CROSSFIT TRAINING CHANGES BRAIN-DERIVED NEUROTROPHIC FACTOR AND IRISIN LEVELS AT REST, AFTER WINGATE AND PROGRESSIVE TESTS, AND IMPROVES AEROBIC CAPACITY AND BODY COMPOSITION OF YOUNG PHYSICALLY ACTIVE MEN AND WOMEN
2Medical University, Department of Pharmaceutical Biochemistry, Wroclaw, Poland
INTRODUCTION
Regular physical activity brings about beneficial changes in the organism that can be observed on many levels of its organization, including the molecular one (remodeling). It fosters an improvement in overall physical performance as well as in the efficiency of energy processes, cardiovascular fitness, improvement in nerve-muscle coordination and also in cognitive function and memory (1). All the adaptive changes result in an increased tolerance of fatigue, allow faster restitution and performance of longer and of higher-intensity exercise. The appearance of adaptive changes is correlated with the stimulation of a number of molecular mechanisms, which are the basis for physiological changes (2). According to Pedersen (3) locally secreted substances, which participate in intercellular communication working as auto-, para- and hemocrine signals, are responsible for tissue remodeling.
Brain-derived neurotrophic factor (BDNF) is an example of a substance participating in the integration of blood-transmitted signals. It is a protein that stimulates processes of neurogenesis, fosters the survival of neurons and microglia, stimulates neuroplasticity, participates in the differentiation of cells developed in the hippocampus, the main structure of the central nervous system in which neurogenesis takes place. BDNF is responsible for short-term memory and also the maintenance of long-term potentiation (LTP), and can facilitate memorizing, cognition, emotional states, spatial navigation, learning (4-6). The protein is also produced by skeletal muscles in response to physical exercise. BDNF is involved in muscle communication with the nervous system and stimulation of synaptic plasticity and neurogenesis. BDNF is, therefore, also a myokine (3, 7, 8), and muscles are an endocrine organ (9, 10).
According to the research by Bostrom et al. (7), carried out on an animal model, the beneficial changes occurring in skeletal muscles during physical exercise are the result of expression of peroxisome proliferator activated receptor gamma coactivator 1a (PGC-1a). An increase in expression of this coactivator stimulates an increase in expression of fibronectin type III domain containing protein 5 (FNDC5). FNDC5 undergoes proteolytic cleavage, which results in a 112-amino acid fragment called irisin being cleaved from it and secreted into the bloodstream (6). Irisin in the cells of adipose tissue stimulates expression of UPC-1 (uncoupling protein-1) with PGC-1α and takes part in the transdifferentiation of white adipocytes into brown ones (browning). It is capable of inducing UPC-1α, and also numerous genes of the cells of brown adipose tissue. It takes part in the regulation of energy metabolism. It plays an important role in glucose homeostasis. Irisin is also called an effort hormone. It is a myokine secreted by muscles during physical exercise (7) and an adipokine released by white adipose tissue (11). Wrann et al. (6) observed an increase in expression of FNDC5 also in the hippocampus of mice subjected to endurance exercise and stated that PGC-1α and FNDC5 regulate expression of BDNF in the brain.
Over the last few years CrossFit training has become very popular. Its aim is to shape different features of physical fitness - endurance, strength, speed, coordination or power. It consists in doing exercise based on functional movements, whose feature is the complexity of the movement pattern, consistent with the natural form of movement, which engages the whole kinematic chain (12).
CrossFit training units are based, among other things, on multi-joint movements performed as quickly as possible with the workload adjusted to the subject’s abilities, in a limited time period or with a limited number of repetitions. Due to the variety of its movement forms, combinations of exercise and repetition of exercise in series, CrossFit training is classified as HIIT training (High Intensive Interval Training). It contributes to the overall development of a person’s body and mind (13).
More and more often attention is paid to the beneficial influence of high-intensity training on the reduction in body mass, an improvement in the lipid profile, and in aerobic capacity and on similar adaptive changes in comparison with traditional endurance training (14-17). Smith et al. (18) showed that a 10-week CrossFit training program based on HIPT training (High Intensity Power Training) performed by adult men and women resulted in a significant increase in VO2max and a reduction in adipose tissue percentage.
In the literature there are no investigations of the influence of HIIT on BDNF and irisin levels in the bloodstream, and the available ones are mostly concerned with exercise and training of moderate intensity.
The aim of the study was to find out:
- if resting BDNF and irisin levels change after CrossFit training in men and women,
- how BDNF and irisin levels change after Wingate and progressive tests before and after CrossFit training in men and women,
- if physical performance and anthropometric parameters change after CrossFit training in men and women.
MATERIAL AND METHODS
The studied group
At the beginning the studied group consisted of 15 men and 15 women. However, over the course of time the number of participants was gradually reduced, mainly due to the injuries the subjects suffered and the study discipline, which was impossible for some volunteers to cope with. The discipline implied the subjects’ regular participation in training sessions and tests before and after CrossFit training. As a result, the studied group at the end consisted of 12 adults - men (n = 7, age 26.78 ± 6.8 years, body mass 83.96 ± 4.85kg; height 179.71 ± 3.15cm) and women (n = 5, age 24.0 ± 1.82 years, body mass 59.25 ± 5.7kg, height 165.72 ± 8.02 cm), who voluntarily agreed to participate in the CrossFit training program. The subjects were fit and healthy, have not be treated or hospitalized for the last 12 months. For 2 days before the tests, they abstained from any physical activity that might influence the test results.
