BRONCHODILATOR, VASODILATOR AND SPASMOLYTIC ACTIVITIES
OF CYMBOPOGON MARTINII
3Department of Pharmacy, Salerno University, Fisciano 84084, Salerno, Italy
INTRODUCTION
Cymbopogon martinii (Roxb.) Will. Watson (Poaceae), commonly known as palma rosa and Indian geranium, is a lemongrass native to South and Southeast Asia, especially India and Pakistan, and it is often cultivated for its oil (1). Phytochemical studies on volatile components of Cymbopogon martini revealed the presence of mono- amd sesquiterpenes (2-4), togheter with some chatacteristic components as cymbodiacetal (5) and dihemiacetal bismonoterpenoids (6). The inflorescence of the plant is a good source of geraniol-rich essential oil, which is used to impart a rose-like aroma to a wide range of perfumes, soaps, cosmetics, toiletry and tobacco products (7). The plant has folkloric repute as abortifacient, analgesic, and aphrodisiac (8) and native healers use the plant for its astringent, carminative, emmenagogue, vulnerary antispasmodic, stimulant and sudorific properties (9). It has been used to manage ache, snakebite, impotence, sore, cancer of stomach, liver and spleen, guinea worm, and pains (8), amenorrhea, fever, bleeding, wound, rheumatism (9), arthritis, alopecia, dermatosis, lumbago, biliousness, enterosis and spasms (7). Moreover, it has also been known to be beneficial in diabetes, urinary tract infections and the plant is claimed to possess anti-inflammatory and diuretic properties (10).
Scientific studies on Cymbopogon martinii demonstrated anthelmintic (11-12) antiseptic, antifungal (13-14) and insect-repellent (15-16) activities. It inhibits MAO activity in a competitive manner (17) and has wound healing properties (18). Cymbopogon martini has been reported to exert a-glucosidase inhibitory activity and helps in the management of postprandial glucose level (19). Volatile oil obtained from Cymbopogon martinii showed neuroprotective effect against cerebral ischemia and reperfusion-induced oxidative stress in rats, and showed therapeutic potential in cerebro-vascular diseases including stroke (20). Essential oil is reputed to improve stiff joints and lumbago, skin diseases, baldness and manage bilious complaints (21).
Despite its use in cardiovascular and gastrointestinal ailments, no study exists on rationalizing its use in these ailments. In this context, as part of our continuous studies on exploring medicinal flora of Pakistan for various activities (22-24), the present study was undertaken to validate traditional use of Cymbopogon martinii in the management of gastrointestinal, respiratory and cardiovascular.
MATERIALS AND METHODS
Collection, extraction of plant material and fractionation of extract
The leaves of Cymbopogon martinii were collected from Sadaqabad, Pakistan, in March 2012. The plant was identified by the taxonomist, Professor Dr. Altaf Ahmad Dasti, in the Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan and a voucher specimen (P.fl.108-3) was deposited in the herbarium of this Institute.
The plant material was shade dried and was rendered free of possible adulterant through manual picking. The dried herbal material was subsequently grinded into coarse powder. This powder was subsequently subjected to extraction by cold maceration and about 1 kg of the powdered material was soaked in 70% aqueous methanol in an amber glass container at 25°C for 7 days with occasional shaking. The soaked material was passed through a muslin cloth to get rid of the vegetative debris and fluid portion obtained was filtered through Whatman-1 filter paper and the filtrate was evaporated to a thick, semi-solid mass of dark brown color at 37°C under reduced pressure on rotavapour (Buchi R-200 Switzerland) coupled with recirculation chiller (B-740) and vacuum pump (Buchi vac V-500). The approximate yield of the crude methanolic extract (Cm.Cr) was 6%. The fractionations of crude methanolic extract of Cymbopogon martini was achieved by dissolving about 5 g of Cm.Cr in 20 ml of distilled water, followed by vigorous shaking with 20 ml of an immiscible organic solvent (dicloromethane) in a separating funnel. Individual fractions were collected in separate flasks and were evaporated by means of rotary evaporator under reduced pressure obtaining the dichloromethane (Cm.DCM) and the aqueous fractions (Cm.Aq).
Chemicals
Acetylcholine chloride, carbachol, potassium chloride, verapamil hydrochloride, phenylephrine, magnesium chloride, ethylene tetra-acetic acid (EDTA) were purchased from Sigma Chemicals Co. St Louis, MO, USA. Calcium chloride, glucose, magnesium sulphate, potassium dihydrogenphosphate, sodium bicarbonate, sodium dihydrogenphosphate and methanol were obtained from Merck, Darmstadt, Germany. Ammonium hydroxide, sodium chloride, and sodium hydroxide were purchased from BDH Laboratory supplies, Poole, England. The chemicals used in these experiments were of highest purity and the reagents of analytical grade.
