PATHOPHYSIOLOGY AND CLINICAL CHARACTERISTICS
OF PAIN
IN MOST COMMON LOCATIONS IN CANCER PATIENTS
INTRODUCTION
An effective treatment of pain is a very important part of management of cancer patients. It is very important from several reasons. From a point of view of pathophysiology pain may induce several disturbances of the function of a respiratory, circulatory and nervous system. Pain limits patients’ activities with a negative effect on physical and psychological functioning, continuously reminds about the disease, may lead to a development of a depression and deepen hopelessness. It should be kept in mind that unrelieved pain induces suffering of not only patients but also families and careers. Therefore, an obligation of pain treatment that leads to an improvement of patients’ and carers’ quality of life (QOL) concerns each physician (1).
EPIDEMIOLOGY OF PAIN IN CANCER PATIENTS
Pain is one of the most common and feared symptoms in patients with cancer. In 2012 worldwide 14 million new cases of cancer were diagnosed with annual death of over 8 million. Over half of new cases and two thirds of deaths took place in low- and middle-income countries (2). It is well known that pain frequency increases with a progression of the disease and that type of pain and its intensity depend on a location of the tumour and an extent of the disease. According to Foley pain is present in 30 – 50% patients during anticancer therapy and in 70 – 90% of patients with an advanced disease (3). Twycross found pain in approximately 75% patients with advanced disease with 80% of those with pain having at least two or more types of pain and over 30% four or more types of pain in the course of cancer (4).
Newer epidemiologic data suggest that the frequency of pain during oncology treatment equals approximately 50% and in advanced cancer increases to 65%. An obvious conclusion is that the interest of a physician in a diagnosis, treatment and monitoring of pain therapy in cancer patients should start with a diagnosis of cancer. Epidemiological data indicate that after oncology treatment completion approximately 33% patients suffer from pain that needs to be treated and that is why the therapy should be monitored in cancer patients on all stages of the disease (5).
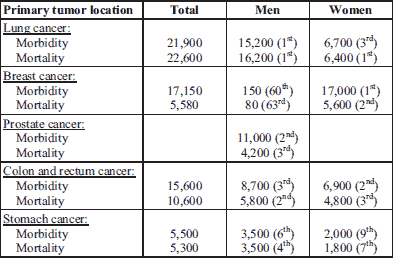
Data gathered in 2012 in Poland (Table 1) indicate that nearly 22,000 patients were diagnosed and over 22,600 died from lung cancer. In women, the most common tumour diagnosed was breast cancer (17,000 patients) and it was cause of death in 5,600 patients. Among men prostate cancer was diagnosed in nearly 11,000 patients and it was a cause of death in 4,100 patients. In gastrointestinal (GI) tract the most frequent tumor was colon and rectum cancer: annual morbidity equalled nearly 15,600 patients and mortality for both tumor taken together was 10,600 patients. Stomach cancer was found in nearly 5,500 patients, while 5,300 patients died this year. In 2012 in Poland approximately 150,000 (nearly equally 76,500 women and 76,500 men) and 95,000 (42,000 women and nearly 53,000 men) patients were diagnosed and died from cancer, respectively. The 5-year morbidity (the number of those living in whom cancer was diagnosed during the former 5 years) equalled 364,000 patients (6). Thus, it may be assumed that in Poland annually at least 300,000 cancer patients require pain treatment.
PATHOPHYSIOLOGY OF PAIN IN CANCER PATIENTS
The mechanism of cancer pain is a complex pathological process that comprises cellular, tissue, and systemic changes that occur during the proliferation, invasion, and metastasis of cancer. It is a result of interactions between cancer cells, the peripheral and central nervous systems, and the immune system (7).
- The presence of tumor or the appearance and/or growth of a metastasis
- Anticancer therapy (diagnostic procedures, surgical interventions, radiotherapy, chemotherapy, immunotherapy, hormone and molecular therapy)
- Mechanisms indirectly related to cancer and its treatment (infections, metabolic imbalance, myofascial pain)
- Mechanisms unrelated to the cancer itself or its treatment (migraine, painful diabetic neuropathy, low-back pain) (8).
Pain in the cancer patient is usually due to several causes:
- nociceptive (somatic or visceral),
- neuropathic,
- mixed.
In terms of pathophysiological criteria, pain in the cancer patient may be divided into:
Nociceptive pain arises as a result of irritation of, or a decreased irritability threshold in nociceptors located in superficial structures: skin, subcutaneous tissues, muscles, and the skeletal-articular system (somatic pain) or in organs located within body cavities, such as the thorax, abdomen, and pelvis (visceral pain). This kind of pain is usually due to the infiltration of tissue by tumor or metastasis or due to tissue injury as a result of anticancer treatment.
On the other hand, tumor growth or treatment may lead to lesions in the structures of the central or peripheral nervous system, which causes neuropathic pain. This type of pain is often poorly tolerated and difficult to control. It needs to be stressed, however, that pain in cancer patients is usually of a mixed origin and rarely manifests itself as a purely (8). In most cases, it is a complex phenomenon that is a consequence of various, concurrently occurring factors, including inflammatory, neuropathic, and ischemic elements, that tend to be located in several places simultaneously (9). The identification of these factors is extremely important due to their therapeutic implications, and, as a consequence, the possibilities of implementing effective pain therapy. The main mechanisms responsible for pain results directly from tumor involvement in cancer patients are shown in Fig. 1.
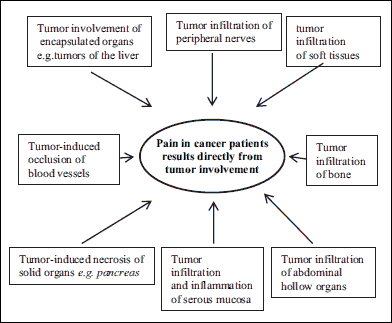 |
Fig. 1. Mechanisms of cancer pain as a direct consequence of tumor. |
SOMATIC PAIN IN CANCER PATIENTS
This kind of pain may be caused by neoplastic invasion of the skin, connective tissue, bone, joint or muscle. It may be subdivided into superficial pain (i.e. cutaneous ulceration by malignant involvement) and deep pain (i.e. bone marrow infiltration by malignant cells). Most often cutaneous pain is well localized, sharp, or pricking. Deep tissue pain usually appears diffuse and is dull or aching in quality (8). The growing tumor triggers local production and release of histamine, bradykinin, cytokines, prostanoids, potassium ions, ATP and other mediators that activate nociceptors - ‘pain receptors’ - located on the primary endings of afferent sensory neurons.
The key function of nociceptors is to detect the mechanical, thermal and chemical stimuli and convert them into electrochemical signals transmitted to the central nervous system (CNS) via sensory fibres (unmyelinated C fibres and myelinated As fibres) (10-12). Moreover, tissue damage apart from the orthodromic transmission of nociceptive stimuli to the central nervous system (CNS) induces the release other mediators such as substance P (SP) from primary afferent of A σ and C fibres along the antidromic pathway. This leads to the dilation of the vascular bed and increased capillary permeability which results in tissue edema and the release of bradykinin (BK) and serotonin (5-HT) from platelets, histamine from mast cells, other tissue mediators such as prostanoids (PG), the nerve growth factor (NGF), and cytokines (TNF-α). These mediators also increase vascular permeability and SP release (13).