CrossFit training
The CrossFit training sessions lasted for 3 months and took place from February (baseline) to April 2013 (state 3), twice a week on Tuesdays and Fridays from 8 to 9 p.m. The training was of a group-class type. The classes took 60 min, and consisted of 2 workouts known as a workout of the day (WOD).
Each training unit started with a warm-up (running round the gym, multiple-joint movements, skipping jumps, jumps, push-ups, knee bends, arms swings etc.). Then, the main part of the training, the WOD, followed. For 10 – 15 minutes, each subject was tasked with performing the maximal number of repetitions of the exercises set by the instructor with their maximal tempo and intensity. Most often they were asked to perform a mix of some strength exercises, for example front squats, kettlebell swings, clean and jerk, lunges, and aerobic exercises: running round the gym, rope jumps, box jumps, burpees, air squats. After the first part of the training, there was a 5-minute break for the subjects to regenerate their strength and then the second WOD started. Most often, the first WOD was of a strength-speed training type developing speed, power and endurance, while the second WOD was dominated by aerobic exercises in which the emphasis was put on their aerobic capacity. In this part, the subjects took turns to exercise on a rowing ergometer, cycle ergometer and mechanical treadmill. During the training sessions the subjects were encouraged by the instructor and their group. After the training there was a cool-down period and intense stretching of the whole body (5 min). The training session lasted for one hour including the break between the WODs. The actual intensity of each training units measured with Sportester (Polar RS 400, Finland) oscillated in the range 85 – 95% HRmax, HRmax was calculated according to the formula 220-age.
To assess the influence of CrossFit training on the body and levels of BDNF and irisin, the subjects performed 2 tests twice before and after the training: a 30-second Wingate test and a week later a progressive test. Both tests were performed on a cycle ergometer, from 7:30 to 10:30 a.m., 2 hours after a meal.
The Wingate test
The Wingate test assesses anaerobic capacity. It was performed on a Monark 828E cycle ergometer. The test was preceded by a 5-minute warm-up that was also performed on a cycle ergometer at a workload of 100 W. The essence of the test is to generate as quickly as possible the maximum power by the muscles, which is achieved when the subject reaches their maximal speed. The test is aimed at achieving the maximal power in the shortest time possible and at maintaining it for 30 seconds. The workload in the test is calculated with regard to the subject’s body mass, (75 g/kg of body mass). In the test the maximal power (Pmax), minimal power (Pmin), time in which maximal power was achieved (TPP) and time in which maximal power was maintained (Tmax) were assessed, as well as the total work performed during the test (Wtot) expressed in kJ or J/kg of body mass.
The progressive test up to exhaustion
The test assesses the subjects’ aerobic capacity. It was performed on an Excalibur (LODE, The Netherlands) cycle ergometer. The idea of the test is to overcome external resistance as long as the subject is able to maintain the same work rhythm despite a steady state increase in the workload and the increasing acidosis of the organism. The subjects breathed through a mask with a flowmeter located at its end (type: Bidirectional digital turbine Φ 28 mm), which recorded the speed of the airflow and the frequency of exhalations and inhalations. Oxygen analysis was carried out with a zirconia oxygen analyser (type: Zirconia temperature controlled) and a CO2 sensor (type: NDIR). The data were analyzed breath-by-breath and respiratory parameters (VO2, VCO2, VE) were registered with a Quark b2 ergospirometer (Cosmed, Italy). The data of each inhalation and exhalation were averaged in 10s intervals. Before each test the ergospirometer was calibrated in standard fashion with reference.
In the progressive test maximal oxygen uptake VO2max (ml/min) and VO2max (ml/min/kg), maximal pulmonary ventilation (VEmax), oxygen input (VO2) and carbon dioxide output (VCO2), energy expenditure (EEtotal) and the time of the test duration were assessed. VO2max and VEmax were defined as the highest 10s average in the last seconds of the test. EE total was calculated by the device according to an algorithm which takes into account the value of oxygen consumption in 1 minute, the value of the respiratory quotient and caloric coefficient of oxygen. The test procedure and conditions in which it was carried out as well as the applied algorithms to calculate energy expenditure all complied with generally accepted rules (19).
The test began with the workload of 50W, which was then increased by 50W every 3 minutes. The pedaling frequency oscillated in the range 70 – 80 rpm. The subject performed the test up to exhaustion.
The test was performed in laboratory conditions, at room temperature according to the instructions for performing exercise tests.
Due to the subjects’ faster restitution, the Wingate test was always performed first. One week later, on the same day of the week, the subjects performed the progressive test. The tests were performed in the same order after the completion of the training program.
Biochemical parameters
BDNF and irisin were assayed in the serum. Before the training and after its completion the subjects had their blood taken from the basilic vein to determine BDNF and irisin levels. Blood samples were collected in the morning, 2 hours after a meal, from 7:30 to 10:30 a.m. on the day of the first control test. Blood was collected before the training (baseline), and then, on the day of the first control test after the completion of the training (state 3). 20 minutes after it had been collected, the blood was centrifuged and the serum was separated. Each subject’s serum was placed in a few small test tubes and immediately frozen at –85°C. After the completion of the tests, when all the subjects’ blood had been collected and the serum had been separated, biochemical analysis was carried out. Two test tubes with the serum of each subject were thawed. BDNF was assayed in one serum sample and irisin in the other one.
BDNF and irisin levels were assayed using the ELISA method. To assay BDNF, the Enzyme-Linked Immunosorbent Assay kit for brain derived neurotrophic factor (BDNF) (Cloud-Clone Corp. USA) was used. Intraassay CV < 10%, interassay CV < 12%. The minimum detectable dose of BDNF that can be detected by this assay is 0.071 ng/mL.
