ANTIANGIOGENIC POTENTIAL OF GRAPE STEM EXTRACT THROUGH INHIBITION OF VASCULAR ENDOTHELIAL GROWTH FACTOR EXPRESSION
2Agricultural University of Athens, Athens, Greece
INTRODUCTION
Angiogenesis, the multistep process by which new vessels arise from pre-existing vasculature, plays a central role in physiological processes, such as embryogenesis and wound healing, and in several pathological conditions including rheumatoid arthritis, aging, proliferative retinopathy, corneal neovascularization and carcinogenesis (1 - 3). Specifically, cancer excessive angiogenesis occurs when diseased cells produce abnormally large amounts of angiogenesis factors overwhelming the effects of natural angiogenesis inhibitors.
Vascular endothelial growth factor (VEGF) is considered as one of the most potent pro-angiogenic factors (4, 5). Human VEGF exists as a result of differential splicing in at least four isoforms, namely VEGF-A, -B, -C and -D (6). The biological activities of VEGF are mediated through two high-affinity receptor tyrosine kinases, VEGF receptor 1 (VEGFR-1) and 2 (VEGFR-2), whose expressions are mainly restricted to endothelial cells (7). High VEGF expression promotes vascular permeability, allowing tumor cells to enter into the bloodstream and metastase (8), stimulating neovascularization from existing vessels and impairing the delivery of chemotherapy to the tumor (9). Thus, VEGF has become an attractive therapeutic target for fighting tumors (6, 10).
VEGF expression appears to be regulated under hypoxia, a prime stimulus for angiogenesis, through the transcription of hypoxia inducible factor 1 (HIF-1) (11). HIF-1 is a heterodimer composed of HIF-la and HIF-1β subunits and functions by inducing the increase in VEGF mRNA copy number by binding to a hypoxia responsive element (HRE) within the VEGF promoter (12). Between the two subunits, HIF-1β is constitutively expressed while HIF-la is induced by hypoxia (12). Although HIF-1α regulation occurs during the transcriptional, translational, nuclear translocation and trans-activation steps, modulation of degradation is the primary process through which HIF-1α protein levels change. During normoxia, HIF-1α is degraded, while, during hypoxia, degradation ceases favoring HIF-1α accumulation (12).
Moreover, VEGF expression has been shown to be regulated by cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes (13) that produce prostaglandins (PGs) from arachidonic acid (14, 15). Several studies have shown that cyclooxygenase-derived prostaglandins contribute to carcinogenesis by inducing newly formed blood vessels (neoangiogenesis) that sustain tumor cell viability and growth (16, 17). Prostaglandins upregulate the production of several growth factors including VEGF acting directly on endothelial cells, and bFGF stimulating the COX-2 upregulation in fibroblasts (18). COX-2 derived prostaglandins in fibroblasts stimulate VEGF production which acts on endothelial cells in a paracrine fashion upregulating again COX-2 and facilitating vascular permeability and angiogenesis. Thus, cyclooxygenase inhibitors block prostaglandin production, therefore preventing the growth factor-induced angiogenesis (19). Cyclooxygenase-1 and -2 exhibit differential expression patterns. In particular, COX-1 is broadly distributed in normal, as well as in neoplastic, tissues, while COX-2 is expressed within human tumor neovasculature as well as in different neoplastic cells including human colon, breast, prostate and lung cancers (13). Moreover, Olszanecki et al., (20) have shown that COX-1 but not COX-2 is expressed in the endothelial EA.hy926 cells which were used in our study.
Blocking angiogenic factors such as those mentioned above has been suggested as a very effective and promising method for treating malignancy (21-23). For example, in 2003, bevacizumab (Avastin), a monoclonal antibody against VEGF-A, became the first antiangiogenic drug in large-scale clinical trials to inhibit tumour blood vessel growth and prolong survival in patients with metastatic colorectal cancer and other malignancies (24, 25). However, recent genomics-focused drug-discovery efforts have failed to produce the expected large number of compounds aimed at ‘novel’ targets for antiangiogenesis (26, 27). On the other side, there is an increasing body of evidence suggesting that several plant extracts comprise a rich source of antiangiogenic compounds (28, 29).
Grape extracts and their polyphenols (e.g. resveratrol) have been found to inhibit angiogenesis (28). However, most of these studies concern extracts of seeds, skins, juice and pomace, while no literature data on extracts from grape stems exists. Grape stems are scarcely investigated byproducts produced in large amounts (approximately 5% of grape biomass) during the vinification process. Grape stems derived from the wine making process are used for low economic interest purposes such as the production of animal feed or natural organic fertilizers (compost). They are usually discarded in nearby fields along with grape pomace, causing environmental problems. Hence, there is a research interest for the exploitation of their utilization. In this respect, we have previously shown that grape stem extracts are rich in bioactive polyphenols including trans-resveratrol (30) and also display significant antioxidant activity, protective activity against ROS-induced DNA damage and inhibitory activity against human hepatocellular and cervical cancer cells (31).
The aim of the present study was to examine the inhibitory effect of a grape stem extract on tube formation, a marker of angiogenesis, by EA.hy926 human endothelial cells, as well as on the expression of the proangiogenic factors VEGF, HIF-1α, and COX-1.
