SIGNIFICANT DETERIORATION OF ANTI-ATHEROGENIC EFFICACY OF NEBIVOLOL IN A DOUBLE (APOLIPOPROTEIN E AND ENDOTHELIAL NITRIC OXIDE SYNTHASE) KNOCKOUT MOUSE MODEL OF ATHEROSCLEROSIS IN COMPARISON TO SINGLE (APOLIPOPROTEIN E) KNOCKOUT MODEL
INTRODUCTION
Since 1992 the mouse has become an excellent model for experimental atherosclerosis research. Until 1992, the diet - induced atherosclerosis mouse model has been used effectively, but the lesions tended to be small and were limited to early fatty-streak stage. In 1992 the first line of gene targeted animal models, namely apolipoprotein E (apoE)-single knockout mice was developed. Of the genetically engineered models, the apoE-deficient model was the only one that developed extensive atherosclerotic lesions on a chow diet. The creation of apoE-single knockout mice has changed the face of atherosclerosis research (1).
In 2001, more sophisticated model of atherogenesis: apoE and endothelial nitric oxide synthase (eNOS)-double knockout mice has been created independently in two laboratories (2, 3). It has shown that chronic deficiency of eNOS increases atherosclerosis in apoE-knockout mouse model. Furthermore, in the absence of eNOS, peripheral coronary disease, chronic myocardial ischemia, heart failure, and an array of vascular complications develop that have not been observed in apoE-knockout animals. However, soon it has occurred that there was a big problem with the model: apoE and eNOS-double knockout mice bred extremely poor. In spite of this (since the model is excellent), we decided that after our experiments with nebivolol in apoE-single knockout mice (4, 5), we would try to use apoE and eNOS-double knockout mice to investigate the mechanism of nebivolol anti-atherogenic action.
MATERIALS AND METHODS
Animals and treatment
All animal procedures were approved by the Jagiellonian University Ethical Committee on Animal Experiments.
Ten female apoE and eNOS-double knockout mice on B6.129P2 background were created from apoE-knockout and eNOS-knockout mice by Jackson Laboratory (Bar Harbor, Maine, USA) (project number 21536_BHSM). Thirteen female apoE-single knockout mice on B6.129P2 background were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Mice were maintained on 12-h dark/12-h light cycles in air-conditioned rooms (22.5 ± 0.5°C, 50 ± 5% humidity) and access to diet and water ad libitum. At the age of 8 weeks mice were put on chow diet made by Ssniff (Soest, Germany) for 4 months. Experimental group received the same diet, mixed with nebivolol (Janssen Pharmaceutica, Geel, Belgium) at a dose 2.0 µmol per kg of body weight per day (five from double knockouts and six from single knockouts).
Therefore, we received four groups: A: control apoE-single knockout (n=7); B: apoE-single knockout treated with nebivolol (n=6); C: control apoE and eNOS-double knockout (n=5); D: apoE and eNOS-double knockout treated with nebivolol (n=5).
Procedures
At the age of 6 months mice were sacrificed under anesthesia and 1000 UI of fraxiparine (Sanofi-Synthelabo, France) was injected into the peritoneum. The blood was collected from the right ventricle. Plasma was separated by centrifugation at 1000 × g at 4°C for 10 min and stored in –80°C. Then, right atrium was incised and the heart was perfused by PBS through the apex of the left ventricle at a constant pressure of 100 mm Hg. Next, the heart was dissected (6, 7).
Plasma lipids
Total cholesterol and triglycerides were assayed using commercially available kits (Roche Molecular Biochemical, USA).
Quantitation of atherosclerosis
The heart and ascending aorta were embedded in OCT compound (CellPath, UK) and snap-frozen. Ten micrometer-thick cryosections were cut from the aortic root using a standardized protocol (8, 9).