Animals and housing conditions
All the experiments performed complied with the rulings of Institute of Laboratory Animal Resources, Commission on Life Sciences (25), approved by the Ethical Committee of Bahauddin Zakariya University, Multan.
Animals (female and male) used in this study were local strain rabbits (1.0–1.8 kg). These were housed under controlled environmental condition (23–25°C) at the animal house of Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The animals were provided with standard food and tap water ad libitum. The animals were deprived of food 24 h prior to the experiments but were given free access to water. Rabbits were sacrificed following a blow on back of head to be used for in vitro studies.
Isolated rabbit jejunum preparations
The crude methanolic extract of C. martinii (Cm.Cr) was tested for the possible presence of either spasmolytic or spasmogenic activity by using isolated rabbit jejunum preparations and responses were recorded through isotonic transducer by Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with Lab Chart Software (Version 7). For isolation of desired tissue, rabbit was dissected to remove jejunum and placed in Tyrode’s physiological salt solution maintained at 37°C and aerated with carbogen (95% O2 and 5% CO2). The tissues was cut into segments about 2 cm in length, rendered free of adhering mesenteries and subsequently suspended in isolated tissue baths containing Tyrode’s solution at 37°C and continuously aerated with carbogen. The composition of the Tyrode’s solution (mM) was: KCl (2.68), NaCl (136.9), MgCl2 (1.05), NaHCO3 (11.90), NaH2PO4 (0.42), CaCl2 (1.8) and glucose (5.55). Under normal physiological environment, isolated rabbit jejunum preparations exhibit spontaneous rhythmic contractions, allowing testing of the antispasmodic (relaxant) effect without application of an agonist (26-33). The possible mechanism of the relaxant activity of the test materials were investigated through the relaxation of the observed sustained spasmodic contractions following exposure to K+ (80 mM) (27). The test materials were applied in a cumulative manner to the sustained contractions to achieve concentration-dependent relaxant effects (26-27).The observed relaxant effect of the test materials on K+ (80 mM)-induced contraction was expressed as percent of the control contractile response.
Calcium channel blocking effect of the test substances were confirmed as reported in literature (31-33). Subsequent to an incubation period of 30 min, cumulative Ca2+ concentrations were applied to the tissue bath to obtain control calcium concentration-response curves (CRCs). The tissues were then washed and allowed to equilibrate with the Cm.Cr for 1 h and then the concentration response curves of Ca2+ were recorded and compared to the control curves. The CRCs of Ca2+ were recorded in the presence of different concentrations of the plant extracts in tissue bath.
Isolated rabbit tracheal preparations
Rabbit trachea was dissected out as described previously (31-33) and kept in Krebs solution having the following composition (mM): NaCl (118.2), NaHCO3 (25.0), CaCl2 (2.5), KCl (4.7), KH2PO4 (1.3), MgSO4 (1.2) and glucose (11.7). The isolated rabbit tracheal preparations were mounted in 20 ml organ bath containing Krebs solution being maintained at 37°C and aerated with carbogen (95% O2 + 5% CO2). A preload tension of 1 g was applied and tissue preparations were allowed to be equilibrated for 1 hour prior to addition of any test material. The sustained contractions produced by carbachol (1 µM) and K+ (80 mM) were subsequently used for testing of different concentrations of the test material in a cumulative fashions. The isometric responses were recorded through a Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with Lab Chart Software (Version 7). The standard drug, verapamil, with Ca2+ channel blocking effect, was tested on carbachol- and K+ (80 mM)-induced spastic contractions for confirmation of possible mechanism of action.
Isolated rabbit aorta preparation
The effect of Cm.Cr on systemic vascular resistance was assessed on isolated rabbit aorta preparations. The descending thoracic aorta of rabbit was cut vertically in 2–3 mm width segments and was mounted in a tissue organ bath (Radnoti) containing Krebs solution aerated with carbogen at 37°C. A pre-load tension of 2 g was applied to each preparation and allowed to equilibrate for a period of 1 hour. The contractile effect of the test substance were studied on addition to tissue organ baths in a cumulative manner, whereas relaxant effect was studied following application to phenylephrine (1 µM)- and K+ (80 mM)-induced contractions. The changes in isometric tension of aortic rings were recorded by a force-displacement transducer (Model FORT100, WPI, USA) coupled to a Power Lab data acquisition system (AD Instruments, Sydney, Australia) and computer running Lab Chart software (version 7).