MATERIALS AND METHODS
Chemicals and reagents
Gallic acid, (+)-catechin, (-)-epicatechin, p-coumaric acid, ferulic acid, caffeic acid, syringic acid, kaempferol, quercetin, rutin and trans-resveratrol, trypan blue, Bradford reagent and cobalt chloride were purchased from Sigma-Aldrich (Steinheim, Germany). The Folin-Ciocalteu reagent was purchased from Fluka (Steinheim, Germany). All solvents used for the qualitative and quantitative polyphenolic determination were purchased from J. T. Baker (Griesheim, Germany) as analytical (polyphenol extraction) or HPLC (high-performance liquid chromatography analyses) grades. All remaining chemicals were of analytical grade and obtained from Sigma-Aldrich.
Grapes and vinification byproducts
The grape stems used were from the white Greek Vitis vinifera grape variety Assyrtiko cultivated on the island of Santorini and were from the 2011 harvest and vinification campaign. They were directly obtained by manual separation from the grape berries, air-dried, mill powdered and stored at room temperature.
Extract preparation
Fifty grams of dried sample stems were poured into a 200 mL mixture of methanol (MeOH)/H2O/1.0 N HCl (90:9.5:0.5 v/v) and sonicated in an ultrasonic bath for 10 min. The solvent was separated by filtration and the remaining solid was re-extracted three additional times, using the same solvent system and procedure. The combined extracts were evaporated under vacuum to provide a slurry, which was dissolved in 30 mL of MeOH/H2O (1:1) and centrifuged for 10 min (7000 rpm). The supernatant liquid was extracted with petroleum ether (3 × 30 mL) in order to remove the contained lipids and concentrated under vacuum. The remaining residue was poured into 30 mL of brine and extracted repetitively with ethyl acetate (EtOAc, 4 × 30 mL). Thus, all sugars contained were separated in the aqueous layer and discarded. The combined organic layers were dried over anhydrous magnesium sulfate and evaporated under vacuum. The remaining solid was weighed and dissolved in MeOH to 1 mg/mL, membrane filtered (0.45 µm) and subjected to HPLC analysis. In order to avoid polyphenol degradation, all the aforementioned activities were performed in the absence of direct sunlight and at temperatures below 35°C.
High-performance liquid chromatography analyses
All HPLC analyses were carried out on a Hewlett Packard HP1100 system equipped with a quaternary pump and degasser. The column used was a Kromasil C18 column (250 × 4.6 mm, particle size 5 µm) with a guard column of the same material (8 × 4 mm). Injection was by means of a Rheodyne injection valve (model 7725I) with a 20 µL fixed loop. For the chromatographic analyses HPLC-grade water was prepared using a Milli-Q system, whereas all HPLC solvents were filtered prior to use through cellulose acetate membranes of 0.45 µm pore size. Chromatographic data were acquired and processed using Chemstation software. The HPLC method used is a modified version of the method developed by Tsao and Yang (32). More specifically, the analysis was carried out at 30°C (maintained by a column thermostat) using samples of 20 µL, which were directly injected by means of a Rheodyne injection valve (model 7725I). The gradient eluted consisted of solvent A (obtained by the addition of 3% acetic acid in 2 mM sodium acetate aqueous solution) and solvent B (acetonitrile, CH3CN). Run time was set at 70 min with a constant flow rate at 1.0 mL/min in accordance with the following gradient time table: at zero time, 95% A and 5% B; after 45 min, the pumps were adjusted to 85% A and 15% B; at 60 min, 65% A and 35% B; at 65 min, 50% A and 50% B; and finally at 70 min, 100% B. This routine was followed by a 30 min equilibration period with the zero time mixture prior to the injection of the next sample. The analysis was monitored at 280, 320, and 360 nm simultaneously. Three replicate experiments were carried out for each sample examined. Peaks were identified by comparing their retention time and UV-vis spectra with the reference compounds, and data were quantitated using the corresponding curves of the reference compounds as standards. All standards were dissolved in methanol.
Assessment of the total phenolic content
The total phenolic content (TPC) of the extracts was determined in accordance with a modified version of the Folin-Ciocalteu method (33). A 100 µL sample of extract was added to a 10 mL flask containing 6 mL of deionized water. One milliliter of Folin-Ciocalteu reagent was added to the mixture, and the flask was stoppered and allowed to stand at room temperature for 3 min. A 1.5mL portion of 20% Na2CO3 was added and the solution was diluted to the desired volume (10 mL) with deionized water. Absorbance was measured at 725 nm versus a blank after 2 hours at room temperature. The results are expressed as gallic acid equivalents (GAE) using the standard curve (absorbance versus concentration) prepared from authentic gallic acid.
Cell culture conditions and reagents
EA.hy926 cells were a gift from Prof. Koukoulis (University of Thessaly, Greece). EA.hy 926 cell line is a hybrid derived by fusion of human umbilical vein endothelial cells (HUVEC) with a thioguanine-resistant clone of A549 by exposure to polyethylene glycol (PEG) (34). Hybrid clones were selected in HAT medium and screened for factor VIII-related antigen (34). EA.hy 926 cells have been characterized regarding morphology and expression of endothelial-specific markers (35, 36) and have been used in many studies on angiogenesis (37-39). Cells were used up to 20 passage. All cells were cultured in normal Dulbecco’s modified Eagle’s medium (DMEM, Gibco, UK), containing 10% (v/v) fetal bovine serum, 2 mM l-glutamine (Gibco, UK), 100 units/mL of penicillin, and 100 µg/mL of streptomycin (Gibco, UK) in plastic disposable tissue culture flasks at 37 °C in 5% CO2.