Irisin was assayed with the Irisin ELISA kit (BioVendor-Laboratorni Medicina, Czech Republic). Intraassay CV = 6.91%, interassay CV = 9.07%. The lowest level of irisin that can be detected by this assay is 0.001 µg/ml.
Three minutes after the completion of the Wingate and progressive tests blood was collected from the fingertip. The level of lactate (LA) and parameters of acid-base balance were assayed in the capillary blood. In the study only the results of hemoglobin saturation with oxygen (satO2) and partial pressure of oxygen in the capillary blood (pO2) after the progressive test at baseline and state 3 were shown. The level of lactate was assayed by means of colorimetry using an LKM 140 Lactate Cuvette Test kit (Dr. Lange, Germany) in a Mini Photometer LP20 Plus (dr Lange Germany). The norm range was 0.6 – 0.9 mmol/l.
Anthropometric measurements
Before the training and after its completion, the subjects had their height, body mass, body composition, chest, waist, hip, arm and thigh circumference measured; their BMI and WHR (waist to hip ratio) were calculated. To measure the height and body mass, medical scales (Radwag, Poland) were used. The assessment of body composition parameters was done by means of spectrometry in near-infrared (Near Infrared Light) using a FUTREX-6100/XL (Futrex Inc.,USA). It is one of the field methods, very often applied in research done in sportspeople. When laboratory methods cannot be used, this is an accepted method for body composition assessment (20-22).
The probe was placed on the biceps brachii, in the middle of the muscle length. As a result, the following were analyzed: the absolute content of adipose tissue (FAT, kg) and its percentage (FAT, %) and the absolute content of lean body mass (LBM, kg). Additionally, the subjects’ waist and hip circumferences were measured. On the basis of the obtained measurements, the value of the waist-to-hip ratio was calculated (WHR). Circumferences of upper (right and left arm) and lower limbs (right and left thigh) were also measured. A SECA 201 measuring tape was used.
The tests were carried out at a Certificated Exercise Physiology Laboratory (licence No: PL-1374-a/3/2009) in compliance with the conditions and procedures applicable to research laboratories of that kind. The researchers were given consent to carry out their research by the University Ethics and Research Committee. All the subjects signed an informed consent agreeing to participate in the study.
Statistical methods
Statistica PL Stat Soft version 10.0 was used for statistical analysis. In all applied statistical tests the level of P < 0.05 was considered statistically significant. The normality of the distribution was measured with the Shapiro-Wilk test. The following tests were used for the data analysis. The ANOVA test was used to assess the differences in parameters at baseline and 3 months after the training, and also to assess the differences in parameters before and after the Wingate and progressive tests. Student’s t-test for independent trials was used to assess differences between men and women. To measure the correlation between the variables, Spearman’s rank correlation coefficient was calculated.
RESULTS
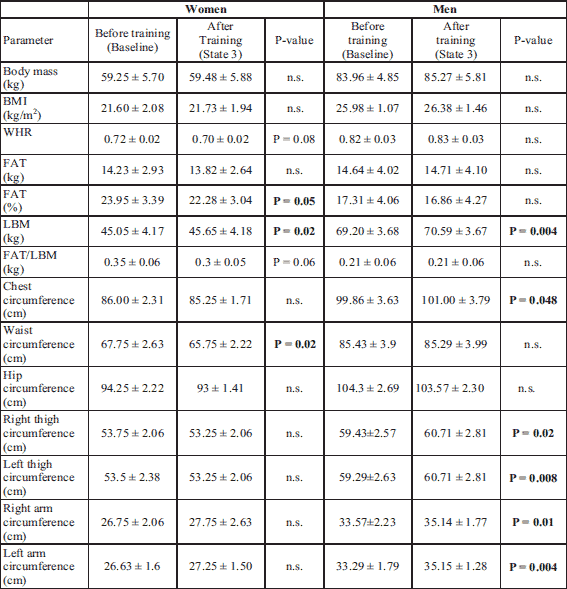
While analyzing the subjects’ anthropometric parameters, changes in body composition and limb circumferences were noted. In women there was a decrease in the value of the WHR indicator and a significant reduction in the waist circumference. LBM significantly increased both in men and women. In women, % FAT in the organism decreased after CrossFit training. What is more, an increase in the circumferences of lower and upper limbs and of the chest was observed in men (Table 1).
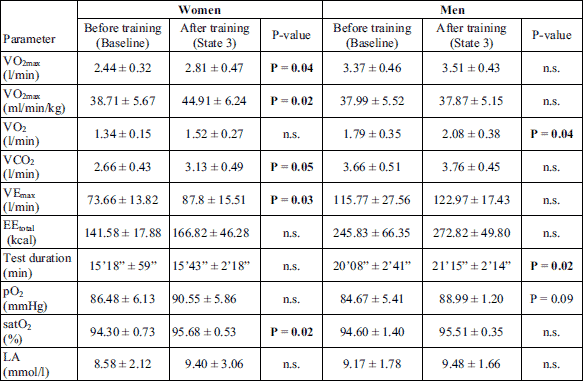
After the 3-month CrossFit training, the subjects’ aerobic capacity measured in the progressive test improved. There was an increase in the value of VO2max, VEmax, and significantly in the duration of exercise performed up to exhaustion. Energy expenditure was also increased during the test. No significant changes were observed in the level of lactate after the progressive test carried out after the completion of the Cross Fit training in comparison to its value before the training (baseline). An increase in pO2 and satO2 was observed after the progressive test carried out 3 months after the CrossFit training (Table 2).