Serial sections were cut from the proximal 1 mm of the aortic root. Eight adjacent sections were collected at 100-µm intervals starting at a 100-µm distance from the appearance of the aortic valves. Sections were thaw-mounted on poly-L-lysine coated slides and air dried. After fixation in 4% paraformaldehyde (pH = 7), sections were stained with Meyer's hematoxylin and oil red-O (Sigma-Aldrich, USA). Oil red O-stained sections were examined under Olympus BX50 (Olympus, Tokyo, Japan) microscope and used for quantitative evaluation. Images of the aorta were recorded using Olympus Camedia 5050 digital camera and stored as TIFF files of resolution 1024 × 768 pixels. Total area of the lesion was measured semiautomatically in each slide using LSM Image Browser 3 software (Zeiss, Jena, Germany). For each animal a mean lesion area was calculated from eight sections, reflecting the cross-section area covered by atherosclerosis.
Statistical analysis
Results are expressed as mean ± S.E.M. The nonparametric Mann-Whitney U test was used for analysis of the data. P<0.05 was considered as statistically significant.
RESULTS
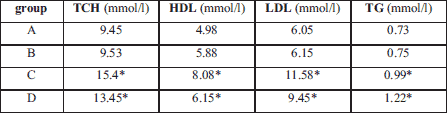
Nebivolol did not change the level of cholesterol and triglycerides in blood, as compared to the control group, both in apoE-single knockout as well as in apoE and eNOS-double knockout groups. However, lipid level was significantly higher in double knockout mice (Table 1).
In apoE-single knockout mice, lesion area measured by "cross-section" of aortic roots was 79,244 ± 6,143 µm2 in the control group versus 65,347 ± 6,152 µm2 in nebivolol-treated group (P<0.05).
In apoE & eNOS-double knockout mice, lesion area measured by "cross-section" of aortic roots was 92,319 ± 8,876 µm2 in the control group versuss 98,609 ± 9,164 µm2 in nebivolol-treated group (P>0.05).
The comparison between apoE-single knockout mice and apoE and eNOS-double knockout mice without treatment, showed statistically significant difference: 81,232 ± 8,264 µm2 versus 92,319 ± 8,876 µm2 (P<0.05) (Fig. 1 and 2).
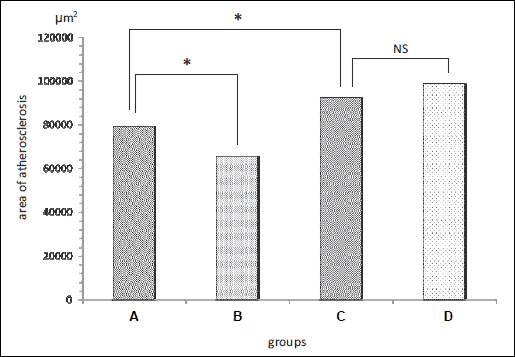 |
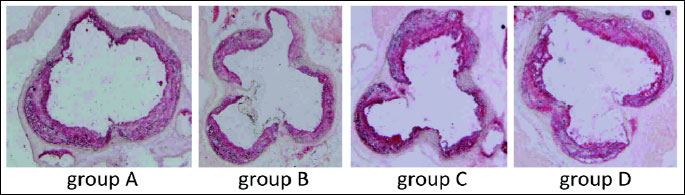
Fig. 1. Atherosclerostic lesions, presented as mean in (A) control apoE-single knockout mice, (B) nebivolol-treated apoE-single knockout mice, (C) control apoE and eNOS-double knockout mice and (D) nebivolol-treated apoE and eNOS-double knockout mice. *P<0.05; NS - non statistically significant difference between groups. |
DISCUSSION
Nebivolol is a third-generation β-blocker with vasorelaxation properties (10). It has been suggested that this effect is mediated by increased nitric oxide (NO) production, because it can be abrogated by inhibitors of NO synthase (NOS) (11).
In vivo metabolized nebivolol increases vascular NO production. This phenomenon involves endothelial b2-adrenergic receptor ligation, with a subsequent rise in endothelial free [Ca2+]i and endothelial NO synthase-dependent NO production. This may be an important mechanism underlying the nebivolol-induced, NO-mediated arterial dilation in humans.