Trypan blue exclusion assay in grape extract-treated EA.hy926 cells
Cell viability of EA.hy926 cells treated with grape stem extract was determined by trypan blue exclusion assay. EA.hy926 cells were grown to 90% confluency (~250,000 cells/well) in 24 well plates in complete DMEM. Prior to treatment, all medium was removed and replaced with DMEM containing 5% FBS and different concentrations (20–320 µg/ml) of grape stem extract. After culturing cells for 24 hours, the medium was removed, and the remaining cells were washed once with 1 ml of 1X PBS. 25 µl of trypsin solution 0.25% was added to each well, and the mixture was incubated at 37°C for approximately 7 min. Cells were transferred to microcentrifuge tubes, and then centrifuged at 5000 rpm for 10 min. The supernatant was removed and cells were resuspended in 1 ml of DMEM, which was mixed thoroughly and appropriate dilutions were performed. Then, equal volumes (1:1) of resuspended cells and trypan blue solution (0.4% wt/vol) were mixed, and trypan blue exclusion by living cells was scored using a hemocytometer and phase contrast microscope. Each experiment was repeated three times in duplicate.
Tube formation assay
Tube formation was performed as described previously (40). Briefly, 250 µl of growth factor reduced matrigel (MatrigelTM, BD Biosciences, Franklin Lakes, NJ, USA) was added to each well of a 24-well plate and allowed to solidify for at least 30 min at 37°C. Afterwards, EA.hy926 cells were plated (16 × 104 cells/well) on the surface of the matrigel and treated with grape stem extract at different concentrations (20, 50 and 100 µg/ml) diluted in DMEM medium with 5% fetal bovine serum (FBS). A negative control, untreated cells, was used in each experiment. Cells were incubated for 16 hours and then the effect of the grape stem extract on tubular morphogenesis was documented microscopically and photographed. Total tube length was evaluated in 6 optical fields per well using ImageJ (NIH) software. Each experiment was repeated at least three times in duplicate. The percentage of inhibition of tube formation by the grape extract was calculated as follows:
where Tube lengthcontrol and Tube lengthsample are the tube lengths of negative control and sample respectively.
ELISA analysis for vascular endothelial growth factor expression and assessment of apoptosis in grape extract-treated EA.hy926 cells
VEGF protein concentration was determined on cultured EA.hy926 cells by ELISA (Quantikine Human VEGF Immunoassay ELISA kit; R&D Systems, Minneapolis, MN, USA). Cells were plated (3 × 105 cells) in 6-well plates in 10% FBS-containing media for 24 hours to adhere. Then, they were kept for 16 hours in either 5% FBS-containing media, for an untreated control, or with 5% FBS-containing media and different concentrations (50 and 100 µg/ml) of grape stem extract. Afterwards, the supernatants of the cell cultures were collected and centrifuged at 15,000 rpm for 30 min to remove cells. VEGF levels were determined according to the manufacturer’s instructions of the ELISA kit. All the controls suggested by the manufacturer were used. Moreover, it was tested if the extract alone affects ELISA assay, namely if the extract changes a known VEGF concentration. Absorbance was measured using an automatic microplate reader at 450 and 540 nm. The concentration of VEGF in each sample was interpolated from the plate specific standard curve after subtracting the background staining at 540 nm from the absorbance measured at 450 nm. Total protein from all the treated wells was harvested after cell lysis with sonication and used to normalize the VEGF levels from ELISA. Total protein concentration was measured using a Bradford reagent from Sigma-Aldrich.
A cell death detection ELISAPLUS kit (Roche, Germany) was used according to manufacturer’s instructions for the evaluation of grape stem extract-induced apoptosis in endothelial EA.hy926 cells. This assay is based on the determination of cytoplasmic histone-associated-DNA-fragments (mono- and oligo-nucleosomes) after induced apoptosis. As known, DNA fragmentation is a key feature of apoptosis, that is activated by endogenous endonucleases with subsequent cleavage of chromatin DNA into internucleosomal fragments (41). Cells were plated (1 × 104 cells per well) in 96-well plates in 10% FBS-containing media for 24 hours to adhere. Afterwards, the cells were treated for 16 hours with the grape stem extract (50 and 100 µg/ml) in 5% FBS containing media. Each experiment included a negative control (untreated cells). After treatment, the cell apoptosis was evaluated by ELISA according to manufacturer’s instructions. All experiments were repeated at least three times in duplicate.
Western blot analysis for hypoxia inducible factor-1a protein under hypoxia-mimicking conditions induced by CoCl2 and cyclooxygenase-1 protein expression in grape stem extract-treated EA.hy926 cells
EA.hy926 cells were plated (1.5 × 105 per plate) in Petri dishes and after 24 hours, were treated with 250 µM cobalt chloride (CoCl2), a chemical inducer of HIF-1α, for 16 hours. At the end of the treatment period, cells were extracted in cell lysis buffer [1% Triton X-100, 1% deoxycholate, 10 mM Tris (pH 7.2), 150 mM NaCl, 1 mM sodium vanadate, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 50 mg/ml aprotinin, and 50 mg/ml leupeptin]. Protein concentrations in cell lysates were measured using a Bradford reagent from Sigma-Aldrich. Cell lysates containing 50 µg of proteins were then subjected to 8% SDS/PAGE and immunoblotted. HIF-1α protein was identified by incubation with mouse anti-human HIF-1α monoclonal antibody (BD, CA, USA) in a dilution of 1:500 for 1 hour at room temperature. Membranes were incubated with goat anti-mouse IgG (Sigma, St. Louis, MO, USA) G-conjugated horseradish peroxidase secondary antibody (1:2000) for 30 min at room temperature.