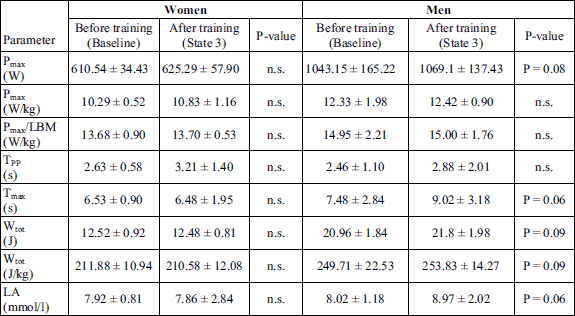
No significant changes were noted in the values of parameters measured in the Wingate test and determining the subjects’ anaerobic capacity; however, in men the growing tendency of Pmax, Wtot and Tmax was observed (Table 3). No significant changes were also observed in the level of lactate after the Wingate test carried out after the completion of the Cross Fit training in comparison to its value at baseline.
While analyzing the changes in BDNF levels, it was noticed that the resting BDNF levels increased after the CrossFit training both in men and women (Fig. 2). What is more, the resting BDNF level was higher in men than in women, both before and after the training.
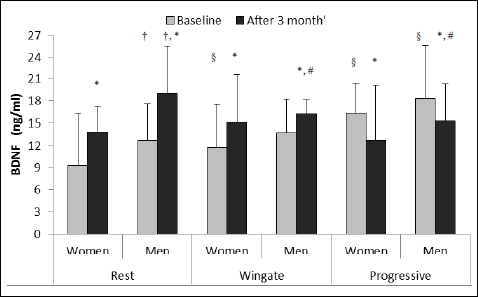 |
Fig. 2. BDNF level at rest and after Wingate and progressive test in women and men at baseline and after 3 months of CrossFit training (values shown as a mean and standard deviation, women n = 5, men n = 7). †P < 0.05 in comparison to the resting value in women (t-Student); §P < 0.05 in comparison to the resting value before training (ANOVA); *P < 0.05 in comparison to the value before training (ANOVA); #P < 0.05 in comparison to men’s resting value after training (ANOVA). |
After the Wingate test, before the CrossFit training BDNF level in men did not change, contrary to the progressive test, after which BDNF level increased significantly in men and in women. The values of BDNF were also significantly higher after the progressive test in comparison with the Wingate test.
After 3 months of the training the values of BDNF after the Wingate test in men and women decreased in relation to the resting values established for the post-training period, but they were higher than the values noted after the Wingate test before the training. Besides, in men higher values of BDNF were noted after the Wingate test in comparison with women. After the progressive test performed after the completion of the training, lower values of BDNF were also observed in both groups. The recorded values for men were significantly higher than those for women.
Before the training, the resting irisin level was significantly higher in women than in men (Fig. 3). Irisin level after the CrossFit training significantly decreased in women. In men, resting irisin level increased but the differences were not statistically significant. After the Wingate and progressive tests, irisin levels did not change in any of the studied groups. The resting irisin level in women after the CrossFit training was slightly higher than in men (P = 0.079). We also observed a little higher level of irisin in women than in men after Wingate and progressive tests in both studied periods but the differences were not statistically significant (P = 0.087, P = 0.082).
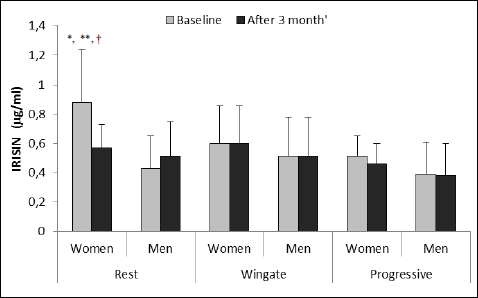 |
Fig. 3. Irisin level at rest and after Wingate and after progressive test in women and men at baseline and after 3 months of CrossFit training (values shown as a mean and standard deviation, women n = 5, men n = 7). *P < 0.01 in comparison to the resting value in men before training (t-Student), **P < 0.01 in comparison to the value after CrossFit training (ANOVA); †P < 0.05 in comparison to the value after Wingate and progressive tests before training (ANOVA). |
In the whole studied group in both periods at rest correlation of irisin with BMI (r = 0.48; P = 0.020) and % FAT (r = 0.56; P = 0.014) and with VO2max in men (r = 0.43; P = 0.012) was noted.
DISCUSSION
In recent years more and more often the positive influence of HIIT on the organism’s aerobic and anaerobic capacity, a reduction in adipose tissue and an increase in muscle tissue has been shown (18, 23-25). CrossFit training is also becoming a very popular free time activity. Thanks to its short-time, varied and attractive functional exercises, it has become an interesting alternative for all those who do not like long, low-intensity exercise sessions aimed at a reduction of body mass or an improvement in physical performance. The example may be provided by the research done by Corte de Araujo et al. (26), in which HIIT was used in obese children, and which proved that the effects of this training are comparable with the effects of endurance training.
After our 3-month CrossFit training the subjects’ physical performance significantly improved. In all subjects, and especially in women the value of VO2max increased. An increase in LBM and in upper and lower limb circumferences was observed in both studied groups. In addition, in women a reduction in % FAT was noted and a downward trend in all subjects.