Statistical analysis
In isolated tissue experiments, data were expressed as the mean ± standard error of the mean (S.E.M.) and the median effective concentrations (EC50 values) with 95% confidence intervals (CI) were calculated by using the computer software Graphpad Prism Program (version 5.0), San Diego CA, USA. Concentration response curves were analyzed by non-linear regression of sigmoid response curve (variable slope). The statistic applied was the student’s t-test and P<0.05 was considered as significant.
RESULTS
Effect on isolated rabbit jejunum preparations
The Cm.Cr on application to spontaneous contractions of isolated rabbit jejunum preparations caused relaxation of spontaneous contractions at tissue bath concentration range of 0.01–5.0 mg/ml with an EC50 value of 1.33 mg/ml (95% CI: 0.1707–1.8422 mg/ml; n=5) (Fig. 1a and b). Verapamil also inhibited the spontaneous contractions in isolated rabbit jejunum (Fig. 1b) with EC50 value of 0.214 µM (95% CI: 0.1765–0.9601 µM; n=5). When tested against K+ (80 mM)-induced contractions, Cm.Cr relaxed the K+ (80 mM)-induced contractions at a tissue bath concentrations range of 0.01–5.0 mg/ml with an EC50 value of 1.88 mg/ml (95% CI: 1.2801–2.2289 mg/ml; n=5) (Figs 1c and 2a). Verapamil also exhibited a similar pattern of relaxant effect against K+ (80 mM)-induced contractions with an EC50 value of 0.0357 µM (95% CI: 0.1765–0.9601 µM; n=5) (Fig. 1b). The pretreatment of isolated rabbit jejunum preparations with Cm.Cr (0.3–1.0 mg/ml) caused rightward shift of concentration response curves of Ca2+ similar to that produced by verapamil (Fig. 3).
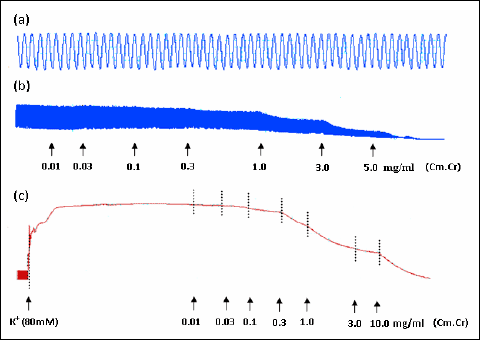 |
Fig. 1. Tracings presenting (a) spontaneous contraction of isolated rabbit jejunum and relaxant effect of the methanol extract of Cymbopogon martinii (Cm.Cr) on (b) spontaneous- and (c) high K+ (80 mM)- induced tissue contraction. Extract was added in cumulative manner and values listed were the final tissue bath concentrations (n=5). |
Activity directed fractionation of Cm.Cr revealed that the relaxant activity was more potent in the non-polar dichloromethane fraction (Cm.Dcm) on K+ (80 mM)-induced contractions with EC50 values of 0.863 mg/ml (95% CI: 0.2577–1.2983 mg/ml, n=5) in comparison with Cm.Aq which showed minor relaxation of the K+ (80 mM)-induced contractions with EC50 values of 9.313 mg/ml (95% CI: 6.3270–10.7805 mg/ml, n=5) (Fig. 2c).