EA.hy926 cells were seeded (1.5 × 106 cells per plate) in 100 mm culture plates, allowed to adhere for 24 hours and then exposed to either a 5% FBS-containing medium, for untreated control, or to 50 or 100 µg/ml of grape stem extract for 16 hours. At the end of the treatment period, cells were extracted in cell lysis buffer. Protein concentrations in cell lysates were measured using a Bradford reagent. Cell lysates were then subjected to SDS/PAGE and immunoblotted. COX-1 protein was identified by incubation with rabbit anti-human COX-1 (1:400; R&D Systems, Minneapolis, MN, USA) for 3 hours at room temperature. Membranes were incubated with goat anti-rabbit IgG (Calbiochem, Gibbstown, NJ, USA) G-conjugated horseradish peroxidase secondary antibody (1:2000) for 30 min at room temperature.
Labeled protein bands were detected by enhanced chemiluminescence (Perkin-Elmer, Waltham, MA, USA). All membranes were reprobed for β-actin (antibody from Sigma; dilution 1:10.000) to permit loading correction. Densitometry data were normalized to β-actin and analyzed using the Alpha View quantification software (Alpha Innotech, CA, USA). Each experiment was repeated at least three times.
Statistical analysis
All experiments were carried out at least three times. Statistical analysis was made by ANOVA followed by Dunnett’s post hoc test for multiple pair wise comparisons. Differences were considered significant at P <0.05.
RESULTS
Extract polyphenols
The polyphenol composition and the total polyphenolic content (TPC) of the tested extract are presented in Table 1. These data have been published previously in Sahpazidou et al., (42) and are presented herein for reader’s convenience. The results from the analysis of polyphenolic composition showed the presence of relatively large amounts of various flavanols (e.g. (-)-epicatechin and (+)-catechin), flavonols (e.g. quercetin and rutin), phenolic acids (e.g. gallic acid) and stilbenes (e.g. trans-resveratrol). This finding was revealed by the high TPC value determined for a white grape variety (372 mg GAE/gr of dry weight).
Grape stem extract inhibits tube formation by endothelial EA.hy926 cells
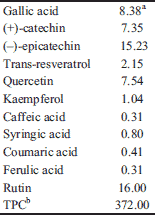
The grape stem extract was examined in vitro for its antiangiogenic potential using tube formation assay at non-cytotoxic concentrations selected according to the results from the cytotoxicity assay of trypan blue exclusion assay. The results from the cell viability assay showed that the grape extract exhibited cytotoxicity against EA.hy926 cells at concentrations higher than that of 160 µg/ml (Fig. 1). Thus, the extract concentrations used in the tube formation assay were 20, 50 and 100 µg/ml. Moreover, at these concentrations the extract did not change significantly either the pH or the electrical conductivity of the cultured medium (data not shown). The results showed that, at concentrations of 50 and 100 µg/ml, the grape stem extract inhibited statistically significant tube formation by 63 and 94% respectively (Fig. 2).
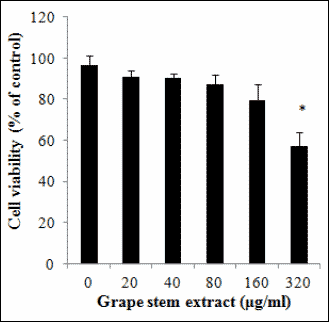 |
Fig. 1. Trypan blue exclusion assay was used to assess the cytotoxicity of grape stem extract against EA.hy926 cells. Cells were treated with different concentrations of grape stem extract for 24 hours. Values are means ± S.D. from three independent experiments carried out in triplicate. * P<0.05 when compared with the control (untreated cells). Statistical analysis was made by ANOVA followed by Dunnett’s post hoc test. |
Grape stem extract inhibits for vascular endothelial growth factor expression in EA.hy926 cells
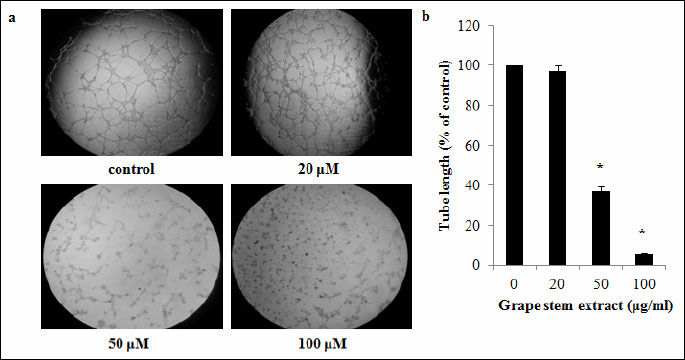
Since VEGF protein is considered one of the most important pro-angiogenic factors, the possible inhibition of VEGF expression in supernatants of EA.hy926 cell cultures treated with the grape stem extract was examined. The ELISA analysis for VEFG levels demonstrated that the grape stem extract treatment significantly reduced VEGF expression by 61% at 100 µg/ml, compared to control cultures (untreated cells) (Fig. 3).
 |
Fig. 3. (A) Effects of grape stem extract on VEGF levels. EA.hy926 cells were treated for 16 hours with 5% FBS-containing media and different concentrations of grape stem extract. Afterwards, the supernatants of the cell cultures were collected and subjected to VEGF ELISA. VEGF levels were normalized to total protein concentration. (B) Effects of grape stem extract on EA.hy926 cell apoptosis. Cells were treated with different concentrations of grape stem extract for 16 hours. Apoptosis induction was quantified using a cell death ELISA (Cell Death Detection ELISA, Roche Diagnostics).Values are means ± S.D. from three independent experiments carried out in duplicate. * P<0.05 when compared with the control (untreated cells). Statistical analysis was made by ANOVA followed by Dunnett’s post hoc test. |
Grape stem extract induces apoptosis in EA.hy926 cells
One of the roles of VEGF protein is to prevent apoptosis (43). Therefore, since grape extract reduced VEGF expression, its effect on EA.hy926 cell apoptosis was also examined. The results from the Cell death detection ELISA assay showed that the grape stem extract induced significant apoptosis by about 5.4-fold at 100 µg/ml in EA.hy926 cells (Fig. 3).