Dessy et al. identified β3-adrenoreceptors in the endothelium of human coronary resistance microarteries, where they mediate an endothelium-dependent relaxation to both endogenous catecholamines and 3-adrenoreceptor-preferential agonists (12).
The expression of β3-adrenoceptor mRNA and protein was demonstrated in extracts of human coronary microarteries. Immunohistochemical analysis revealed their exclusive localization in the endothelium, with no staining of vascular smooth muscle. In contractility experiments in which videomicroscopy was used, the nonspecific - β agonist isoproterenol and the β3-preferential agonist BRL37344 evoked an 50% relaxation of endothelin-1 - preconstricted human coronary microarteries. Relaxations were blocked by the 1/2/3-adrenoceptor antagonist bupranolol, but were insensitive to the 1/2-adrenoceptor antagonist nadolol, confirming a β3-adrenoceptor-mediated pathway.
Dessy et al. also showed this response to be mediated through the production of both NO and a hyperpolarizing factor that partly maintains vessel relaxation when eNOS is inhibited. Such a dual mechanism would be particularly suitable in circumstances of reduced NOS activity or NO bioavailability, as commonly found in atherosclerotic and ischemic diseases (12).
They, therefore, examined also whether nebivolol could activate b3-adrenoreceptors to mediate NO-dependent vasodilatory effects in isolated human and rodent coronary microvessels and characterized the transduction pathway leading to eNOS activation (13). Moreover, comparative use of rings from β3-AR(–/–) and eNOS(–/–) mice again confirmed that this property depends on the presence of both eNOS and β3-adrenoreceptors.
Hypertension and atherosclerosis are major factors in the etiology of ischemic heart disease and cerebrovascular disease - the 2 leading causes of death worldwide - and are important in the development of kidney dysfunction, congestive heart failure, and angina (2).
Considerable epidemiological evidence suggests that high blood pressure (BP) may have a direct role in enhancing atherosclerotic lesion formation (14). Atherosclerosis is 3 times more common in patients with hypertension, and there is a positive, although not linear, correlation between BP and atherosclerosis. In addition, atheroma formation occurs in muscular arteries but not in lower-pressure veins, and hypertension promotes lesion formation in the presence of hypercholesterolemia (14). Clinically, many antihypertensive drugs are effective in reducing morbidity and mortality from atherosclerotically mediated cardiovascular events (15). Despite this body of evidence, the effect of hypertension on atherosclerosis has been difficult to study in humans because of confounding variables and the complexity of the genetics underlying each condition. Animal studies have been equally hindered by the lack of appropriate models with simultaneous genetic predispredispositions for atherosclerosis and hypertension.
In 2000, Knowles et al. created for the first time such a model, generated by breeding mice that spontaneously develop atherosclerosis due to apoE-deficiency with mice that are hypertensive due to lack of the endothelial nitric oxide synthase gene (eNOS-knockout) (2).
The eNOS serves important basal regulatory functions in the vasculature. In response to stimuli such as shear stress or acetylcholine, eNOS catalyzes the production of nitric oxide (NO) from L-arginine. The NO diffuses across the endothelial cell membrane into neighboring smooth muscle cells and induces vasodilation. NO also acts locally to prevent platelet and leukocyte aggregation and inhibits vascular smooth muscle cell proliferation (16). Direct evidence that eNOS mutations can cause hypertension has been presented by Shesely et al. (17) and Huang et al. (18), who showed that mice lacking eNOS have increased BP, decreased heart rate, and increased plasma renin activity, but no atherosclerosis. Although linkage between genetic polymorphisms in the eNOS gene and essential hypertension has not been conclusively documented in humans, there is substantial evidence that NO pathways are disrupted in both hypertension and atherosclerosis (19, 20).