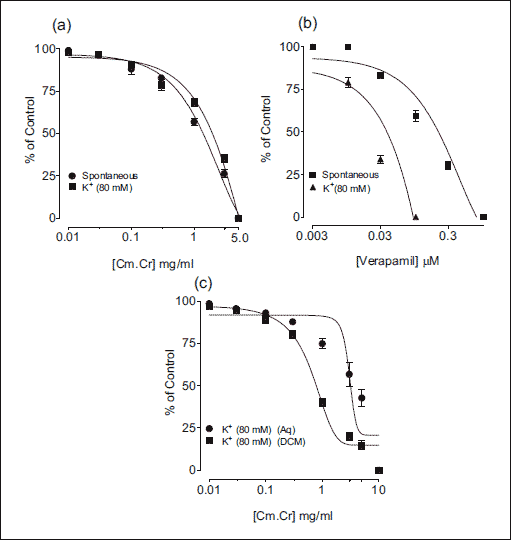 |
Fig. 2. Concentration dependant spasmolytic effect of (a and c) methanol, aqueous and dichloromethane fraction of the methanol extract of Cymbopogon martinii (Cm.Cr) and (b) verapamil on spontaneous- and high K+ (80 mM)-induced contractions in isolated rabbit jejunum preparations (values are the mean ± S.E.M., n=5). |
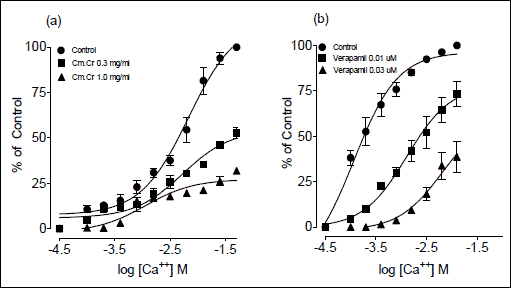 |
Fig. 3. Effect of (a) methanolic extract of Cymbopogon martinii (Cm.Cr) and (b) verapamil on concentration response curves of Ca2+ in isolated rabbit jejunum preparations. Values are the mean ± S.E.M., n=5. Control Ca2+. |
Effect on isolated rabbit tracheal preparations
The Cm.Cr at respective tissue bath concentrations of 0.01–10.0 mg/ml and (0.01–5.0 mg/ml) exerted a relaxant effect on carbachol (CCh; 1 µM)- and K+ (80 mM)-induced contractions (Fig. 4) in isolated rabbit tracheal preparations with respective EC50 values of 2.84 mg/ml (95% CI: 1.223–2.595 mg/ml; n=5) and 0.805 mg/ml (95% CI: 1.414–1.990 mg/ml; n=5) (Fig. 5a). Verapamil also caused the relaxation of CCh
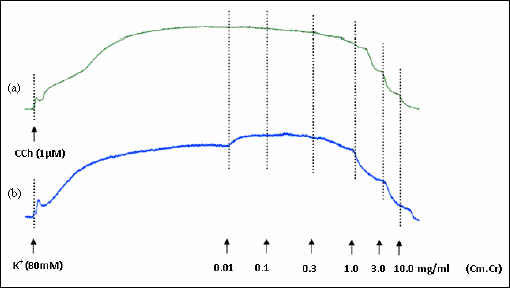 |
Fig. 4. Tracings showing relaxant effect of methanolic extract of Cymbopogon martinii (Cm.Cr) on (a) carbachol (1 µM)- and (b) high K+ (80 mM)-induced contractions in isolated rabbit tracheal preparation. |
(1 µM)- and K+ (80 mM)-induced contractions with respective EC50 values of 0.220 µM (95%: 0.04187–0.1862 µM; n=5) and 0.0721 µM (95% CI: 0.7150–1.062 µM; n=5) (Fig. 5b).
The organic fraction; dichloromethane (Cm.Dcm) exhibited complete relaxation of high K+ (80 mM)-induced contractions of tracheal tissue at the dose of 1 mg/ml with EC50 values of 0.3577 mg/ml (95% CI: 0.23464–0.6169 mg/ml, n=5), while aqueous fraction (Cm.Aq) did not exert complete relaxant effect even at the tissue bath concentration of 5 mg/ml with EC50 values of 1.874 mg/ml (95% CI: 0.7014–3.100, n=5) (Fig. 5c). Similarly when these fractions were tested against carbachol (1 µM) induced contraction, Cm.Dcm exhibited more potent bronchodilation on tracheal tissue with EC50 values of 0.2225 mg/ml (95% CI: 0.1659–0.2983 mg/ml, n=5) in comparison with Cm.Aq fraction which showed minor relaxation at higher concentration contractions with EC50 values of 7.5052 mg/ml (95% CI: 5.3270–8.7805 mg/ml, n=5) (Fig. 5d).