Grape stem extract did not affect hypoxia inducible factor-1a and cyclooxygenase-1 expression levels in EA.hy926 cells
HIF-1α protein is a transcription factor induced by hypoxia, that up-regulates VEGF. Since, the grape stem extract reduced VEGF expression, it was also examined by using Western blot analysis and the hypoxia-mimicking agent CoCl2, if it reduces HIF-1α expression levels. The results showed that grape extract did not affect HIF-1α levels (Fig. 4). Moreover, it was examined if grape stem extract modulates COX-1 protein levels, another protein that is involved in regulation of VEGF expression. The Western blot analysis demonstrated that grape extract had no effect on COX-1 expression (Fig. 4).
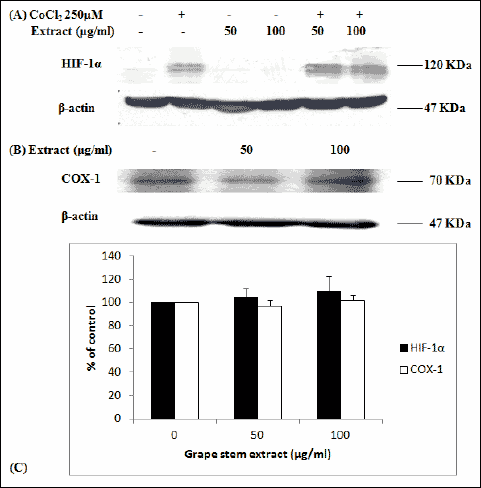 |
Fig. 4. (A) Representative Western blots showing expression of HIF-1α in EA.hy926 cells following treatment with the hypoxia-mimicking agent CoCl2 (250 µM) and grape stem extract (50 and 100 µg/ml) for 16 hours. The same membrane was reprobed for b-actin to permit loading correction. (B) Representative Western blots showing expression of COX-1 in EA.hy926 cells following treatment with grape stem extract at concentrations of 50 and 100 µg/ml for 16 hours. The same membrane was reprobed for b-actin to permit loading correction. (C) Densitometric quantifications for HIF-1α and COX-1 are reported. Values represent means ± S.D. values (n=3). Statistical analysis was by ANOVA followed by Dunnett’s test. |
DISCUSSION
The identification of antiangiogenic compounds is currently considered one of the most important and effective strategies for cancer treatment (44). It is important to note that our previous studies have demonstrated that grape stem extracts, a scarcely investigated byproduct produced in large amounts during the wine-making process, are rich in bioactive polyphenols (30) and possess important biological anti-carcinogenic properties (31). Thus, in an effort to further investigate the anticancer effects of grape stem extracts, we examined their possible antiangiogenic effects in the present study.
The findings showed that the tested grape stem extract inhibited at low (i.e. 50 and 100 µg/ml) and non-cytotoxic concentrations tube formation by human endothelial EA.hy926 cells, suggesting that it possesses antiangiogenic potential. Other studies have also shown that grape seed extracts inhibited tube formation by HUVECs at concentrations similar to those used in our study (45), while there are also studies reporting the opposite effect (46, 47). These contradictions could be explained by the different chemical composition of different grape extracts, since their polyphenolic composition affects their activity. This emphasizes the need to investigate the biological properties of local grape varieties as was done in the present study.
The fact that the chosen concentrations were not cytotoxic indicates that the inhibition of tube formation was due to a molecular mechanism and not just to cell death. According to the results, grape stem extract treatment inhibited VEGF levels in EA.hy926 cells, and consequently its antiangiogenic effect may be exerted through the down-regulation of VEGF expression, one of the most important proangiogenic factors (4). Moreover, in other studies, grape seed extracts inhibited VEGF expression in DU145 prostate cancer cells, U251 human glioma cells and MDA-MB-231 human breast cancer cells, at concentrations from 25 to 80 µg/ml, comparable to those of our study (45, 48). In addition, the administration of a red wine polyphenolic extract to BALB/c mice implanted with C26 colon carcinoma cells reduced vascularization and VEGF levels in tumors (49).
Moreover, the grape stem extract-induced apoptosis in EA.hy926 cells supports further the finding that VEGF down-regulation accounts for the tube formation inhibition. Since VEGF prevents apoptosis (43), the inhibition of its expression results in increased apoptosis (50, 51). Similarly, in other in vitro and in vivo studies grape seed and red wine extract-induced decrease in VEGF levels are associated with increased apoptosis (45, 49). VEGF induces apoptosis, at least in some cell types, through activation of mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) and suppression of the stress-activated protein kinase/c-jun-NH2-kinase (SAPK/JNK) signaling pathways (52, 53). However, it should be noted that the tested extract reduced VEGF levels and induced apoptosis only at 100 µg/ml and not at 50 µg/ml, although the latter concentration exhibited inhibition of tube formation. An explanation for this may be that the extract inhibits tube formation not only by reducing VEGF levels but also by other mechanisms.