Knowles et al. demonstrated that mice lacking both eNOS and apoE have significantly increased BP, develop larger plaques, and have more severe kidney damage than do apoE-deficient mice with intact eNOS function (2). The effects were ameliorated by treatment with the angiotensin-converting enzyme (ACE) inhibitor enalapril. However, Chen et al. in 2001 showed that the effects of eNOS gene deficiency on accelerating atherogenesis are not solely due to hypertension, in that lowering the blood pressure to normal in the apoE and eNOS-double knockout mice did not reduce the lesion area (21).
Besides the effects on BP and heart rate, the eNOS system is potentially antiatherogenic through many other mechanisms. For example, a direct role for NO in decreasing vascular smooth muscle cell proliferation after a remodeling stimulus has been described in eNOS-knockout mice (22). In addition, NO inhibition of platelet aggregation and monocyte adherence to the vessel wall are potentially antiatherogenic (16, 19), and antioxidant functions of NO may prevent proatherogenic changes in lipoproteins (23). Many of the local functions of NO counterbalance proatherogenic effects of angiotensin II, which mediates vasoconstriction, smooth muscle cell migration and proliferation, and monocyte adhesion. It is important to note that eNOS-deficient mice have increased plasma renin activity (17), and probably also have increased production of angiotensin II.
Moreover, Kuhlencordt et al. described that mice total cholesterol, and lipoprotein profile did not differ between apoE-single knockout mice and apoE and eNOS-double knockout mice apoE/eNOS-DKO mice fed the Western-type diet for 16 weeks. This is in contradiction to our observations. However, in our experiment we fed mice only by chow diet, which can explain the differences.
Anti-atherogenic action of nebivolol, first shown in rabbit model of atherosclerosis (13, 14) and than confirmed in apoE-single knockout mouse model (4, 24), can be explained by its beneficial effect on endothelium. We described also that nebivolol suppresses the inflammatory processes in the plaque and enhances its stability (5). The results of our experiment confirmed the role of eNOS in the action of nebivolol. In apoE-single knockout mice treated with nebivolol (group B) the atherosclerosis was statistically significant decreased by the action of nebivolol, compared to apoE-single knockout control (group A). However, that was not the case in apoE & eNOS-double knockout mice treated with nebivolol (group D), compared to apoE and eNOS-double knockout control (group C) (Fig. 1 and 2). Moreover, as was described in earlier articles (2, 3, 21, 25-27), total atherosclerosis in apoE and eNOS-double knockout mice is bigger than in apoE-single knockout mice.
Our results show clearly that only in the presence of eNOS, nebivolol significantly decreases atherogenesis. Although this finding confirms the conclusion drawn previously, here, thanks to the use of eNOS knockout animals, our evidences gain a strong direct character which is reflected in the fact that the knockout of eNOS gene is able to destroy the anti-atherosclerotic effect of nebivolol. Nebivolol, but not atenolol, is known to reverse endothelial dysfunction in arterial hypertension both in experimental models as well as in patients (28, 29). Moreover, nebivolol prevents vascular nitric oxide synthase (NOS) III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells (30).
Of note, despite our plans, we were able to receive only 10 female apoE and eNOS-double knockout mice. It was caused by extremely poor breeding of such animals. One can see that until now worldwide, there are only four articles available about apoE and eNOS-double knockout mice, comparing to thousands, relating to apoE-knockout. Naturally, so poor breeding of double knockout mice strongly limited our previous plans, including the number of experiments as well as our plans on molecular studies. However, despite this, hoping that our results may open new area of research on atherogenesis and the mechanism of anti-atherogenic activity of nebivolol, we have decided to submit our results in the state in which we managed to achieve till now.
To our knowledge, this is the first report that describes the effect of nebivolol on atherogenesis in apoE and eNOS-double knockout mice, proving directly the necessity of the presence of eNOS for nebivolol to show an anti-atherogenic potency.