Prostaglandins play an important role in the modulation and perception of nociceptive stimuli, since their release at the site of tissue damage and inflammation lowers the excitation threshold for the activation of sensory fibres in response to nociceptive stimulation (8). NGF, which acts via tyrosine kinase (TrkA) receptors and p74 receptors located on the neuronal membrane, ensures local growth and survival of afferent sensory fibres. However, under pathological conditions this factor can also facilitate cancer invasion and trigger the development of thermal or mechanical hyperalgesia. Moreover, continuous exposure to NGF action increases the expression of acid-acid-sensing ion channels (ASIC) and acid-sensitive receptors: vanilloid receptors (TRPV1) receptors in afferent sensory fibres, both of which contribute to the development of pain in the cancer patient (14). Nerve growth factor (NGF) released by cancer and associated stromal cells also appears to drive sprouting primary afferent nerve fibers in the tumor-bearing bone and this sprouting is blocked by the administration of anti-NGF (15, 16). Recently, in an experimental study conducted in rats it was demonstrated that the mechanism of pain behavior and the cutaneous blood flow are caused by the activation of the spinal transient receptor potential ankyrin (TRPA1) channel promoting the mechanical pain hypersensitivity (17).
The presence of a tumor is accompanied also by cytokine production with the tumor necrosis factor-α (TNF-α) that stimulates the immune cells to produce pronociceptive mediators (16). Furthermore, the response of inflammatory cells that accompanies neoplastic infiltration of tissue may cause the release of extracellular protons (H+) that generate local acidosis. This leads to the activation of acid-sensitive receptors: vanilloid receptors TRPV1 and acid-sensing ion channels ASIC-3, which are expressed by nociceptors and also induce pain (18).
One of the critical factors in inducing pain in the cancer patient is proteolytic activity. Proteases (e.g., trypsin) activate the receptors (protease-activated receptors, PARs) expressed on nociceptors, on the primary afferent fibres within the cancer environment. This, in turn, is caused by the fact that many proteases that activate PARs, either directly or via their peptide products, are synthesized during tissue damage due to the tissue’s infiltration by malignant cells (19, 20).
As a result of the above mechanisms, neurogenic inflammation develops in damaged tissues. This leads to peripheral sensitization. It is caused and exacerbated by a direct activation of nociceptors and ‘extravasation’ of algesiogenic and sensitizing factors. This sensitization of nociceptors is responsible for the occurrence of primary hyperalgesia, involves increased sensitivity to noxious stimulation at the injury site, and is mediated by peripheral mechanisms.
Nociceptive information, encoded as electrochemical signals, reaches the dorsal root ganglion (DRG) and causes the release of, among others, excitatory amino acids (EAA), substance P (SP), neurokinin A (NKA), and likely other peptides, which are transported from the DRG to the dorsal horn of the spinal cord. Together with other mediators, they act as neurotransmitters or modulators of sensory information (12, 20).
The induction of these mediators and activation of receptors, by acting on adjacent cells in the structures of the spinal cord, cause the spread of the activation process and change the properties of adjacent neurons. Positive feedback occurs between microglia and nerve cells, which brings about changes that manifest themselves clinically as hyperalgesia and allodynia. The above-described process of central sensitization is a likely cause of secondary hyperalgesia that extends beyond the injury site, referred pain, and the so-called pain memory associated with the hyperexcitability of nociceptive system and WDR (Wide Dynamic Range) cells (21).
From the dorsal horn of the spinal cord, nociceptive information is transmitted to the higher levels of the CNS, with the final stage of the nociceptive process being the perception that occurs in the brain, which plays a cognitive role and is responsible for the awareness of pain stimulation, its assessment, and affective and emotional response to it. This is where anxiety, aggression, and anger occur and where behavior models related to the remembered pain are shaped (8).
CANCER BONE PAIN
The most common type of pain caused by cancer is bone pain. Skeleton is the third most common metastatic site (after the lung and the liver) (23). Metastases may invade bones in 30 – 69% of cancer patients, especially in patients with advanced breast, lung, and prostate cancers (24).
The majority of patients with metastatic bone disease experience moderate to severe pain (25, 26). Tumor growth in bones results in pain, and it may also be the consequence of skeletal fractures or compression of the spinal cord. Pathological fractures are the most frequent in patients with myeloma and breast cancer and affect primarily vertebral bodies, ribs, and long bones (27). The most serious complications of vertebral metastases include spinal cord compression caused by vertebral body collapse or spinal cord injury secondary to vascular supply compromised. The collapse of vertebral bodies as a consequence of metastases is frequent in the thoracic spine and may cause compression of thoracic nerve roots and radicular pain localized to the chest or the upper abdomen (28). The main causes responsible for bone pain in cancer patients are shown in Fig. 2.
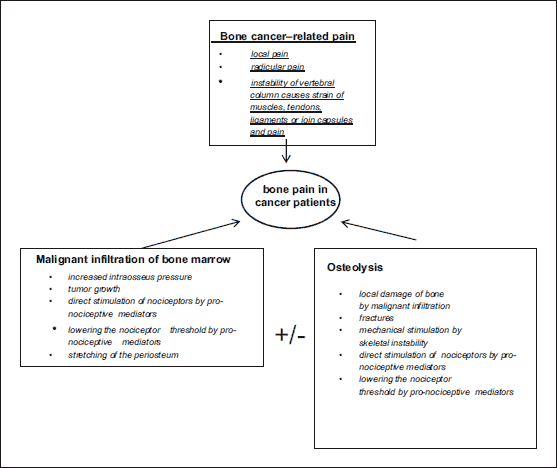 |
Fig. 2. Causes of bone pain in cancer patients. |
Studies on animals and humans have suggested that osteoclasts play a significant role in the mechanism of bone cancer pain development. Osteoclasts are activated both by tumor products and by osteoclast-stimulating substances (RANKL - receptor activator for nuclear factor κB ligand) released by osteoblasts and immune cells (i.e. lymphocytes T) that are activated by mediators, which are themselves produced by the tumor (14). Osteoclasts degrade bone minerals by secreting protons through the vacuolar H+ ATPase and creating acidic microenvironments. Extracellular protons are known to be potent activators of nociceptors; hence osteoclasts cause pain through proton secretion (14, 29).
Two acid-sensing ion channels - the acid-sensitive vanilloid receptor TRPV1 and acid-sensing ion channels 3 (ASIC-3) - are expressed on primary afferents of the sensory neurons that innervate the marrow, mineralized bone, and periosteum. It is believed that osteoclasts play a significant part in the induction of bone pain in the cancer patient through the activation of TRPV1 and ASIC-3 (26), because both of these receptors are excited by a decrease in pH in the range of 4.0 – 5.0. This is confirmed by the findings of experimental studies, in which the administration of TRPV1 receptor antagonists was found to attenuate nociception in bone pain in mice, in the cancer patient model (30). Furthermore, immune and inflammatory cells also stimulate tumor cells and tumor stromal cells to release protons that generate local acidosis (29).