Since VEGF expression is induced by hypoxia conditions through the HIF-1 transcription factor (11), it was also examined if the extract could reduce the expression of HIF-la, the subunit of HIF-1 that is induced by hypoxia. However, the results showed that the extract could not reduce HIF-1α expression, and thus grape stem extract-induced VEGF down-regulation may be due to an HIF-1-independent mechanism. However, it should also be examined if the extract inhibits the binding of HIF-1 to the VEGF promoter. Lu et al., (48) have reported that a grape seed extract might inhibit VEGF expression through HIF-1α down-regulation in U251 human glioma cells but their data do not rule out the possibility that grape seed extract may modulate VEGF expression by other molecular pathways. Moreover, it is plausible that grape extracts having different polyphenolic composition may down-regulate VEGF expression by different molecular mechanisms, while this mechanism may also be cell type-dependent.
Furthermore, it was examined if the grape stem extract-induced VEGF down-regulation is exerted through the decrease of COX-1 expression, a molecule that increases VEGF levels (13) and exhibits high expression in EA.hy926 cells (20). A number of studies have shown that cyclooxygenase-derived prostaglandins up-regulated the production of VEGF which acts on endothelial cells in a paracrine fashion to facilitate vascular permeability and angiogenesis (18). Like HIF-1α levels, the tested extract did not affect COX-1 levels in EA.hy926 cells suggesting that its effect on VEGF expression is mediated through other molecules. Although a red wine polyphenol extract administration has been demonstrated to reduce COX-2 levels in a colon tumorigenesis mouse model (49), there is not any study showing that grape extracts affect COX-1 expression.
Thus, our results indicate that other molecules apart from HIF-1 and COX-1 are involved in the observed grape stem extract-induced VEGF down-regulation. For example, Barthomeuf et al. (54) have suggested that a grape skin extract reduced VEGF levels through down-regulation of sphingosine-1-phosphate (S1P) factor playing important role in angiogenesis (55), and platelet activating factor (PAF), and reduced phosphorylation of p38/MAPK and ERK. Furthermore, whole grape fruit extracts have been reported to inhibit tube formation by HUVEC cells through a decrease in metalloproteases (MMPs) levels, the proteins facilitating the endothelial cell migration through the extracellular matrix during angiogenesis (56). It has also been shown that free radicals increase VEGF expression (57, 58). Since we have shown that grape stem extracts exhibit strong antioxidant activity (31), the grape stem extract-induced VEGF down-regulation may also be due to its free radical scavenging effects.
The observed antiangiogenic effect of the grape stem extract may be attributed to its high polyphenolic content, as it has been previously shown for other grape extracts (49, 54, 56). From the polyphenols found at high concentrations in the tested extract, the antiangiogenic activity of the stilbene trans-resveratrol has been very well-studied (59). Trans-resveratrol has been shown to inhibit VEGF-induced angiogenesis by the disruption of ROS-dependent Src kinase activation and VE-cadherin tyrosine phosphorylation in HUVECs (60). Moreover, trans-resveratrol has been reported to inhibit multiple myeloma angiogenesis by regulating expression and secretion of VEGF, basic fibroblast growth factor (bFGF), MMP-2 and MMP-9 (61). Also, Srivastava et al., (62) have demonstrated that regulation of FOXO transcription factors by trans-resveratrol mediated through PI3K/AKT and MEK/ERK pathways in HUVECs may play an important role in angiogenesis. Another polyphenol with important bioactivities, the flavonol quercetin, has been shown to inhibit tumor growth and angiogenesis by targeting VEGF-R2 regulated AKT/mTOR/P70S6K signaling pathway in vitro, ex vivo and in vivo (63), as well as COX-2-mediated angiogenesis in human endothelial cells through inhibition of the p300 signaling (64). Moreover, quercetin has been shown to reduce HIF-1α levels (65) but in different cells, the MCF-7 breast cancer, than those used in our study. The glycosylated form of quercetin, rutin, has also been shown to inhibit VEGF levels in cancer cells (66). Similarly, gallic acid inhibited tube formation and VEGF levels in cancer cells through suppression of ADAM17 and downregulation of PI3K/Akt and Ras/MAPK signaling pathways (48). However, conflicting results have been reported for the two flavanols, (+)-catechin and (-)-epicatechin, concerning their role in angiogenesis. In particular, (-)-epicatechin inhibited tube formation by HUVECs (67) but its administration to mouse induced angiogenesis (68). Similarly, although (+)-catechin inhibited tumour-specific angiogenesis by regulating the production of pro- and antiangiogenic factors (e.g. nitric oxide, VEGF, IL-2 and TIMP-1) (69), it seems that it may act either as an antiangiogenic or as a proangiogenic factor depending on the presence or absence of angiogenic stimuli (70).
Although, the present in vitro results indicate that grape stem extract may possess antiangiogenic potential, it should be bear in mind that there are no superior models for endothelial research in vitro. While in vitro assays are often useful for screening antiangiogenic agents due to their rapid nature and ease of quantification, care must be taken in interpretation. Thus, although in vitro assays are considered a valuable tool for studying angiogenesis, the validity of cultured cells as a model of physiological function in vivo has been criticized as being too different from the natural cellular environment. As known, the vasculature is such a complex system, and so knowledge about interactions within and among endothelium and their surrounding cells can be gathered by implementing the results of in vitro research to animal models to see effects on the whole vasculature (71). Thereafter, in vitro research should be used again to study the underlying mechanisms in physiology and pathology (71).