Acknowledgements: This article was supported by the grant from Polish National Center of Science (NCN) No 2012/05/B/NZ4/02743 for years 2013-2015.
Conflict of interests: None declared.
REFERENCES
- Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol 2004; 55: 503-517.
- Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(–/–) Apoe(–/–) mice are ameliorated by enalapril treatment. J Clin Invest 2000; 105: 451-458.
- Kuhlencordt PJ, Gyurko R, Han F et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double knockout mice. Circulation 2001; 104: 448-454.
- Kus K, Gajda M, Pyka-Fosciak G, et al. The effect of nebivolol on atherogenesis in apoE-knockout mice. J Physiol Pharmacol 2009; 60: 163-165.
- Pyka-Fosciak G, Jawien J, Gajda M, Jasek E, Litwin JA. Effect of nebivolol treatment on atherosclerotic plaque components in apoE-knockout mice. J Physiol Pharmacol 2013; 64: 745-750.
- Jawien J, Gajda M, Olszanecki R, Korbut R. BAY x 1005 attenuates atherosclerosis in apoE/LDLR - double knockout mice. J Physiol Pharmacol 2007; 58: 583-588.
- Olszanecki R, Suski M, Gebska A, et al. The influence of angiotensin-(1-7) peptidomimetic (AVE 0991) and nebivolol on angiotensin I metabolism in aorta of apoE-knockout mice. J Physiol Pharmacol 2013; 64: 317-320.
- Toton-Zuranska J, Gajda M, Pyka-Fosciak G, et al. AVE 0991-angiotensin-(1-7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol 2010; 61: 181-183.
- Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol 2012; 63: 77-85.
- Bowman AJ, Chen CP, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol 1994; 38: 199-204.
- Howlett JG. Nebivolol: vasodilator properties and evidence for relevance in treatment of cardiovascular disease. Can J Cardiol 2014; 30 (Suppl. 5): S29-S37.
- Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL. Endothelial 3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation 2004; 110: 948 -954.
- Dessy C, Saliez J, Ghisdal P et al. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation 2005; 112: 1198-1205.
- Chobanian AV, Alexander RW. Exacerbation of atherosclerosis by hypertension. Potential mechanisms and clinical implications. Arch Intern Med 1996; 156: 1952-1956.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265: 3255-3264.
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329: 2002-2012.
- Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1996; 93: 13176-13181.
- Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995; 377: 239-242.
- Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis 1995; 38: 87-104.
- Cox DA, Vita JA, Treasure CB, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation 1989; 80: 458-465.
- Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation 2001; 104: 2391-2394.
- Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium- derived nitric oxide in vascular remodeling. J Clin Invest 1998; 101: 731-736.
- Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension 1995; 25: 155-161.
- Baumhakel M, Schlimmer N, Buyukafsar K, Arikan O, Bohm M. Nebivolol, but not metoprolol, improves endothelial function of the corpus cavernosum in apolipoprotein e-knockout mice. J Pharmacol Exp Ther 2008; 325: 818-23.
- Thakur NK, Hayashi T, Sumi D et al. Anti-atherosclerotic effect of beta-blocker with nitric oxide-releasing action on the severe atherosclerosis. J Cardiovasc Pharmacol 2002; 39: 298-309.
- de Nigris F, Mancini FP, Balestrieri ML, et al. Therapeutic dose of nebivolol, a nitric oxide-releasing beta-blocker, reduces atherosclerosis in cholesterol-fed rabbits. Nitric Oxide 2008; 19: 57-63.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2009; 202: 48-57.
- Lekakis JP, Protogerou A, Papamichael C, et al. Effect of nebivolol and atenolol on brachial artery flow-mediated vasodilation in patients with coronary artery disease. Cardiovasc Drugs Ther 2005; 19: 277-281.
- Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double-blind, crossover study. Circulation 2001; 104: 511-514.
- Mollnau H, Schulz E, Daiber A, et al. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol 2003; 23: 615-621.
A c c e p t e d : October 29, 2014