With disease progression, tumor destroys distal ends of the sensory nerve fibres that innervate the bone (26). But there is not the only mechanism by which neuropathic skeletal pain may be generated. Another one is an active and pathological sprouting and neuroma formation by sensory and sympathetic nerve fibers that innervate the bone (15, 31). This sprouting is remarkable and these newly sprouted nerve fibres which are observed in the periosteum, mineralized bone and marrow, have a unique morphology, organization and high density that is never observed in normal bone. Results of many studies suggest that release nerve growth factor (NGF) from tumor cells, and also from immune and inflammatory cells induces this marked and pathological remodeling of sensory nerve fibres that may contribute to cancer neuropathic pain. Therapies that target NGF or its cognate receptor TrkA may be efficacious at impeding this pathological nerve sprouting and attenuating cancer bone pain (15).
VISCERAL PAIN IN CANCER PATIENTS
Visceral pain is caused by pathological processes occurring within the internal organs in the chest, the abdominal cavity, and the pelvis. Pain may result from the distention, impaction, ischemia, inflammation, or traction on the mesentery. In comparison with somatic pain, visceral pain is poorly localized due to both fewer receptors participating in the process of visceral pain and ‘scarce representation’ within the primary somatosensory cortex (32). The diffuse nature of visceral pain and its referral to superficial structures are caused by the convergence of visceral and somatic afferents on the same neurons in the dorsal horn of the spinal cord. These observations also explain why visceral pain is difficult to localize and is often referred to other areas of the body (33).
Viscera are innervated by two distinct classes of nociceptive sensory receptors. High-threshold receptors are activated by stimuli within the noxious range and contribute to the peripheral encoding of noxious events in the viscera. Nevertheless, the low-threshold receptors are activated by a range of stimulation intensity from innocuous to noxious.
Visceral afferents are also called polymodal since they generate excitatory responses to inflammation, ischemia, stretching, and distention, in other words excitatory responses to different kinds of stimulation to somatic pain. Inflammation or hypoxia that may occur within the organs affected by the pathology induces the sensitization and activation of receptors that under normal conditions are not stimulated by innocuous stimuli (e.g., distention) (32).
Obstruction due to neoplastic infiltration or inflammation within the biliary tract or pancreatic duct induced an increase in intralaminar pressure which causes both pain and a release of pronociceptive mediators, which additionally exacerbates the pain (34). Tumors within certain organs (liver, spleen, kidneys) cause pain due to the stretching of the organ capsule especially in the case of rapidly growing liver tumors, which may lead even to a several folds’ increase in the dimensions of this organ. This results in a significant increase in the intraorgan pressure and the ensuing stimulation of intracapsular mechanoreceptors. Likewise, distention or traction on the gallbladder leads to deep epigastric pain. Renal colic is usually secondary to urethral obstruction and the subsequent distention of renal pelvis and the ureter, as is usually the case with tumors located in the lower abdomen (8). The main mechanisms responsible for visceral pain in cancer patients are shown in Fig. 3.
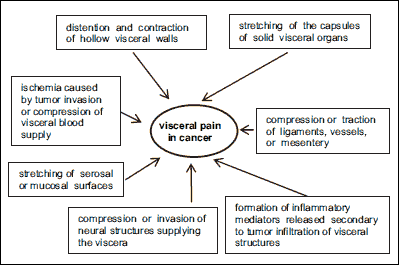 |
Fig. 3. Mechanisms responsible for visceral pain in cancer patients. |
In pancreatic cancer, pain is caused by the obstruction of the pancreatic ducts, infiltration of pancreatic connective tissue, capillaries, and afferent nerves, and the invasion of adjacent organs by intra- and extra pancreatic invasion by cancer cells. This is observed in 70 – 97% of pancreatic cancer patients. It was found that the levels of pronociceptive mediators are markedly increased in pancreatic cancer and have been associated with a higher degree of pain. Moreover, overexpression of vanilloid receptors TRPV1 observed in pancreatic cancer patients significantly correlates with the severity of pain (34). The presence of back pain in pancreatic cancer patients may indicate that it has spread into retroperitoneum and para-aortic nodes as well as penetrated into paravertebral muscles. However, it needs to be underscored that an important pain mechanism in cancer patients may involve peritoneal carcinomatosis. In such cases, pain is caused by peritoneal irritation, mesenteric compression/distention, abdominal wall distention with ascites, and bowel obstruction (8).
NEUROPATHIC PAIN IN CANCER PATIENTS
Neuropathic pain is defined as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system. This type of pain develops if the nervous system is damaged, which, in the case of cancer, may occur due to tumor infiltration of nerve structures as a result of tumor associated or therapy-related toxin activity or surgical damage (8).
The prevalence of cancer patients with neuropathic pain ranges from a conservative estimate of 19% to a liberal one of 39% (if patients with mixed cancer pain were included). The prevalence of pain with a neuropathic mechanism ranged from 18.7 to 21.4% of all recorded pain in cancer patients. Findings of the etiology of neuropathic pain in cancer patients indicate that in 63% of cases it is caused directly by cancer and in 20.3% by cancer treatment, in 3.5% it is associated with cancer, in 10.2% it is unrelated to the cancer, and in 2% its etiology remains unknown (35). The mechanisms involved are presented in Fig. 4.
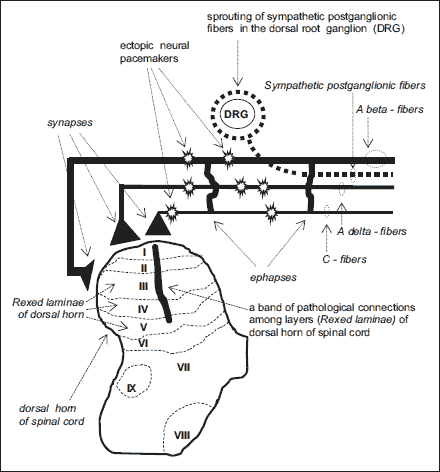 |
Fig. 4. Mechanisms of neuropathic pain. (modified after Wordliczek J, et al. Cancer Pain., Springer 2013, 47-70 (8). With permission). |
Nerve damage leads to a pathological interaction between the somatic and autonomous systems. This interaction is caused by the development of pathological points of contact - ephapses - between Aβ afferent fibres that conduct the sensation of touch and nociceptive fibres A σ and C and efferent sympathetic fibres, both along the nerve and within the neuroma. Additionally, sympathetic fibres sprout and wrap around the DRG in a way that resembles a basket. Thus, mutual excitation may occur directly or indirectly by endogenous catecholamines. The above-mentioned changes in the proximal part of the damaged nerve cause the sympathetically maintained pain component (36).
In recent years, researchers underlined the role of glia cell excitation and an increased release of proinflammatory cytokines in the development of chronic pain syndromes, both following spinal cord damage and peripheral nerve damage. It is postulated that the excitation of glia cells causes referred pain distant from the damage site (37).