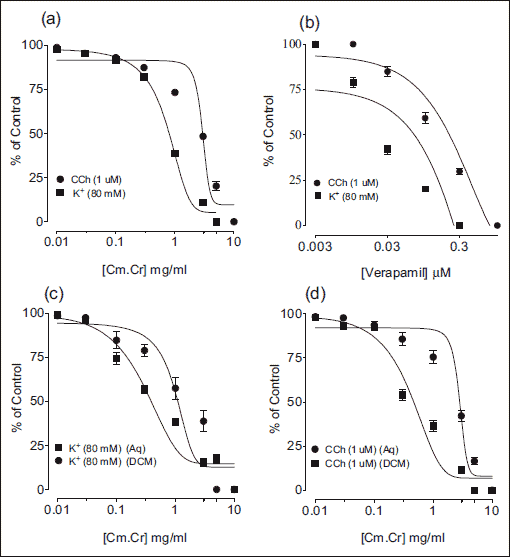 |
Fig. 5. Concentration dependant inhibitory effect of (a) methanolic extract of Cymbopogon martinii (Cm.Cr) (b) verapamil and (c and d) aqueous and dichloromethane fractions of Cm.Cr on carbachol (1 µM) and high K+ (80 mM)- induced contractions in isolated rabbit tracheal preparations (Values are the mean ± S.E.M., n=5). |
Effect on isolated rabbit aorta preparations
The Cm.Cr exerted contractile effect after application to the isolated rabbit aortic preparations at concentration ranging from 0.01 to10.0 mg/ml (Fig. 6a). However, the Cm.Cr at tissue bath concentration of 0.01–3.0 mg/ml exerted initially a contractile effect and then a relaxant effect (>5mg/ml) on K+ (80 mM)-induced contractions in isolated rabbit aortic preparations with an EC50 value of 0.219 mg/ml (95% CI: 3.285–3.905 mg/ml; n=3–4), whereas it caused a partial relaxation of phenylephrine (PE; 1 µM)-induced contractions (Fig. 6b).
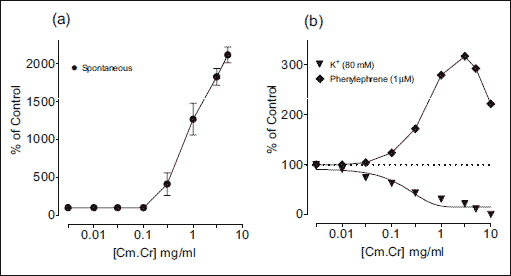 |
Fig. 6. Graphs showing (a) contractile effect of Cymbopogon martinii (Cm.Cr) on isolated rabbit aortic tissue (without any pretreatment) in a concentration dependent manner (0.01–10.0 mg/ml) (b) application of Cm.Cr on pretreatment with high K+ (80 mM) aortic tissue caused dual effect; initial vasoconstriction (0.01–3.0 mg/ml) and then vasorelaxation (>5mg/ml). While against phenylephrine (1 µM) induced contraction Cm.Cr completely relaxed the tissue. (Values are the mean ± S.E.M., n=3–4). |
DISCUSSION
Cymbopogon martinii is a species of grass in the lemongrass genus and possess numerous traditional applications in the south Asians communities for the relief of abdominal discomforts including diarrhea, dysentery and abdominal pain. The essential oil (palmarosa) extracted from the leaves of Cymbopogon martini has shown clinical usefulness in anthelmintic and antibacterial activities with the predominating component geraniol (65–83%), a monoterpenoid and an alcohol (11, 26). Although other components like citral, citronellol and linalool are present and possibly could contribute significantly in biological activities. In the recent years many pharmacological activities pertaining to garaniol and citral has been pointed out ranging from Ca2+ channel blockage to interference with enteric nervous system of gastrointestinal tract (27, 28). This voltage gated channel blockage potential of essential oil of Cymbopogon martinii drew out attention to validate the folkloric uses of this lemongrass genus in specific types of visceral tissues in which calcium is a key player. Therefore, the possible presence of spasmolytic constituent(s) was investigated on isolated rabbit jejunum preparations, a model that permits the study of spasmolytic activity without the use of an agonist (27-33). Addition of Cm.Cr to spontaneously contracting isolated rabbit jejunum preparation inhibited spontaneous contractions, thus demonstrated an antispasmodic potential. It has been reported that spasmolytic effect on the part of medicinal plants likely can be mediated through blockade of Ca2+ channels (29, 36). The contractile elements in smooth muscle preparations including isolated rabbit jejunum preparations are activated on increased cytoplasmic free Ca2+ concentration (34). The increased intracellular Ca2+ level is likely to be mediated either influx through voltage dependent Ca2+ channels (VDCs) or released of Ca2+ from sarcoplasmic stores (38). The spontaneous movement of the intestine is regulated by the periodic depolarization and repolarization and when tissue is at maximal depolarization, the action potential is mediated via rapid influx of Ca2+ through VDCs (39). Thus, the observed relaxant effect of the Cm.Cr on the hyperactive smooth muscle preparation can be possibly mediated either through blockade of VDCs or through inhibition of Ca2+ released from sarcoplasmic stores. This finding is in agreement with the previously conducted study in which other variety of lemongrass i.e. Cymbopogan citratus and chief constituent citral has shown spasmolytic effect on isolated rabbit ileum (26).