In conclusion, this is the first study showing that grape stem extract exhibits antiangiogenic potential that involves VEGF down-regulation. The identification of novel compounds with antiangiogenic activity is currently considered very important for anticancer treatment (7, 2). In addition, the grape stem extract-induced VEGF down-regulation suggests that it may also have anti-inflammatory and antiatherogenic activity, since high VEGF levels induce inflammation and atherosclerotic lesion (72). Moreover, grape stems are by-products generated in large amounts from the wine-making process and their disposal causes environmental problems. Thus, the present findings suggest that grape stem extracts possess important bioactivities such as anti-angiogenic potential, and hence they could be exploited for developing chemopreventive and anticancer agents, and at the same time protecting the environment.
Acknowledgments: The present work was funded by Research Committee of University of Thessaly Grant 4290.01.08 (D. Stagos) and by the MSc programmes „Biotechnology-Nutrition & Environment“ and „Molecular Biology and Genetics Applications“ in the Department of Biochemistry and Biotechnology at University of Thessaly. The present work was funded by Research Committee of University of Thessaly Grant 4290.01.08 (D. Stagos) and by the MSc programmes 'Biotechnology-Nutrition & Environment' and 'Molecular Biology and Genetics Applications' in the Department of Biochemistry & Biotechnology at University of Thessaly. This research has also been co-financed by the European Union (European Social Fund - ESF) and Greek national funds through the Operational Program 'Education and Lifelong Learning' of the National Strategic Reference Framework (NSRF) - Research Funding Program: „Heracleitus II. Investing in knowledge society through the European Social Fund“.
Conflict of interests: None declared.
REFERENCES
- Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008; 73: 751-762.
- Folkman J. Angiogenesis. Annu Rev Med 2006; 57: 1-18.
- Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol 2014; 65: 209-215.
- Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2012; 2: a006593.
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw 2009; 20: 158-163.
- Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266: 11947-11954.
- Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem 2000; 275: 16986-16992.
- Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDAMB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 2003; 278: 5277-5284.
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58-62.
- Chen L, Wu YY, Liu P, Wang J, Wang G, Qin J, et al. Down-regulation of HPV18 E6, E7, or VEGF expression attenuates malignant biological behavior of human cervical cancer cells. Med Oncol 2011; 28 (Suppl )1: S528-S539.
- Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med 2007; 85: 1301-1307.
- Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis 2008; 11: 121-140.
- Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem 2000; 7: 1163-1170.
- Wong SL, Wong WT, Tian XY, Lau CW, Huang Y. Prostaglandins in action indispensable roles of cyclooxygenase-1 and -2 in endothelium-dependent contractions. Adv Pharmacol 2010; 60: 61-83.
- Fortier MA, Krishnaswamy K, Danyod G, Boucher-Kovalik S, Chapdalaine P. A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. J Physiol Pharmacol. 2008; 59 (Suppl. 1): 65-89.
- von Rahden BH, Brucher BL, Langner C, Siewert JR, Stein HJ, Sarbia M. Expression of cyclo-oxygenase 1 and 2, prostaglandin E synthase and transforming growth factor beta1, and their relationship with vascular endothelial growth factors A and C, in primary adenocarcinoma of the small intestine. Br J Surg 2006; 93: 1424-1432.
- Konturek PC, Kania J, Burnat G, Hahn EG, Konturek SJ. Prostaglandins as mediators of COX-2 derived carcinogenesis in gastrointestinal tract. J Physiol Pharmacol 2005; 56 (Suppl 5): 57-73.
- Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett 1995; 372: 83-87.
- Wang FL, Sun JY, Wang Y, et al. Oldhamianoside II, a new triterpenoid saponin, prevents tumor growth via inducing cell apoptosis and inhibiting angiogenesis. Oncol Res 2013; 20: 369-376.
- Olszanecki R, Gebska A, Korbut R. Production of prostacyclin and prostaglandin E2 in resting and IL-1beta-stimulated A549, HUVEC and hybrid EA.HY 926 cells. J Physiol Pharmacol 2006; 57: 649-660.
- Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 2013; 123: 3190-3200.
- Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat 2010; 13: 16-28.
- Brezillon S, Zeltz C, Schneider L, et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol 2009; 60 (Suppl. 4): 15-22.
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21: 60-65.
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005; 333: 328-335.
- Szymkowski DE. Chemical genomics versus orthodox drug development. Drug Discov Today 2003; 8: 157-159.
- Lindsay MA. Target discovery. Nat Rev Drug Discov 2003; 2: 831-838.
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci 2006; 27: 297-309.
- Dulak J. Nutraceuticals as anti-angiogenic agents: hopes and reality. J Physiol Pharmacol 2005; 56 (Suppl. 1): 51-67.
- Anastasiadi M, Chorianopoulos NG, Nychas GJ, Haroutounian SA. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J Agric Food Chem 2009; 57: 457-463.
- Apostolou A, Stagos D, Galitsiou E, et al. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem Toxicol 2013; 61: 60-68.
- Tsao R, Yang R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography. J Chromatogr A 2003; 1018: 29-40.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1999; 299: 152-178.
- Edgell CJ, Reisner HM, Graham JB. Endothelial cell hybrids and the suspension of factor VIII related antigen expression. Br J Haematol 1980; 46: 613-620.
- Edgell CJ, Haizlip JE, Bagnell CR, et al. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. in vitro Cell Dev Biol 1990; 26: 1167-1172.
- Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIIIrelated antigen established by hybridization. Proc Natl Acad Sci USA 1983; 80: 3734-3737.