Under normal conditions, pain is transmitted by afferent neuron fibres, whose afferent dendrocytes reach Rexed laminae I and II in the dorsal horn of spinal cord. Aβ fibres that transmit the sensation of touch reach layer III. However, in the case of peripheral nerve damage, some neurons in the areas of the spinal cord that are not responsible for pain transmission (i.e., Aβ) also sprout into lamina II of the dorsal horn of the spinal cord, which is the area involved in the transmission of nociceptive afferent inputs. As a result of these processes, a band of nerve fibres grow that combine neurons from layer II to layer V. This is followed by increased neuronal activity, expansion of the neuronal receptive field, and hyperexcitability of other regions. This fact enables us to explain the central mechanism behind allodynia or, in other words, why the irritation of neurons Aβ (that transmit the sensation of touch) causes pain (38).
Central neuropathic pain syndromes are relatively infrequent in the cancer population. Painful cranial neuralgias may occur secondary to head and neck (especially nasopharynx) cancers, skull and leptomeningeal metastases and spinal cord injury (39, 40).
Epidural spinal cord metastasis is a common complication and occurs in 5 – 8% of all cancer patients. It usually accompanies advanced stages of breast, prostate, and lung cancers. In about 69% of cases, compression of spinal cord occurs in the thoracic region, in 20% of cases it involves the lumbar spine, and in 10% of cases it involves the cervical spine. Multiple sites of compression occur in about 20% of patients. Metastatic pathways involve haematogenous or cerebrospinal fluid spread, or direct invasion from paravertebral tumors (41, 42). Pain is the first symptom in 89% of spinal cord compression cases. It results from vertebral metastases, root compression (radicular pain), and/or compression of the long tracts of the spinal cord (funicular pain). Therefore, spinal metastases may be accompanied by local pain usually described as aching or gnawing within the segment invaded by the tumor or back pain that exacerbates as the patient moves or during spinal weight-bearing, especially where vertebral bodies have been damaged by the disease. This damage causes instability of the vertebral column, which results in muscle, tendon, ligament, or joint capsule strain and subsequent pain. Radicular pain occurs when metastases compress a nerve root inducing sharp and shooting pain (42).
Although generally neuropathic pain is difficult to achieve effective analgesia when treated exclusively with opioid analgesics in cancer patients, several newer therapies are tried with promising results. A combination of an opioid with adjuvant analgesic, especially newer class of anticonvulsants (gabapentinoids) is often effective. Recently, a prominent role of pregabalin in combination with opioid decreasing symptoms of neuropathic hyperalgesia has been demonstrated in an experimental animal model (43). Among experimental methods of neuropathic pain management in rats melatonin demonstrated inhibitory effect on mechanical allodynia and a slight inhibitory effect on thermal hyperalgesia. This effect was induced by an activation of type 1 and 2 of melatonin receptors and opioid system involvement in neuropathic pain that was demonstrated in an experimental study conducted in rats. Newer promising methods of neuropathic pain management include glia inhibitors such as minocycline (44).
TUMOR-RELATED MONONEUROPATHY
The most often observed tumor-related painful mononeuropathy is intercostal nerve neuropathy secondary to rib or chest wall metastases. Rib metastases and their pathological fractures are usually observed in the case of breast, prostate, stomach, and colon cancers and multiple myeloma. Their incidence is estimated at 1 – 5%. Main symptom is pain that increases intensity with deep inhalation, body movement, coughing, sneezing, and changing body posture (45).
TUMOR-RELATED PLEXOPATHY
Cervical plexus pathologies usually develop in the case of primary head and neck tumors or metastases to lymph nodes in the neck. Pain in this patient group usually has lancinating or dysesthetic components referred to retroauricular and nuchal areas, the periauricular area, anterior neck, shoulder and supraclavicular nerve and jaw. They may be accompanied by other symptoms such as ipsilateral Horner syndrome and diaphragmatic nerve palsy (45).
Brachial plexopathy usually develops as a result of compression or infiltration of the brachial plexus from tumor originating in the adjacent structures, such as axillary or supraclavicular lymph nodes, or from tumor in the apex of the lung (Pancoast tumor), metastatic spread of tumors to the plexus, or post-radiation injury causing sensory or motor symptoms. In neoplastic brachial plexopathy, pain is the first symptom in 84% of patients and often precedes neurological deficits. Pain involves the arm, shoulder, and axilla. Its intensity increases substantially during shoulder movement. The pain is neuropathic in nature and is accompanied by numbness, paresthesias, dysesthesias, allodynia, and hyperesthesia (46).
Lumbosacral plexopathy is caused by either a direct tumor infiltration from adjacent tissues or lymph nodes, compression from metastases in the bony pelvis, or post-radiation injury (46). It is estimated that lumbosacral plexopathy due to metastases to retroperitoneal lymph nodes is the most often diagnosed neurological complication in patients with advanced cervical cancer. The lumbosacral plexus is usually locally infiltrated or invaded by metastases of pelvic neoplasms. It may develop as a result of a local extension or nodal metastasis from colorectal cancer and other pelvic tumors (prostate, testicle, cervix, uterus, bladder), sarcomas, and lymphomas. It may also occur with metastases from breast and lung cancer or melanoma (8).
In one-third of cancer patients, neoplastic infiltration may occur involving the upper part of the plexus (L1 – L4), and the pain may occur in the back, lower abdomen, iliac crest, or anterolateral thigh. In one-half of cancer patients with infiltration involving the lower plexus (L4 – S3), pain occurs in the buttocks and perineum with referral to the posterolateral leg and thigh; pain is commonly severe burning, cramping, or lancinating (46).
PAIN CAUSED BY ANTICANCER THERAPY
Certain pain syndromes diagnosed in cancer patients are due to treatment modalities including surgery, chemotherapy, and radiation therapy. In most cases, pain is the predominant symptom. Its source is in the damaged structures of the peripheral or central nervous system. Sometimes, symptoms such as pain and concomitant neurological deficits develop with a delay of several weeks or even months, which may cause difficulties in differential diagnosis between complications of therapy and recurrent disease (8). The main causes responsible for pain caused by anticancer therapy are shown in Fig. 5.
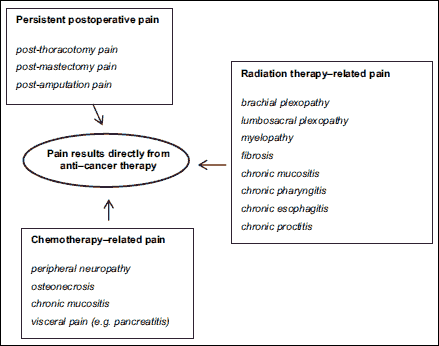 |
Fig. 5. Mechanisms of pain in cancer patients as a direct consequence of anti-cancer treatment. |
PERSISTENT POSTSURGICAL PAIN
Persistent postoperative pain is a chronic, pathological pain that develops as a result of a prior surgical procedure, is related to the disruption of tissue continuity, and persists for over 3 months of the surgery despite tissue healing at the site of the surgery. Most often it is observed in cancer patients following thoracotomy, mastectomy, or amputation (47).