It has been reported that K+ (80 mM) causes the opening of VDCs, modifying the extracellular Ca2+ and resulting in the contraction of smooth muscle (38); therefore the substances capable to relax K+ (80 mM)-induced contractions is presumed to act as Ca2+ channel blocker (CCB). In order to assess whether antispasmodic effect of Cm.Cr is also mediated via a similar mechanism, the Cm.Cr was tested on K+ (80 mM)-induced contractions and the addition of Cm.Cr to tissue bath in a cumulative fashion (33) resulted in the relaxation of K+ (80 mM) induced contractions in isolated rabbit jejunum preparations. These findings were confirmed considering that the administration of Cm.Cr to isolated rabbit jejunum preparation resulted in a decrease in tissue response to Ca2+, resulting in rightward shift of the concentration response curves of Ca2+, similar to verapamil, a standard Ca2+ channel blocker (40). The Ca2+ channel blockers are known to be effective in hyperactive diseases of the gut (34) and this may validate the folkloric use of Cymbopogon martinii. The fractions of Cm.Cr, when explored for Ca2+ channel blocking activity, resulted in appearance of more pronounced activity in dichloromethane fraction as compared to aqueous, indicating that Ca2+ channel blocking activity is present among the non-polar plant constituents.
The leaves of Cymbopogan martini in the form of herbal tea has been used for the management of respiratory diseases like asthma and bronchitis and therefore, require validation. High K+ was used to induce contraction by opening of VDCs in a similar mechanism of jejunum tissue whereas, carbachol which is a M1 muscarinic receptor agonist induce contraction of tracheal tissue by stimulating predominantly Gq protein of trimeric G proteins of class that use upregulation of phospholipase C and, therefore, inositol trisphosphate and intracellular calcium as a signalling pathway (42). The Cm.Cr exerted relaxant effect on carbachol
(1 µM)- and K+ (80 mM)-induced contractions in isolated rabbit tracheal preparations in a manner similar to verapamil and it is possible to hypothesize that this activity can be mediated through Ca2+ channel blocking effect. Recently, it has been reported by de Menezes-Filho and colleagues (28) about the blockage of calcium and potassium channels in myocardium by garaniol, therefore we could possibly speculate that inhibition of calcium channels in trachea is mediated by this chief component of lemon grass. As Ca2+ channel blockers are known to be useful as bronchodilator in ailments due to increased sensitivity of respiratory tract (36), this study provided a scientific basis for the traditional uses of the plant in the management of respiratory disorders including asthma, cough, and bronchitis.
Surprisingly, Cm.Cr when applied to isolated spontaneously contracting aortic tissue caused further contraction of vascular smooth muscles. The contraction of vascular smooth muscle by Cm.Cr indicates the ability of this extract to stimulate possibly α1 adrenergic receptors which downstream augment calcium response and thus contraction of vascular smooth muscles (39). Further, Cm.Cr showed a dual effect (vasoconstriction-vasorelaxation) of the K+ (80 mM)-induced contractions in isolated rabbit aorta, whereas phenylephrine-induced contractions were partially relaxed. At higher dosage (>5.0 mg/ml) of Cm.Cr vasorelaxation effect might be mediated through the blockage of L-type Ca2+ and voltage gated K+ channels. This finding is in agreement with the recent published work by de Menezes-Filho et al., which demonstrated the blockage of Ca2+ and voltage gated K+ channels by geraniol (major component of Cymbopogan martini leaves) in mammalian myocardium (28). The isolated rabbit aorta preparations have been used for characterization of Ca2+ channel blocking activities (33) and tissues exposed to K+ (80 mM) showed contractions of smooth muscles via opening of voltage dependent Ca2+ channels (VDCs). The increase in intracellular Ca2+ due enhanced influx of Ca2+ can cause further Ca2+ release from sarcoplasmic reticulum (43, 44). Similarly, phenylephrine (PE) causes contraction of vascular smooth muscles due to raised cytsoplasmic Ca2+ through two possible means, i.e., Ca2+ influx via receptor operated channels (ROCs) and subsequent release of Ca2+ from intracellular stores (37). The partial relaxation of phenylephrine-induced contractions on the part of Cm.Cr can be explained by focusing the fact that Cm.Cr, like other Ca2+ channel blockers, can only block Ca2+ influx through only VDCs and sparing other possible mechanism involved (38). Recently Su and colleagues (45) provided strong evidences of vasorelaxation of aortic ring by an ethanolic extract of Rubus chingii Hu (Rosaceae) with involvement of other mechanisms like release of vasodilator substances from intact endothelium, or nitric oxide (NO) synthase or stimulation of muscarinic receptors. Therefore, vasodilator activity mediated by Cm.Cr against phenylephrine and high K+ could be the result of multiple other mechanisms apart from calcium antagonism. Nevertheless, the observed relaxant effect of Cm.Cr on vasculature may provide a scientific basis for the folkloric use of Cymbopogon martini in the management of cardiovascular ailments.