CrossFit training is characterized by very high intensity of performed exercises, its stimulation of all skeletal muscles and a duration time which is shorter than traditional strength or endurance training because it lasts 20 to 60 minutes. It is claimed that the number and duration of training sessions per week have the biggest influence on body mass reduction and changes in body composition, because it is connected with higher energy expenditure and a negative energy balance. In their research done in a group of professional bicycle racers, Lucia et al. (27) suggest that HIT training has a positive influence on recruitment of slow twitch muscle fibres activated in aerobic exercise. Majerczak et at. (28) claim that the higher oxygen cost and also the higher energetic cost of an exercise below and above the lactate threshold during cycling is dependent on the composition of myosin heavy chains. The authors stated that the higher the content of the fast myosin heavy chain (MyHC2) in vastus lateralis, the higher the oxygen cost of the effort and the more disturbance there is in the metabolic stability of type II muscle fibres, which results in decreased muscle efficiency and a bigger increase in heart rate at a given power output.
HICT (High Intensity Circuit Training) has also been proved to significantly influence a reduction in body mass and adipose tissue in obese adults leading to a considerable increase in LBM. The authors claim that the high intensity of strength training results in an increase in muscle mass and a reduction in body fat mass at the same time (29).
In our study we did not observe any reduction in body mass but there was a significant increase in LBM. Our results demonstrate that CrossFit training has a positive influence on aerobic capacity. The mean value of VO2max in women increased by 14%. The measurements of acid-base balance indicate a post-training increase in pO2 in the bloodstream and blood saturation at rest and after exercise, which might increase oxygen capacity of the blood and the body’s capability of performing long-term exercise as a result of an increase in the number of capillaries (angiogenesis) in the organism. Tang et al. (30) showed that physical effort increases expression of VEGF (vascular endothelial growth factor) in the brain, lungs and skeletal muscles. In our study the duration of the progressive test was, on average, longer by 4%, and maximal pulmonary ventilation achieved by the subjects after the CrossFit training increased by 9%.
Against the background of these desired training changes, it was concluded that the 3-month CrossFit training resulted in a significant increase in resting BDNF level both in men and women. It proves that the exercises used in the training exerted an influence that stimulated physiological adaptation, which can be seen in the aforementioned increase in muscle mass and in limb circumferences as well as in an improvement in physical performance. An improvement in VO2max and an increase in BDNF level was also observed by Ericson et al. (31) in elderly people with an average age of 66 or Laske et al. (32) in elderly diabetic patients.
In our CrossFit training program we included different types of exercise, both resistance and power-shaping speed exercises, besides typical endurance ones. Such a variety of exercise stimuli activating different physiological mechanisms could be an underlying factor for an increase in resting secretion of BDNF after the training. Exercises used in CrossFit training engage the whole body and all joints, contrary to exercises done at a gym that isolate certain groups of muscles.
Our observations suggest that the type of exercise and of the dominant changes that occur due to it can be a decisive factor as far as the dynamics of post-exercise changes in BDNF is concerned. The period of time in which a study is carried out also seems to play a part. After the progressive test aimed at assessing the subjects’ aerobic capacity, before the training, we noted a significant increase in BDNF level, while after the Wingate test and an intensive anaerobic exercise, we did not notice any changes in BDNF. However, after the CrossFit training we noticed higher values of BDNF at rest in comparison with the changes observed after the Wingate and progressive tests.
In the literature more attention is definitely paid to the influence of aerobic exercise on BDNF secretion in comparison with anaerobic exercise. Some authors (33) observed, just as we did, an increase in BDNF level after a single aerobic exercise as well as after regular aerobic training (34). Others did not observe any changes in BDNF levels (35). There is also little mention of strength and power anaerobic exercise. In our training program resistance, strength and power exercises were performed alternately with conditioning exercises, therefore, it is difficult to unequivocally state which exercises stimulated BDNF. It seems that these were exercise stimulating both aerobic and anaerobic capacity, even though we did not manage to register any significant changes in BDNF after the Wingate test. We think that our assumptions can be correct because Coelho et al. (36) noted an increase in BDNF level after resistance training in elderly women who exercised for 10 weeks. Similarly, an increase in resting BDNF levels after resistance training in young, physically inactive men was noticed by Yarrow et al. (37). However, the results provided by Rojas Vega et al. (38) are in opposition to the aforementioned studies. Goekint et al. (35), after doing their research in a group of young volunteers, also came to the conclusion that strength training does not influence BDNF level after 10 weeks of training. While analyzing the influence of resistance exercise on the level of growth factors in people, Rojas Vega et al. (39) noticed an increase in IGF-1 in the bloodstream without any significant changes in BDNF. In our research we did not determine the level of IGF-1, which is one of the factors stimulating an increase in muscle mass. Zwetsloot et al. (40) observed a significant increase in the level of cytokines, which are an important factor in muscle adaptation and hypertrophy. Ruas et al. (41) write that PGC-1a participates in the regulation of muscle hypertrophy. It is conceivable that the aforementioned mechanisms are the underlying reason for an increase in LBM and muscle circumferences that we noticed in our study.
While comparing our results with the results provided by other authors, we could conclude that the initial level of physiological adaptation can play a vital part in the assessment of changes in BDNF after a single exercise. In our study a significant increase in BDNF after a single exercise (Wingate and progressive tests) was noticed only before the training. After the training we noted a significant increase in resting BDNF level both in men and women. After the training we also observed a much higher level of BDNF in men than in women. Despite an improvement in VO2max and beneficial changes in body composition and BDNF at rest, BDNF level did not increase after either the Wingate or progressive test, even though the resting values of the protein were higher in comparison with the values before the CrossFit training. Thus, it can be assumed that the lower level of physiological adaptation is, the more significant changes there are in BDNF level after a single exercise. On the other hand, in young people, with a high level of physiological adaptation and big muscle mass (that is what our subjects are considered to be), a single exercise does not induce BDNF secretion. Interestingly enough, after the CrossFit training we could notice higher values of BDNF in men than in women. It might be connected with the higher content of LBM in men’s body composition, but also with its increased value after the training in this group.