The incidence of persistent postoperative pain after thoracotomy varies widely from 30 to 65% (48). Traction on the ribs and rib resection are the most common causes of intercostal nerve injury during surgical procedures involving the chest. Pain usually occurs in the distribution of one or more intercostals nerves close to the thoracotomy scar. Pain usually gradually diminishes after months or years. It should be underlined, however, that whenever pain intensity increases (especially in the thoracotomy area) or occurs more than 3 months after surgery, it is usually due to the recurrence of the cancer (49).
The incidence of persistent postoperative pain after mastectomy varies widely from 20 to 50% (50). This pain syndrome most frequently occurs in women who, apart from the surgery, were subjected to radiotherapy or chemotherapy, in those after post-mastectomy breast reconstruction surgery and in female patients with postoperative complications, such as wound infection or local fibrosis (51).
Persistent post-mastectomy pain may present as postaxillary dissection pain which typically develops after surgery and is usually due to the damage of a section of the intercostobrachial nerve during axillary lymph node dissection. Brachial plexus damage during surgery may also occur and lead to pain and paresthesias in the upper limb. Persistent post-mastectomy pain may also affect the postoperative scar. This form of pain is characterized by allodynia, which frequently makes it impossible for patients to wear the breast prosthesis. In 7 – 13% of patients, chronic pain after mastectomy is present as phantom pain (52, 53).
Phantom pain disables a significant number of patients undergoing amputation of different body parts for malignancy. It is estimated that phantom breast pain occurs in 7 – 13% of patients (53), and phantom rectal pain in up to 18% of patients after surgery for rectal carcinoma (8). After limb amputation for cancer, the prevalence of phantom limb pain in patients was 46.7%, phantom sensation 90.7%, and surgical stump pain 32.0% (54). The incidence of post-amputation pain is greater in cases in which pain was present in the body part before the amputation (55). Phantom sensation may also be related to visceral organs and may be associated with functional sensations, e.g., the urge to urinate or defecate (8).
POST-RADIATION THERAPY PAIN
Radiotherapy used in cancer treatment may cause damage to CNS structures, which may manifest as focal radionecrosis. They constitute a response to radiation that primarily affects the white matter of the brain or the spinal cord. It produces necrosis and vascular injuries as well as axonal and oligodendrocyte loss with gliosis and demyelination. Edema, mass effect, increased intracranial pressure, pain, and cognitive dysfunction are also observed (56, 57).
Radiotherapy may also lead to the damage of peripheral nervous system structures. Typical lesions include brachial and lumbosacral plexopathies (58, 59). Post-radiation plexopathy occurs more often in patients who, apart from radiotherapy, were subjected to chemotherapy (57). Irradiation of the chest walls and the anatomic structures within the axilla may lead to the development of post-radiation brachial plexopathy (60). It should be underscored that in patients with a plexopathy caused by tumor growth or a metastasis, pain typically occurs earlier and its intensity is greater than in the case with pain following radiotherapy.
Another pain mechanism associated with local radiotherapy or some forms of chemotherapy is mucositis. It results from the damage of the mucosa by radiation or certain medications used in chemotherapy and causes chronic pain syndromes involving the mouth, pharynx, and sometimes the esophagus. Local radiation may also cause colorectal mucositis, colitis, and proctitis (61).
CHEMOTHERAPY-RELATED PAIN SYNDROMES
The most frequently observed consequence of neurotoxicity of medications used in cancer chemotherapy is peripheral neuropathy (CIPN). Chemotherapy induced peripheral neuropathy may be very painful. It is the source of patients’ suffering and also restricts treatment possibilities with potentially useful anticancer drugs (62). The incidence of cancer chemotherapy-induced peripheral neuropathy (CIPN) varies between 3 and 7% in the case of single-agent use and up to 38% when using combined medications (62). It is a common adverse effect of numerous medications used in chemotherapy such as cisplatin, oxaliplatin, vincristine, paclitaxel, and bortezomib (62).
Medications used in cancer chemotherapy have a well-documented direct and indirect neurotoxic action. They affect nerve fibres by altering the amplitude of the action potential and conduction velocity. Medications used in cancer chemotherapy activate the membrane ion channels (sodium, calcium, potassium) or receptors (NMDA) on dorsal root ganglia and dorsal horn neurons to alter the cytosolic ion milieu. A special role in cytotoxic chemotherapy is attributed to the changes in intracellular calcium levels that trigger secondary changes inducing neuropathic pain. Increased intracellular calcium levels, activation of protein kinase C, and the production and release of nitric oxide and free radicals all induce cytotoxicity in axons and neuronal cell bodies (63).
Another group of anti-cancer medications includes hematopoietic growth factors (HGF). They cause neuropathic pain fairly rarely, but may be the source of other types of pain. Thus, patients receiving granulocyte colony-stimulating factors (G-CSF) in the treatment of neutropenia that accompanies chemotherapy may experience bone pain and headaches secondary to the expansion of the hematopoietic matrix and the sensitization of nerve endings in the bone marrow. Bone marrow edema, ischemia, and the stretching of the periosteum due to bone marrow involvement and the activation of sensory and sympathetic nerve fibres that supply the periosteum, mineralized bone, and bone marrow are the traditionally suspected pain mechanisms in this patient group. It is estimated that pain occurs in approximately 20% of patients treated with G-CSF (64).
In turn, the therapy with bisphosphonates may cause severe pain and patient suffering secondary to osteonecrosis of the jaw (65). One of the most painful complications occurring in patients with hematological malignancies under active treatment or in advanced phases of the disease is also oral ulcerative mucositis (66).
ACUTE PAIN ASSOCIATED WITH CANCER MANAGEMENT
It should be underscored that a significant factor which has a negative impact on the quality of life of cancer patients is acute pain caused both by diagnostic and therapeutic procedures such as chemotherapy, radiotherapy, and surgery. The acute pain mechanism in this case is associated with the induction of pathophysiological processes described above, but its nature and generally high intensity leads to very intense pain and patient suffering. For this reason, medical staff involved in cancer management must remember to relieve it as a matter of urgency (8). The mechanisms involved are in Fig. 6.
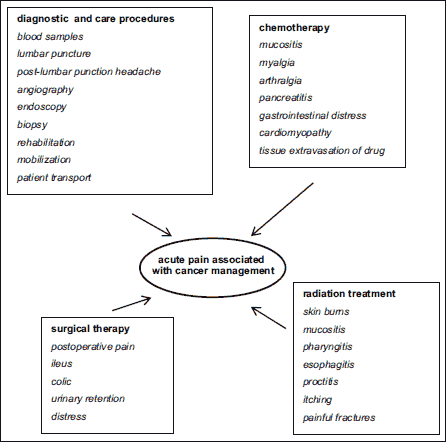 |
Fig. 6. Mechanisms of acute pain as a direct consequence of cancer management. |
PAIN INDIRECTLY RELATED TO CANCER OR ITS TREATMENT
It is estimated that wounds occur in at least one third of cancer patients at the end of their lives (67). Risk factors for tissue breakdown and pressure ulcer development are similar since cancer patients with limited mobility and physical activity are exposed to the highest risk of developing pressure ulcers (68). Pain that accompanies this pathological process is typically exacerbated by the inflammatory process caused by wound infection.