In conclusion, the crude methanolic extract of Cymbopogon martini Roxb. (Cm.Cr) has demonstrated antispasmodic, bronchodilator and vasodilator activities. The observed antispasmodic, bronchodilator and vasodilator properties are likely to be mediated through blockade of voltage dependent Ca2+ channels. Thus the study provided sufficient scientific basis to validate folkloric uses in native systems of medicine.
Conflict of interests: None declared.
REFERENCES
- Cope TA. Poaceae: Flora of Pakistan. Karachi, Pakistan, University of Karachi, 1982.
- Rao BR, Rajput DK, Patel RP. Essential oil profiles of different parts of Palmarosa (Cymbopogon martinii (Roxb.) Wats. var. motia Burk.). J Essent Oil Res 2009; 21: 519-521.
- Rao BR, Rajput DK, Patel RP, Purnanand S. Essential oil yield and chemical composition changes during leaf ontogeny of palmarosa (Cymbopogon martinii var. motia). Nat Prod Commun 2010; 5: 1947-1950.
- Siddiqui N, Garg SC. Chemical composition of Cymbopogon martinii (Roxb.) Wats. var. martinii. J Essent Oil Res 1990; 2: 93-94.
- D’Souza AM, Paknikar SK, Dev V, Beauchamp PS, Kamat SP. Biogenetic-type synthesis of (+)-cymbodiacetal, a constituent of Cymbopogon martinii. J Nat Prod 2004; 67: 700-702.
- Bottini AT, Dev V, Garfagnoli DJ, Hope H, Joshi P, Lohani H. Isolation and crystal structure of a novel dihemiacetal bis-monoterpenoid from Cymbopogon martinii. Phytochemistry 1987; 26: 2301-2302.
- Dubey VS, Luthra R. Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martini Roxb. Wats. var. motia) inflorescence development. Phytochemistry 2001; 57: 675-680.
- Burkill HM. The Useful Plants of West Tropical Africa, London, UK, Royal Botanic Gardens; 1985.
- Boulos L. Medicinal Plants of North Africa. Indiana University, 1983.
- Duke JA, DuCellier JL. Duke’s Handbook of Medicinal Plants of the Bible. Boca Raton, CRC Press, 2008.
- Kumaran AM, D’Souza P, Agarwal A, Bokkolla RM, Balasubramaniam M. Geraniol, the putative anthelmintic principle of Cymbopogon martinii. Phytother Res 2003; 17: 957.
- Katiki LM, Chagas AC, Bizzo HR, Ferreira JF, Amarante AF. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet Parasitol 2011; 183: 103-108.
- Prashar A, Hili P, Veness RG, Evans CS. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry 2003; 63: 569-675.
- Prasad CS, Shukla R, Kumar A, Dubey NK. in vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses 2010; 53: 123-129.
- Tyagi BK, Shahi AK, Kaul BL. Evaluation of repellent activities of Cymbopogon essential oils against mosquito vectors of malaria, filariasis and dengue fever in India. Phytomedicine 1998; 5: 324-329.
- Das MK, Ansari MA. Evaluation of repellent action of Cymbopogan martinii Stapf. var sofia oil against Anopheles sundaicus in tribal villages of Car Nicobar Island, Andaman and Nicobar Islands, India. J Vector Borne Dis 2003; 40:100-104.
- Gacche RN, Shaikh RU, Chapole SM, Jadhav AD, Jadhav SG. Kinetics of inhibition of monoamine oxidase using Cymbopogon martinii (Roxb.) Wats.: a potential antidepressant herbal ingredient with antioxidant activity. Indian J Clin Biochem 2011; 26: 303-308.
- Tamuli P, Saikia M, Boruah P. Post-infectional biochemical changes in Cymbopogon martinii (Roxb.) Wats and Cymbopogon citratus (DC) Stapf. due to leaf rust disease. Am J Plant Sci 2013; 4: 1666-1668.
- Ghadyale V, Takalikar S, Haldavnekar V, Arvindekar A. Effective control of postprandial glucose level through inhibition of intestinal alpha glucosidase by Cymbopogon martinii (Roxb.). Evid Based Complement Altern Med 2012; 2012: 372909.