Mechanisms underlying an increase in resting BDNF level in the bloodstream can be different. Hypoxia and the production of lactate in response to physical exercise can be one of them. Schiffer et al. (42) noticed a considerable increase in BDNF level while administering sodium lactate to their students. The authors claim that lactate participates in the regulation of BDNF level in the bloodstream. If it is so, why did BDNF level not change in our study after a very intense exercise, especially the Wingate test, after which the level of lactate often exceeded 9.50 mmol/l.
Oxidative stress could be another factor stimulating BDNF expression. Sakr et al. (43) observed a significant increase in the levels of cortical malondialdehyde, a decrease in antioxidant activity (reduced glutatione, superoxide dismutase and catalase) and a significant increase in BDNF expression in rats subjected to chronic effort under hypoxic conditions.
Chen and Russo-Neustadt (44) in their experimental research prove that norepinephrine and serotonin are responsible for inducing embryonic hippocampal neurons to eliciting BDNF expression. Serotonin elicits an earlier but brief expression while norepinephrine elicits a more delayed and sustained release of BDNF in comparison with serotonin. What is more, from the research by Counts and Musfan (45) it can be concluded that norepinephrine induces phosphorylation of cAMP- response element binding protein (CREB) through β1 and β2 receptors and stimulates expression of BDNF and/or of nerve growth factor (NGF). Chen and Russo-Neustadt (46) also claim that norepinephrine induces BDNF and activates phosphatidylinositol-3 kinase (PI-3K)/Akt and mitogen-activated protein kinase (MAPK) and NO/cGMP pathways, in embryonic hippocampal neurons. Both neurotrophins activate the Trk receptor and participate in neuron plasticity and promote growth, development and survival of neurons. Similarly, Leal et al. (47) claim that BDNF participating in eliciting and sustaining LTP acts through the tropomyosin-related kinase B (TrkB) receptor, which activates such pathways as Ras/ERK, PI-3K/Akt and phospholipase C-γ (PLC-γ).
Another mechanism that induces an increase in BDNF may be connected with expression of FNDC5 and secretion of irisin - the effort hormone. The role of the hormone is to improve glucose oxidation and induce the conversion of white to brown adipocytes. Irisin elicits expression of the UPC-1 protein which is indispensable in the oxidation of energy components, brown adipocytes, whereas expression of FNDC5 is regulated by PGC-1a, whose level increases during physical exercise in response to hypoxia (40). Expression of PGC-1a is induced by AMP kinase (AMPK) activated by a decrease in ATP level (iATP/AMP) in the cell. Like PGC-1-α, AMPK participates in the intensification of oxidation processes by, among other things, initiating mitochondrial biogenesis through stimulation of UPC-1 expression (6, 7, 48, 49). AMPK expression is also stimulated by BDNF (6).
We find that before the training the values of irisin were much higher in women than in men. After the training no such differentiation was noticed. However, after the training we observed a significant reduction in resting level of the hormone in women, but not in men. Norheim et al. (50) also noted a reduction in irisin level after 12 weeks of the training. After the Wingate and progressive tests we did not notice any changes in irisin level either at baseline or after the training. CrossFit higher level of irisin in women before the training might have been a result of higher % FAT, which was reduced after the training. Roca-Rivada et al. (11) claimed that 28% of irisin circulating in the bloodstream may come from adipose tissue, and 72% comes from the muscles. We observed a correlation between irisin, BMI and % FAT at rest at baseline in all participants. We did not noticed any correlation of irisin with LBM, even though after the training LBM increased both in men and women. We know that irisin level is higher in obese patients in comparison with patients with the normal body mass and patients with anorexia (51). What is more, in that research irisin correlates, like in our studies, with body fat mass, BMI, and also with insulin levels. Although Huh et al. (52) and other authors noted a positive correlation between irisin and free-fat mass, in our research we observed an increase in LBM in both studied groups but we did not notice any correlation with irisin level. Similarly, Hecksteden et al. (53) did not observe an increase in irisin level after 26 weeks either in a group performing aerobic endurance training or in a group performing strength endurance training, despite an increase in maximum performance in both groups. Lack of changes in irisin level was also reported by Hofmann et al. (54) in patients with anorexia nervosa, Norheim et al. (50) after 12 week of training or Pekkala et al. (55).
Timmons et al. (56) claim that irisin is not always exercise-induced and that physically active and older, not younger, people show a 30% higher expression of FNDC5 than people having a sedentary lifestyle. Kurdiova et al. (57) did not notice that intense exercise or regular 3-month training in overweight and obese subjects lead to an increase in FNDC5 expression in skeletal muscles and irisin level in the bloodstream, despite a 6-fold increase in Pgc-1α expression. It has been noticed that an increase in irisin levels in muscles is limited and depends on the type of muscle fibres and the training status. Czarkowska-Paczek et al. (58) noticed an increase in irisin levels in red fibres 3 hours after an acute effort in relation to its levels in samples taken directly after the effort, and a decrease in irisin levels in white fibres 3 hours after an effort in comparison to its levels in these fibres before the effort.
However, Huh et al. (52) claim that irisin level correlates inversely with age. In our research, after acute (intense) exercise (Wingate and progressive tests) we observed no changes in men in both studied periods. In women a reduction in irisin level in the blood was noted in relation to the pre-exercise values.