Myofascial pain frequently occurs in cancer patients. It is generally described as aching and cramp-like. It can be difficult to localize and may be referred to other deep somatic structures. Inactivity, which is common in cancer patients in an advanced stage of the disease, predisposes to the development of muscle-related pain (8).
PAIN IN SELECTED TUMOR LOCATIONS
Lung cancer
Lung cancer may induce pain associated with local growth of a tumor in the thorax, most commonly infiltrating pleura and associated severe pain. Tumor invasion of a pleura may lead to pleural effusion, which may induce cough and dyspnea (69). In the advanced stage of cancer, the tumor may infiltrate chest wall, as well as brachial plexus, the latter in the location of a cancer in the lung apex (Pancoast tumor). In both situations, an injury of nerve structures and neuropathic pain component appear, moreover an infiltration of ribs and intercostal nerves may be present that induce severe pain (70).
In an advanced stage of lung cancer distant metastases to other organs may appear, which may also induce pain. Most commonly metastases are located in the lungs, bones, suprarenal glands and brain. It should be kept in mind that patients diagnosed with lung cancer are especially vulnerable group of patients and the suffering of these patients is associated with number of distressing symptoms such as pain, dyspnea, cough, anorexia-cachexia-asthenia syndrome and poor prognosis, which may impact psychosocial and spiritual dimensions of patients’ life (71).
Breast cancer
In advanced, inoperable breast cancer dissemination to other organs, most commonly to bones, and also to visceral organs (most commonly liver, lungs and pleura) and brain may appear, which may accompany pain in these locations. Bone metastases are commonly cause of pain and may lead to hypercalcemia and more severe complications such as bone fractures, spinal cord compression and need for palliative radiotherapy (72). In the case of lung metastases and pleura infiltration pleural effusion may induce apart from pain also dyspnea and cough.
In few patients, a local primary tumor growth may appear, which induces damage to the skin, subcutaneous tissue and chest wall. Pain may be present in approximately 30 – 40% of patients followed mastectomy or other breast surgical procedures (73). This type of neuropathic pain is usually associated with a damage of nerves in the axillar region, which is difficult to treat effectively. Pain in breast cancer patients is a frequent symptom that may significantly decrease patients’ QOL (74).
Prostate cancer
Pain in patients diagnosed with prostate cancer may be induced by a local tumor growth and distant metastases. A local tumor growth refers to prostate cancer that may induce pain associated with an infiltration of surrounding tissues and organs such as urethra, urinary bladder and ureters. A typical location of metastases is spine, especially in the lumbo-sacral region, which requires appropriate pain management (75). In some patients’ pain is associated with dissemination to other organs (lungs, brain). Pain in patients diagnosed with prostate cancer is a frequent phenomenon and significantly decrease patients’ QOL.
Gastric cancer
Advanced, inoperable gastric cancer or local tumor recurrence induce pain associated with a local tumor growth. Along with visceral pain dyspepsia, lack of appetite, nausea, retching and vomiting, sometimes bleeding and in consequence anaemia. In some patients’ gastrointestinal obstruction may develop, especially when a tumor is located in the cardia region, which may unable feeding by a natural route and requires insertion of a stent or feeding gastrostomy. A frequent location of gastric metastases are liver and peritoneum, which may induce visceral pain often with a colicky component. The management of pain, apart from typical analgesics with a significant role of spasmolytics (76).
Colon cancer
Colon tumors for a long time may develop locally and at the beginning without pain. Commonly the first symptom of the tumor is severe pain associated with bowel obstruction. Similarly, in the advanced stage of the tumor visceral pain associated with a local tumor growth appear, which may lead to bowel obstruction development (77). Often, metastases to liver, sometimes to lungs and bones and brain may appear (78).
In patients with bowel obstruction pain may be relieved by a surgical intervention, which restores gastrointestinal passage and alleviates intractable nausea and vomiting (79). In advanced tumors of the rectum infiltration of surrounding nerve structures and sacral bone, which induce very severe pain with neuropathic component (80).
Pancreatic cancer
Usually pain is localized in epigastrium and is radiating to back. The tumor may infiltrate coeliac plexus and there is a neuropathic pain component present. If metastases to retroperitoneal space are present pain may be localized in the lumbar region. Pancreatic pain is often of severe intensity. Apart from analgesics including opioids celiac plexus block often significantly alleviates pancreatic pain (35).
BREAKTHROUGH (EPISODIC) PAIN
Background pain is constantly present (for over 12 h per 24 h) and requires a constant analgesia provision. Breakthrough (episodic) pain is a transient exacerbation of pain, which appears most commonly in patients who have effectively treated background pain with regular administration of opioid analgesics (81). Breakthrough pain episodes most commonly appear in the form of short lasting pain attacks (median time 30 minutes) with a quickly intensifying pain within few minutes and severe intensity (over 5 on numerical rating scale (NRS: 0 - no pain, 10 - the most severe pain) (82).
Breakthrough pain may be divided into spontaneous (idiopathic) and incident pain. The former has no clear cause of pain exacerbation. Incident pain is evoked by a certain type of patients’ activity. Therefore, incident pain may be further divided into voluntary depending on patients’ willingness e.g. moving in the bed, standing up, walking and involuntary, which does not depend on patients’ will e.g. pain induced by cough, pain during bowel movement, pain evoked by bowel or ureters distention (colicky pain) (83). The prevalence of breakthrough pain is quite high among cancer patients. According to Portenoy and Hagen breakthrough pain appears in 63% (84) and according to Zech et al. in approximately 50% of cancer patients (85). The management of breakthrough pain should start with appropriate assessment of the type and pathophysiology of pain.
Treatment comprises rescue doses of opioids that render quick pain relief. Opioids with a rapid onset and short period of analgesia, which matches time characteristics of breakthrough pain episodes are usually recommended for spontaneous and involuntary incident pain. His condition is fulfilled by transmucosal fentanyl formulations (intranasal spray, buccal and sublingual tablets). However, all transmucosal fentanyl formulations are registered for opioid-tolerant patients (at least 7 days of the treatment with oral morphine of daily doses equalled at least 60 mg per day or an equivalent dose of morphine administered by other routes or other opioids). The dose of any fentanyl formulations must be always titrated until effective analgesia and acceptable adverse effects regardless of background opioid dose and former dose of traditional opioid or another fentanyl formulation used for breakthrough pain management. In predictable incident, voluntary pain episodes traditional short-acting oral formulations of opioids may be administered some time before pain appearance. Alternatively, subcutaneous and intravenous route of opioid administration may be utilised for both spontaneous and predictable pain episodes. The dose of traditional opioid should be determined individually, in most patients the dose for control of breakthrough pain episode equals 10 – 20% of the daily dose of opioid administered on regular basis to control background pain.