- Buch P, Patel V, Ranpariya V, Sheth N, Parmar S. Neuroprotective activity of Cymbopogon martinii against cerebral ischemia/reperfusion-induced oxidative stress in rats. J Ethnopharmacol 2012; 142: 35-40.
- Khare CP. Indian Medicinal Plants an Illustrated Dictionary. Springer, 2007.
- Zia-Ul-Haq M, Riaz M, De Feo V, Jaafar HZ, Moga M. Rubus fruticosus L.: constituents, biological activities and health related uses. Molecules 2014; 19: 10998-11029.
- Zia-Ul-Haq M, Ahmad S, Bukhari SA, Amarowicz R, Ercisli S, Jaafar HZ. Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Biol Res 2014; 47: 23.
- Zia-Ul-Haq M, Stankovic M, Rizwan K, De Feo V. Grewia asiatica L., a food plant with multiple uses. Molecules 2013; 18: 2663-2682.
- Guide for the Care and Use of Laboratory Animals. National Research Council. Washington DC, National Academy Press, 2011.
- Raina VK, Srivastava SK, Aggarwal KK, Syamasundar KV, Khanuja SP. Essential oil composition of Cymbopogon martinii from different places in India. Flavour Fragr J 2003; 18: 312-315.
- Devi RC, Sim SM, Ismail R. Spasmolytic effect of citral and extracts of Cymbopogon citratus on isolated rabbit ileum. J Smooth Muscle Res 2011; 47: 143-156.
- de Menezes-Filho JE, Gondim AN, Cruz JS, et al. Geraniol blocks calcium and potassium channels in the mammalian myocardium: useful effects to treat arrhythmias. Basic Clin Pharmacol Toxicol 2014; 115: 534-544.
- Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci 2005; 76: 3089-3105.
- Imran I, Hussain L, Zia-Ul-Haq M, Janbaz KH, Gilani AH, De Feo V. Gastrointestial and respiratory activities of Acacia leucophloea. J Ethnopharmacol 2011; 138: 676-682.
- Kitic D, Brankovic S, Radenkovic M, Savikin K, Zdunic G, Kocic B, Velickovic-Radovanovic R. Hypotensive, vasorelaxant and cardiodepressant activities of the ethanol extract of Sideritis raeseri spp. raeseri Boiss & Heldr. J Physiol Pharmacol 2012; 63: 531-535.
- Farre AJ, Colombo M, Fort M, Gutierrez B. Differential effects of various Ca2+ antagonists. Gen Pharmacol 1991; 22: 177-181.
- Van Rossum JM. Cumulative dose-response curves. II. Technique for the making of dose response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther 1963; 143: 299-330.
- Janbaz KH, Haider S, Imran I, Zia-Ul-Haq M, De Martino L, De Feo V. Pharmacological evaluation of Prosopis cineraria (L.) Druce in gastrointestinal, respiratory, and vascular disorders. Evid Based Complement Alternat Med 2012; 2012: 735653.
- Chaudhary MA, Imran I, Bashir S, Mehmood MH, Rehman NU, Gilani AH. Evaluation of gut modulatory and bronchodilator activities of Amaranthus spinosus Linn. BMC Complement Altern Med 2012; 12: 166.
- Ghayur MN, Gilani AH. Studies on cardio-suppressant, vasodilator and tracheal relaxant effects of Sarcococca saligna. Arch Pharm Res 2006; 29: 990-997.
- Karaki H, Ozaki H, Hori M, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 1997; 49: 157-230.
- Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev 1986; 38: 321-416.
- Brading AF. How do drugs initiate contraction in smooth muscles? Trends Pharmacol Sci 1981; 2: 261-265.
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol 1977; 17: 149-166.
- Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J 1995; 9: 619-625.
- Pang S, Tsuchiya S, Horie S, Uchida M, Murayama T, Watanabe K. Enhancement of phenylephrine-induced contraction in the isolated rat aorta with endothelium by H2O-extract from an Oriental medicinal plant Leonuri herba. Jpn J Pharmacol 2001; 86: 215-222.
- Gurney AM. Mechanisms of drug-induced vasodilation. J Pharm Pharmacol 1994; 46: 242-251.
- Ghayur MN, Gilani AH, Ahmed T, et al. Muscarinic, Ca++ antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol 2008; 60: 1375-1383.
- Su XH, Duan R, Sun YY, et al. Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: an in vivo and in vitro approach. J Physiol Pharmacol 2014; 65: 417-424.
A c c e p t e d : September 24, 2014