The results we obtained could be caused by e.g. the period of time that had elapsed between the completion of the exercise and the collection of the blood. In our research blood was collected 15 minutes after the completion of both tests. In the research by Daskalopoulou et al. (59) irisin level increased after exercise of different intensity but only just after exercise (3 minutes after exercise) and then, it decreased and remained at the level comparable with the pre-exercise values up to 24 hours. Norheim et al. (50) also claim that irisin level is higher just after exercise.
The post-exercise decrease in resting irisin level in women and no changes in men can be a result of a reduction in fat mass observed in women. We did not observe any reduction in adipose tissue in men. It may be the reason for different observations. It also seems that the time of year is not unimportant in research on irisin. It has been shown that there is a yearly rhythm of the secretion of the hormone, which is at its peak in the winter (January-February) and in the summer (July-August) Kerstholt et al. (60). Our study was commenced in January and finished in April, when according to the authors, lower levels of irisin are noted.
Another reason why there are no changes in irisin level after intense exercise and after a 3-month training program might be the factors suppressing the expression of FNDC5 and inhibiting the cleavage of irisin and its secretion into the blood. SMAD3 seems to be such a factor because in experimental research it suppresses FNDC5 and PGC-1a in cultured skeletal muscle cells. It seems to negatively regulate irisin production and/or its secretion from skeletal muscle (61).
In our research, irisin level was a little higher than in research done by other authors, e.g. by Daskalopoulou et al. (59). Similar values, or even higher ones were obtained by Kerstholt et al. (60) and Pekkala et al. (55). Differences in the values of the obtained results may be the result of the authors using different tests available on the market and the fact that the samples are stored for a very long time (53).
Because of the fact that our studied group was rather small it is difficult for us to come to any decisive conclusion; however, the differences in irisin and BDNF levels between men and women suggest that there is sexual dimorphism as far as the secretion of both hormones is concerned. The observations, however, need further research which could explain the observed whys and wherefores. Nowadays, it seems that anthropometric factors - muscle mass and fat content - play the most important role.
Due to the discrepancy between the results of the very few investigations there have been so far, further research should be done to answer the following questions: what kind of training is the most appropriate stimulus to induce secretions of BDNF and irisin in people of different ages, health and physical performance levels. From the previous studies, it can be concluded that BDNF secretion in response to the contraction of skeletal muscles and its ability to stimulate neurogenesis can be convincing evidence that regular physical exercise improves physiological processes that occur in the nervous and muscular systems. It is an important issue in the context of treatment through physical effort of an aging and obese population and patients with diabetes mellitus type 2 (9, 10).
Conflict of interests: None declared.
REFERENCES
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus 2009; 19: 973-980.
- Scheele C, Nielsen S, Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metabol 2009; 20: 95-99.
- Pedersen BK. The diseasome of physical inactivity - and the role of myokines in muscle - fat cross talk. J Physiol 2009; 587: 5559-5568.
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, denditic complexicity and spine density. J Comp Neurol 2005; 486: 39-47.
- Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. Biochem Mol Biol Rep 2009; 42: 239-244.
- Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 2013; 18: 649-659.
- Bostrom P, Wu J, Jedrychowski MP, et al. PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463-468.
- Lefenetre P, Leske O, Wahle P, Heumann R. The beneficial effects of physical activity on impaired adult neurogenesis and cognitive performance. Front Neurosci 2011; 5: 51. doi: 10.3389/fnins.2011.00051
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379-1406.
- Lancaster GI, Febbraio MA. Skeletal muscle: not simply an organ for locomotion and energy storage. J Physiol 2009; 587: 509-510.
- Roca-Rivada A, Castelao C, Senin LL, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 2013; 8: e60563. doi: 10.1371/journal.pone.0060563.
- Kozub FM. Using the snatch and CrossFit principles to facilitate fitness. JOPERD 2013; 84: 13-16.
- Glassman G. Seminars training guide. CrossFit level 1 training guide. CrossFit J 2010; May 15; http://journal.crossfit.com/2010/05/crossfit-level-1-training-guide.tpl
- Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 2006; 575: 901-911.
- Gibala MJ, McCee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev 2008; 36: 58-63.
- Herrera L, Kravitz L. Yes! You do burn fat during resistance exercise. J Appl Physiol 2007; 102: 1767-1772.
- Laursen PB. Training for intense exercise performance: high-intensity or high-volume training? Scand J Med Sci Sports 2010; 20 (Suppl. 2): 1-10.
- Smith MM, Sommer AJ, Starkoff BE, Devor ST. Crossfit-based high-intensity power training improves maximal aerobic fitness and body composition. J Strength Condit Res 2013; 27: 3159-3172.
- Quark b2 User manual, XVIII Edition 05/2008. Cosmed Srl, Italy, Part N. C00827-02-91. http://internetmed.com /sites/default/files/Cosmed_Quark_b2_Spirometer_-_Service_manual.pdf
- Moon JR, Hull HR, Tobkin SE, et al. Percent body fat estimations in college women using field and laboratory methods: a three-compartment model approach. J Int Soc Sports Nutr 2007; 4: 16. doi:10.1186/1550-2783-4-16
- Moon JR, Tobkin SE, Smith AE, et al. Percent body fat estimations in college men using field and laboratory methods: a three-compartment model approach. Dyn Med 2008, 7: 7. doi:10.1186/1476-5918-7-7.
- Hicks VL, Stolarczyk LM, Heyward VH, Baumgartner RN. Validation of near-infrared interactance and skinfold methods for estimating body composition of American Indian women. Med Sci Sports Exerc 2000; 32: 531-539.