- Aranda E, Owen GI. A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res 2009; 42: 377-389.
- Majumder S, Sinha S, Siamwala JH, et al. A comparative study of NONOate based NO donors: spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 2014; 36: 76-86.
- Sun ZJ, Cai Y, Chen G, et al. LMO2 promotes angiogenesis probably by up-regulation of bFGF in endothelial cells: an implication of its pathophysiological role in infantile haemangioma. Histopathology 2010; 57: 622-632.
- Stagos D, Zhou H, Ross D, Vasiliou V. 4-HNE inhibits tube formation and up-regulates chondromodulin-I in human endothelial cells. Biochem Biophys Res Commun 2009; 379: 654-658.
- Nagata S. Apoptotic DNA fragmentation. Exp Cell Res 2000; 256: 12-18.
- Sahpazidou D, Geromichalos GD, Stagos D, et al. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol Lett 2014; 230: 218-224.
- Ji Y, Chen S, Li K, Xiao X, Xu T, Zheng S. Up-regulated autocrine VEGF/VEGFR-2 loop prevents apoptosis in hemangioma-derived endothelial cells. Br J Dermatol 2013; 170: 78-86.
- Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 2012; 320: 130-137.
- Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer 2004; 108: 733-740.
- Khanna S, Roy S, Bagchi D, Bagchi M, Sen CK. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic Biol Med 2001; 31: 38-42.
- Khanna S; Venojarvi M, Roy S, et al. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic Biol Med 2002; 33: 1089-1096.
- Lu Y, Jiang F, Jiang H, et al. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol 2010; 641: 102-107.
- Walter A, Etienne-Selloum N, Brasse D, et al. Intake of grape-derived polyphenols reduces C26 tumor growth by inhibiting angiogenesis and inducing apoptosis. FASEB J 2010; 24: 3360-3369.
- Singh R, Kim WJ, Kim PH, Hong HJ. Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med 2013; 45:e52.
- Mabeta P. Decreased secretion of vascular endothelial growth factor is associated with increased apoptosis in vascular tumor derived endothelial cells. J Physiol Pharmacol. 2013; 64: 473-477.
- Gupta K, Kshirsagar S, Li W, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 1999; 247: 495-504.
- Kiec-Wilk B, Grzybowska-Galuszka J, Polus A, Pryjma J, Knapp A, Kristiansen K. The MAPK-dependent regulation of the Jagged/Notch gene expression by VEGF, bFGF or PPAR gamma mediated angiogenesis in HUVEC. J Physiol Pharmacol 2010; 61: 217-225.
- Barthomeuf C, Lamy S, Blanchette M, Boivin D, Gingras D, Beliveau R. Inhibition of sphingosine-1-phosphate- and vascular endothelial growth factor-induced endothelial cell chemotaxis by red grape skin polyphenols correlates with a decrease in early platelet-activating factor synthesis. Free Radic Biol Med 2006; 40: 581-590.
- English D, Welch Z, Kovala AT, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J 2000; 14: 2255-2265.
- Liu M, Liu RH, Song BB, et al. Antiangiogenetic effects of 4 varieties of grapes in vitro. J Food Sci 2010; 75: T99-T104.
- Kuroki M, Voest EE, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest 1996; 98: 1667-1675.
- Fay J, Varoga D, Wruck CJ, Kurz B, Goldring MB, Pufe T. Reactive oxygen species induce expression of vascular endothelial growth factor in chondrocytes and human articular cartilage explants. Arthritis Res Ther 2006; 8: R189.
- Kraft TE, Parisotto D, Schempp C, Efferth T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit Rev Food Sci Nutr 2009; 49: 782-799.
- Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 2003; 64: 1029-1036.
- Hu Y, Sun CY, Huang J, Hong L, Zhang L, Chu ZB. Antimyeloma effects of resveratrol through inhibition of angiogenesis. Chin Med J (Engl) 2007; 120: 1672-1677.
- Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem 2010; 337: 201-212.
- Pratheeshkumar P, Budhraja A, Son YO, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One 2012; 7: e47516.
- Xiao X, Shi D, Liu L, et al. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS One 2011; 6: e22934.
- Oh SJ, Kim O, Lee JS, et al. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells. Food Chem Toxicol 2010; 48: 3227-3234.
- Freitas S, Costa S, Azevedo C, et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother Res 2011; 25: 916-921.
- Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett 2002; 180: 139-144.
- Ramirez-Sanchez I, Nogueira L, Moreno A, et al. Stimulatory effects of the flavanol (–)-epicatechin on cardiac angiogenesis: additive effects with exercise. J Cardiovasc Pharmacol 2012; 60: 429-438.
- Guruvayoorappan C, Kuttan G. (+)-Catechin inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-alpha in LPS-stimulated macrophages. Innate Immun 2008; 14: 160-174.
- Negrao R, Costa R, Duarte D, Gomes TT, Azevedo I, Soares R. Different effects of catechin on angiogenesis and inflammation depending on VEGF levels. J Nutr Biochem 2013; 24: 435-444.
- Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol 2009; 90: 195-221.
- Ruef J, Hu ZY, Yin LY, et al. Induction of vascular endothelial growth factor in balloon-injured baboon arteries. A novel role for reactive oxygen species in atherosclerosis. Circ Res 1997; 81: 24-33.
A c c e p t e d : September 21, 2014
Dr. Dimitrios Stagos, Department of Biochemistry and Biotechnology, University of Thessaly, Plutonos 26 & Ailou, Larissa, Greece; e-mail: stagkos@med.uth.gr