Repeated pain episodes before the next opioid dose administration (end of dose pain) usually indicate for to low dose of a baseline opioid, thus it is necessary to modify its dosing and are not classifies as breakthrough pain. Pain, which appear between succeeding opioid doses during dose titration, similarly as is the case for end of dose pain, also is not classified as breakthrough pain episode. More controversies exist regarding patients with pain exacerbations and uncontrolled baseline pain as they may be classified as breakthrough pain episodes (86) or just inappropriate management of background pain (87). However, a recent proposal of Experts from European Association for Palliative Care Research Network suggest that episodic pain is much wider concept of pain exacerbations which may appear not only in patients with controlled background pain but also in those with uncontrolled background pain, those not using opioids and those without background pain. However, there was no clear consensus among researchers which value of increase in NRS would render episodic pain diagnosis. It seems that an increase of 2 or more points in NRS would diagnose episode of pain exacerbation (episodic pain) (88).
CONCLUSION
The knowledge of pain pathophysiology and appropriate, complex pain assessment in cancer patients is a base of an effective pain management. Clinical evaluation should encompass different possible causes of pain in cancer patients including tumor progression, oncology treatment and co-morbidities. The knowledge of pain mechanisms may be useful for a rationale treatment plan.
Pain management should be included in a complex plan of cancer patients’ treatment (89). Apart from appropriate treatment of background pain in a significant percentage of patients’ evaluation and treatment of breakthrough (episodic) pain flares should be implemented. An important role of a palliative oncology treatment, especially radiotherapy, and interventional techniques of pain management should be strongly considered along with pharmacotherapy. The management of other symptoms and meticulous monitoring of effectiveness of pain therapy with possible drug interactions should be taken strongly into account. An appropriate communication with patients, families and careers with a comprehensive psychosocial and spiritual support play significant role in patients and family QOL improvement.
Conflict of interests: None declared.
REFERENCES
- Leppert W, Majkowicz M, Ahmedzai SH. The adaptation of the Sheffield Profile for Assessment and Referral for Care (SPARC) to the Polish clinical setting for needs assessment of advanced cancer patients. J Pain Symptom Manage 2012; 44: 916-922.
- Ferlay JF, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide, IARC Cancer Base. Lyon, France, International Agency for Research on Cancer 2013, (14.01.2014). http://globocan.iarc.fr, access 20th October 2016.
- Foley KM. Pain assessment and cancer pain syndromes. In: Oxford Textbook of Palliative Medicine. Doyle D, Hanks GW, MacDonald N (eds.). Oxford, Oxford University Press 1997, pp. 148-165.
- Twycross R. The extent of the problem. In: Pain Relief in Advanced Cancer. Twycross R (ed.). Churchill Livingstone, Edinburgh 1994, pp. 1-15.
- Van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437-1449.
- Zatonski W, Sulkowska U, Didkowska J. Some notes on cancer epidemiology in Poland. Nowotwory 2015; 65: 179-196 [in Polish].
- Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. Mechanism of cancer pain. Mol Interv 2010; 10: 164-178.
- Wordliczek J, Zajaczkowska R. Mechanisms in cancer pain. In: Cancer Pain. Hanna M, Zylicz B, (eds.). London, Heidelberg, New York, Springer 2013, pp. 47-70.
- Urch CE. Pathophysiology of cancer pain. In: Palliative Medicine. Walsh D (ed.). Philadelphia, Saunders Elsevier 2009, pp. 1378-1384.
- Gold MS, Gebhart GF. Peripheral pain mechanisms and nociceptor sensitization. In: Bonica’s Management of Pain. Fishman SM, Ballantyne JC, Rathmell JP, (eds.). Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins 2010, pp. 24-34.
- Stein C, Clark JD, Oh U, et al. Peripheral mechanisms of pain and analgesia. Brain Res Rev 2009; 60: 90-113.
- Basbaum A, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267-284.
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: Wall and Melzack’s Textbook of Pain. McMahon SB, Koltzenburg M, (eds.). Philadelphia, Elsevier 2008, pp. 3-34.
- Mantyh PW. Bone cancer: from mechanism to therapy. Curr Opin Support Palliat Care 2014; 8: 83-90.
- Bloom AP, Jimenez-Andrade JM, Taylor RN, et al. Breast cancer-induced bone remodeling, skeletal pain and sprouting of sensory nerve fibres. J Pain 2011; 12: 698-711.
- Chang DS, Hsu E, Hottinger DG, Cohen SP. Anti-nerve growth factor in pain management: current evidence. J Pain Res 2016; 9: 373-383.
- Wei H, Saarnilehto M, Falck L, et al. Spinal transient receptor potential ankyrin 1 channel induces mechanical hypersensitivity, increases cutaneous blood flow, and mediates the pronociceptive action of dynorphin A. J Physiol Pharmacol 2013; 64: 331-340.
- Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol 2007; 170: 427-435.
- DeClerck YA, Mercurio AM, Stack MS, et al. Proteases, extracellular matrix, and cancer: a workshop of the path B study section. Am J Pathol 2004; 164: 1131-1139.
- Lam DK, Schmidt BL. Serine proteases and protease activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain 2010; 149: 263-272.
- Mercadante S. Pathophysiology of chronic pain. In: Textbook of Palliative Medicine. Bruera E, Higginson IJ, Ripamonti C, von Gunten C, (eds.). London, Edward Arnold 2006, pp. 359-366.
- Randich A, Ness T. Modulation of spinal nociceptive processing. In: Bonica’s Management of Pain. Fishman SM, Ballantyne JC, Rathmell JP, (eds.). Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins 2010, pp. 48-60.
- Buga S, Sarria JE. The management of pain in metastatic bone disease. Cancer Control 2012; 19: 154-166.
- Li BT, Wong MH, Pavlakis N. Treatment and prevention of bone metastases from breast cancer: a comprehensive review of evidence for clinical practice. J Clin Med 2014; 3: 1-24.
- Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol 2011; 23: 387-392.
- Zhu XC, Zhang JL, Ge CT, et al. Advances in cancer pain from bone metastasis. Drug Des Devel Ther 2015; 9: 4239-4245.
- Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann NY Acad Sci 2010; 1198: 173-181.
- Reddy SK. Causes and mechanisms of pain in palliative care patients. In: Textbook of Palliative Medicine. Bruera E, Higginson IJ, Ripamonti C, von Gunten C, (eds.). London, Edward Arnold 2009, pp. 368-379.
- Yoneda T, Hata K, Nakanishi M, et al. Involvement of acidic microenvironment in the pathophysiology of cancer associated bone pain. Bone 2011; 48: 100-105.
- Ghilardi JR, Rohrich H, Lindsay TH, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 2005; 25: 3126-3131.
- Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci 2010; 30: 14649-14656.
- Abraham J, Ross E, Klickovich RJ. Cancer-related visceral pain. In: Bonica’s Management of Pain. Fishman SM, Ballantyne JC, Rathmell JP, (sds.). Philadelphia, Wolters Kluwer/Lippincott Williams &Wilkins 2010, pp. 635-644.
- Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol 2012; 233: 49-67.
- Ceyhan GO, Michalski CW, Demir IE, Muller MW, Friess H. Pancreatic pain. Best Pract Res Clin Gastroenterol 2008; 22: 31-44.
- Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 2012; 153: 359-365.
- Xua Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 2011; 24: 400-407.
- Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 2008; 60: 297-307.
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807-819.
- Ostgathe C, Voltz R. Brain metastases. In: Palliative Medicine. Walsh D, (ed.). Philadelphia, Saunders-Elsevier 2009, pp. 1240-1244.
- Law M. Neurological complications. Cancer Imaging 2009; 9: 71-74.
- Ribas ES, Schiff D. Spinal cord compression. Curr Treat Options Neurol 2012; 14: 391-401.
- Janjan N, Lin E, McCutcheon I, et al. Vertebral metastases and spinal cord compression. In: Palliative Medicine. Walsh D, (ed.) Philadelphia, Saunders-Elsevier 2009, pp. 1247-1260.
- Popa G, Mititelu Tartau L, Stoleriu I, Lupusoru RV, Lupusoru CE, Ochiuz L. The effect of pregabalin - codeine combination on partial sciatic nerve ligation - induced peripheral mononeuropathy in rats. J Physiol Pharmacol 2016; 67: 465-469.
- Zurowski D, Nowak L, Machowska A, Wordliczek J, Thor PJ. Exogenous melatonin abolishes mechanical allodynia but not thermal hyperalgesia in neuropathic pain. The role of the opioid system and benzodiazepine–gabaergic mechanism. J Physiol Pharmacol 2012; 63: 641-647.
- Griffo Y, Obbens EA. Neurological complications. In: Palliative Medicine. Walsh D, (ed.). Philadelphia, Saunders-Elsevier 2009, pp. 1237-1240.
- Jaeckle KA. Neurologic manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol 2010; 30: 254-262.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618-1625.
- Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth 2011; 107: 25-29.
- Fitzgibbon DR. Mechanisms, assessment, and diagnosis of pain due to cancer. In: Bonica’s Management of Pain. Fishman SM, Ballantyne JC, Rathmell JP, (eds.). Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins 2010, pp. 559-582.
- Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer 2008; 99: 604-610.
- Hansen DM, Kehlet H, Gartner R. Phantom breast sensations are frequent after mastectomy. Dan Med Bull 2011; 58: A4259.
- Vadivelu N, Schreck M, Lopez J, Kodumudi G, Narayan D. Pain after mastectomy and breast reconstruction. Am Surg 2008; 74: 285-296.
- Dijkstra PU, Rietman JS, Geertzen JH. Phantom breast sensations and phantom breast pain: a 2-year prospective study and a methodological analysis of literature. Eur J Pain 2007; 11: 99-108.
- Probstner D, Thuler LC, Ishikawa NM, Alvarenga RM. Phantom limb phenomena in cancer amputees. Pain Pract 2010; 10: 249-256.
- Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain 2007; 8: 102-109.
- Rinne ML, Lee EQ, Wen PY. Central nervous system complications of cancer therapy. J Support Oncol 2012; 10: 133-141.
- Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet 2009; 374: 1639-1651.
- Chamberlain MC. Neurotoxicity of cancer treatment. Curr Oncol Rep 2010; 12: 60-67.
- Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin 2010; 28: 217-234.
- Chen AM, Hall WH, Li J, et al. Brachial plexus-associated neuropathy after high-dose radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012; 84: 165-169.
- Sanguineti G, Sormani MP, Marur S, et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2012; 83: 235-242.
- Jaggi AS, Singh N. Mechanisms in cancer chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012; 291: 1-9.
- Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care 2011; 5: 1-7.
- Niscola P, Tendas A, Scaramucci L, Giovannini M, De Sanctis V. Pain in blood cancers. Indian J Palliat Care 2011; 17: 175-183.
- Hoff AO, Toth B, Hu M, Hortobagyi GN, Gagel RF. Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann NY Acad Sci 2011; 1218: 47-54.
- Niscola P. Mucositis in malignant hematology. Expert Rev Hematol 2010; 3: 57-65.
- Langemo D. General principles and approaches to wound prevention and care at end of life: an overview. Ostomy Wound Manage 2012; 58: 24-34.
- Stephen-Haynes J. Pressure ulceration and palliative care: prevention, treatment, policy and outcomes. Int J Palliat Nurs 2012; 18: 9-16.
- Mercadante S, Vitrano V. Pain in patients with lung cancer: pathophysiology and treatment. Lung Cancer 2010; 68: 10-15.
- Simmons CP, Macleod N, Laird BJA. Clinical management of pain in advanced lung cancer. Clin Med Insights Oncol 2012; 6: 331-346.
- Leppert W, Turska A, Majkowicz M, Dziegielewska S, Pankiewicz P, Mess E. Quality of life in patients with advanced lung cancer treated at home and at a palliative care unit. Am J Hosp Palliat Med 2012; 29: 379-387.
- Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013; 154 (Suppl. 1): S54-S62.
- Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013; 154: 95-102.
- Ganz PA, Stanton AL. Living with metastatic breast cancer. Adv Exp Med Biol 2015; 862: 243-254.
- Fallon M, Hoskin PJ, Colvin LA, et al. Randomized double-blind trial of pregabalin versus placebo in conjunction with palliative radiotherapy for cancer-induced bone pain. J Clin Oncol 2015; 34: 550-556.
- Diciolla A, Cristina V, De Micheli R, Digklia A, Wagner AD. News and perspectives in the treatment of advanced gastric and colorectal cancers. Rev Med Suisse 2015; 11: 1124-1126.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8: 188-191.
- Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK. The characteristics of bone metastasis in patients with colorectal cancer: a long-term report from a single institution. World J Surg 2016; 40: 982-986.
- Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012; 4: 159-169.
- Quraishi NA, Giannoulis KE, Edwards KL, Boszczyk BM. Management of metastatic sacral tumours. Eur Spine J 2012; 21: 1984-1993.
- Cleary JF. Pharmacokinetic and pharmacodynamic issues in the treatment of breakthrough pain. Semin Oncol 1997; 24 (Suppl. 16): S16-S19.
- Mercadante S, Radbruch L, Caraceni A, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer 2002; 94: 832-839.
- Mercadante S, Marchetti P, Cuomo A, Mammucari M, Caraceni A, IOPS MS study Group. Breakthrough pain and its treatment: critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer 2016; 4: 961-968.
- Portenoy RK, Hagen NA Breakthrough pain: definition, prevalence and characteristics. Pain 1990; 41: 273-281.
- Zech D, Petzke F, Radbruch L, et al. Breakthrough pain in cancer patients with chronic pain: prevalence and characteristics. Br J Anest 1995; 74: 215-220.
- Gatti A, Gentili M, Baciarello M, et al. Breakthrough pain in patients with controlled or uncontrolled basal pain: an observational study. Pain Res Manage 2014; 19: e168-e171.
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a Task Group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 2009; 13: 331-338.
- Lore ET, Klepstad P, Bennett MI, et al. From ‘breakthrough’ to ‘episodic’ cancer pain? J Pain Symptom Manage 2016; 51: 1013-1019.
- O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain 2017; 21: 3-19.
A c c e p t e d : December 21, 2016