- Bayati M, Farzad B, Gharakhanlou R, Agha-Alinejad H. A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble ‘all-out’ sprint interval training. J Sports Sci Med 2011; 10: 571-576.
- Gillen JG, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab 2014; 39: 409-412.
- Hottenrott K, Ludyga S, Schulze S. Effects of high intensity training and continuous endurance training on aerobic capacity and body composition in recreationally active runners. J Sports Sci Med 2012; 11: 483-488.
- Corte de Araujo AC, Roschel H, Picanco A, et al. Similar health benefits of endurance and high-intensity interval training in obese children. PLoS One 2012; 7: e42747. doi:10.1371/journal.pone.0042747
- Lucia A, Hoyos J, Pardo J, Chicharro JL. Metabolic and neuromuscular adaptations to endurance training in professional cyclist: a longitudinal study. Jpn J Physiol, 2000; 50: 381-388.
- Majerczak J, Nieckarz Z, Karasinski J, Zoladz JA. Myosin heavy chain composition in the vastus lateralis muscle in relation to oxygen uptake and heart rate during cycling in humans. J Physiol Pharmacol 2014; 65: 217-227.
- Paoli A, Marcolin G, Zonin F, Neri M, Sivieri A, Pacelli QF. Exercising fasting or fed to enhance fat loss? Influence of food intake on respiratory ratio and excess post exercise oxygen consumption after a bout of endurance training. Int J Sport Nutr Exerc Metab 2011; 21: 48-54.
- Tang K, Xia FC, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol 2010; 170: 16-22.
- Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci USA 2011; 108: 3017-3022.
- Laske C, Banschbach S, Stransky E, et al. Exercise-induced normalization of decreased BDNF serum level in elderly women with remitted major depression. Int J Neuropsychopharmacol 2010; 13: 595-602.
- Zoladz, JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008; 59 (Suppl. 7): 119-132.
- Ruscheweyh R, Willemer C, Kruger K, et al. Physical activity and memory functions: an inteventional study. Neurobiol Aging 2011; 32: 1304-1319.
- Goekint M, De Pauw K, Roeland B, et al. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol 2010; 110: 285-293.
- Coelho FM, Pereira DS, Lustosa LP, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr 2012; 54: 415-420.
- Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett 2010; 479: 161-165.
- Rojas Vega, S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res 2006; 1121: 59-65.
- Rojas Vega S, Knicker A, Hollmann W, Bloch W, Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm Metab Res 2010; 42: 982-986.
- Zwetsloot KA, John CS, Lawrence MM, Battista RA, Shanely RA. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J Inflamm Res 2014; 9: 9-17.
- Ruas JL, White JP, Rao RR, et al. A PGC-1a isoform induced by resistance training regulates skeletal muscle hypertrophy Cell 2012; 151: 1319-1331.
- Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Struder HK. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett 2011; 488: 234-237.
- Sakr HF, Abbas AM, El Samanoudy A. Effect of vitamin E on cerebral cortical oxidative stress and brain-derived neurotrophic factor gene expression induced by hypoxia and exercise in rats. J Physiol Pharmacol 2015; 66: 191-202.
- Chen MJ, Russo-Neustadt AA. Nitric oxide signaling participates in norepinephrine-induced activity of neuronal intracellular survival pathways. Life Sci 2007; 81: 280-290.
- Counts S, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem 2010; 113: 649-660.
- Chen M, Russo-Neustadt A. Kinetics of norepinephrine- and serotonin-induced BDNF release in cultured embryonic hippocampal neurons. Neurosci Med 2013; 4: 194-207.
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014; 76: 639-656.
- Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 2006; 30: 145-151.
- Canto C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20: 98-105.
- Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 2013; 281: 739-749.
- Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity - correlation with body mass index. Peptides 2013; 39: 125-130.
- Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61: 1725-1738.
- Hecksteden A, Wegmann M, Steffen A, et al. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med 2013; 11: 235. doi: 10.1186/1741-7015-11-235
- Hofmann T, Elbelt U, Ahnis A, Kobelt P, Rose M, Stengel A. Irisin levels are not affected by physical activity in patients with anorexia nervosa. Front Endocrinol (Lausanne) 2014; 4: 202. doi: 10.3389/fendo.2013.00202
- Pekkala S, Wiklund PK, Hulmi JJ, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol 2013; 591: 5393-5400.
- Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature 2012; 488: E9-E10.
- Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol 2014; 592: 1091-1107.
- Czarkowska-Paczek B, Zendzian-Piotrowska M, Gala K, Sobol M, Paczek L. One session of exercise or endurance training does not influence serum levels of irisin in rats. J Physiol Pharmacol 2014; 65: 449-454.
- Daskalopoulou SS, Cooke AB, Gomez YH, et al. Plasma irisin levels progressively increased in response to increasing exercise workload in young, healthy, active subjects. Eur J Endocrinol 2014; 171, 343-352.
- Kerstholt N, Ewert R, Nauck M, et al. Association of circulating irisin and cardiopulmonary exercise capacity in healthy volunteers: results of the Study of Health in Pomerania. BMC Pulm Med 2015; 15: 41. doi: 10.1186/s12890-015-0035-x
- Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise J Biol Chem 2015; 290: 7671-7684.
A c c e p t e d : October 13, 2015
Dr. Joanna Wojna, University School of Physical Education, Department of Physiology and Biochemistry, 35 I.J. Paderewskiego Ave., 51-612 Wroclaw, Poland. e-mail: wojna.joanna@gmail.com