3: 174-189.
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal 2005; 7: 32-41.
- Sundaram R, Mitra, SK. Antioxidant activity of ethyl ether acetate soluble fraction of Acacia Arabica bark in rats. Ind J Pharmacol 2007; 39: 33-38.
- Ahmed OM, Abdel-Reheem ES. Hypolipidemic, proteogenic and kidney functions effects of curcumin and esculetin in diabetic rats. J Egyptian-German Soc Zoology 2005; 47A: 193-219.
- Venkatanarayana G, Sudhakara G, Sivajyothi P, Indira P. Protective effects of curcumin and vitamin E on carbon tetrachloride-induced nephrotoxicity in rats. EXCLI J 2012; 11: 641-650.
- Okada K, Wangpoentrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr 2001; 131: 2090-2095.
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 2004; 44: 97-111.
- Ahmed OM. The hypoglycemic effect of curcumin and esculetin and their probable mechanisms of action in streptozotocin diabetic albino rats. J Egyptian-German Soc Zoology 2005; 46A: 351-375.
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol 2007; 595: 1-75.
- Buadonpri W, Wichitnithad W, Rojsitthisak P, Towiwat P. Synthetic cucumin inhibits carragennan-induced paw edema in rats. J Health Res 2009; 23: 11-16.
- Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med 1995; 61: 33-36.
- Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res 2000; 41: 283-289.
- Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett 1998; 95: 23-29.
- Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci 2000; 66: 2583-2591.
- Raza M, Alghasham AA, Alorainy MS, El-Hadiyah TM. Beneficial interaction of thymoquinone and sodium valproate in experimental models of epilepsy: reduction in hepatotoxicity of valproate. Sci Pharm 2006; 74: 159-173.
- Alsaif MA. Effect of thymoquinone on ethanol-induced hepatotoxicity in Wistar rats. J Med Sci 2007; 7: 1164-1170.
- Canadian Council on Animal Care (CCAC). Guide to the Care and Use of Experimental Animals, CCAC, Ottawa, Ontario, Canada, 1993. 1-298.
- Esmatparast M, Amniattalab A. A study of histopathologic effects of co-supplementation of vitamins E and C on gentamicin-induced hepatotoxicity in the rat. International Congress on Veterinary Pharmacology and Pharmaceutical Sciences. October 4-5, 2008. Tehran, Iran.
- Khan MR, Badar I, Siddiquah A. Prevention of hepatorenal toxicity with Sonchus asper in gentamicin treated rats. BMC Complement Altern Med 2011; 11: 113.
- Attia A, Ragheb A, Sylwestrowicz T, Shoker A. Attenuation of high cholesterol-induced oxidative stress in rabbit liver by thymoquinone. Eur J Gastroenterol Hepatol 2010; 22: 826-834.
- Sinha M, Mukherjee BP, Mukherjee B, Dasgupta SR. Study on the 5-hydroxytryptamin contents in guinea pig stomach with relation to phenyl butazone induced gastric ulcers and the effects of curcumin thereon. J Indian Pharmacol 1974; 6: 87-96.
- Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effect of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevation in SHR. J Am Coll Nutr 1998; 17: 116-123.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61: 882-888.
- Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. J Eur Biochem 1974; 47: 469-474.
- Kar M, Mishra D. Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 1976; 57: 315-319.
- Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Edinburgh, London, Churchill Livingstone, 2002.
- Pedrycz A, Czerny K. Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following prepregnancy adriamycin administration in the mother. Acta Histochem 2008; 110: 519-523.
- Hussein AM, Ahmed OM. Regioselective one-pot synthesis and anti-proliferative and apoptotic effects of some novel tetrazolo[1,5-a]pyrimidine derivatives. Bioorg Med Chem 2010; 18: 2639-2644.
- Mahmoud AM, Ahmed OM, Galaly SR. Thymoquinone and curcumin attenuate gentamicin-induced renal oxidative stress, inflammation and apoptosis in rats. EXCLI J 2014; 13: 98-110.
- Ademiluyi AO, Oboh G, Owoloye TR, Agbebi OJ. Modulatory effects of dietary inclusion of garlic (Allium sativum) on gentamycin-induced hepatotoxicity and oxidative stress in rats. Asian Pac J Trop Biomed 2013; 3: 470-475.
- Surapaneni KM, Jainu M. Comparative effect of pioglitazone, quercetin and hydroxy citric acid on the status of lipid peroxidation and antioxidants in experimental non-alcoholic steatohepatitis. J Physiol Pharmacol 2014; 65: 67-74.
- Lebda F, Ahmed MA, Abd El Samad AA, Shawky MK. Protective effect of thymoquinone against D-galactosamine-induced liver injury in rats. Aust J Basic Appl Sci 2011; 5: 49-58.
- Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol 2009; 2009: 981963.
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCI4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 2008; 73: 399-409.
- Soetikno V, Harima M, Suzuk K, Watanabe, K. Amelioration of liver injury by curcumin in streptozotocin-induced diabetic rats. International Conference on Medical and Pharmaceutical Sciences, Bangkok, 2012.
- Garcia-Nino WR, Pedraza-Chaverri J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 2014; 69: 182-201.
- Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem 2002; 50: 449-454.
- Tsamandas AC, Thomopoulos K, Zolota V, et al. Potential role of bcl-2 and bax mRNA and protein expression in chronic hepatitis type B and C: a clinicopathologic study. Mod Pathol 2003; 16: 1273-1288.
- Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell 2009; 138: 838-854.
- Lawrence T. The nuclear factor NF-kB pathway in inflammation. Cold Spring Harb Perspect Biol 2009; 1: a001651.
- Olteanu D, Nagy A, Dudea M, et al. Hepatic and systemic effects of rosuvastatin on an experimental model of bile duct ligation in rats. J Physiol Pharmacol 2012; 63: 483-496.
- Tan X, Poulose EM, Raveendran VH, Zhu B, Stechschulte DJ, Dileepan KN. Regulation of the expression of cyclooxygenases and production of prostaglandin I2 and E2 in human coronary artery endothelial cells by curcumin. J Physiol Pharmacol 2011; 62: 21-28.
A c c e p t e d : October 21, 2014