GENERATION OF MEMBRANE-BOUND CATECHOL-O-METHYL TRANSFERASE DEFICIENT MICE WITH DISTINCT SEX DEPENDENT BEHAVIORAL PHENOTYPE
INTRODUCTION
Catechol-O-methyl transferase (COMT) O-methylates compounds with a catechol moiety in the presence of a Mg2+ ion using S-adenosyl-L-methionine (AdoMet, SAM) as a methyl donor (1, 2). The COMT gene (COMT) codes for two isoforms of COMT, soluble (S-COMT) and membrane-bound (MB-COMT) proteins (3, 4). The latter protein is otherwise identical to S-COMT but it incorporates 50 additional hydrophobic amino acids (in humans) that form the membrane anchor (3, 5). COMT contains six exons, the first two of which are non-coding. In exon 3, there are two AUG start codons for two promoters that control expression of the two COMT transcripts (6). The distal P2 promoter regulates the synthesis of a 1.5-kb transcript in humans. Based on the leaky scanning mechanism of translation initiation, this longer transcript can code for both S-COMT and MB-COMT proteins (6-8). The P1 promoter almost completely overlaps exon 3 and falls between the S-COMT and MB-COMT ATG start codons, partially overlapping the MB-COMT coding sequence. Therefore, the shorter mRNA transcript (1.3 kb in humans) regulated by P1 only codes for the S-COMT protein.
S-COMT and MB-COMT share the same kinetic mechanism; however, the kinetic parameters are somewhat different. S-COMT has a high Km value for dopamine but very high enzymatic capacity (Vmax up to nearly 15,000 pmol/min per mg protein in liver) (1, 9). MB-COMT, on the other hand, has low Km value and low capacity (only 2 – 40 pmol/min per mg protein). Furthermore, MB-COMT has a higher affinity for catecholamines than S-COMT (10). Therefore, it has been postulated that S-COMT has an important role in O-methylation when substrate levels are high (e.g. food-derived catechol compounds in gut), whereas MB-COMT gains more importance at low substrate levels (e.g. brain catecholamine neurotransmitters) (11-13). In addition, the two enzyme isoforms differ by the regioselectivity of O-methylation: MB-COMT strictly prefers 3-O-methylation (meta/para ratios between 22 and 88, depending on substrate), whereas S-COMT allows slightly more 4-O-methylation (meta/para ratio between 4 and 15) (14).
COMT is an intracellular enzyme, and in the brain it has been localized to glial cells and short postsynaptic neurons (13, 15, 16). Subcellularly, S-COMT has been found in the cytosol as well as in the nucleus while MB-COMT is associated with intracellular membranes but - notably - not with the cell membrane (16-18). Recently, however, these unanimous views have been disputed by one study giving some evidence for an extracellular position of COMT and for its localization in presynaptic catecholaminergic neurons (19). Nevertheless, due to the requirements of the chemical environment (pH, concentrations of Mg2+, Ca2+ and SAM), it is unlikely that COMT enzyme reactions could effectively happen outside cells (13).
COMT is widely distributed throughout the body, with the highest protein and activity levels found in the liver, kidneys and gut (2, 20). COMT gene expression is fairly uniformly distributed in the body. The highest levels are found in the adrenal gland followed by liver, esophageal mucosa, spinal cord and peripheral nerves. Notably, COMT gene expression levels are at medium level in the brain with only slight fluctuation between the areas (21).
Until recently, it has been difficult to gain reliable information on the tissue distribution of enzyme isoforms because isoform-selective antibodies are not available. Using subcellular fractionation, Lundstrom and coworkers (22) suggested that S-COMT is the predominant isoform in the periphery, whereas MB-COMT may dominate in the human but not rat brain. Analysis of COMT distributions in mutant mice selectively lacking S-COMT (17, 23) confirmed the importance of MB-COMT in brain tissue and the dominance of S-COMT in peripheral tissues (17). Nevertheless, the distribution of COMT isomorphs seems to be species-dependent, as Lundstrom et al. (22) and Ellingson et al. (24) suggested that S-COMT is dominant at least in certain areas of the rat brain (cerebellum, cortex). An important role for COMT in the brain is supported by the fact that the well-known COMT Val/Met polymorphism has been associated with different psychiatric and neurological conditions, for example with depression (25), schizophrenia (26), pain sensitivity (27), and memory impairment (28). There is an optimum level of COMT activity for the maintenance of optimum catecholaminergic tone, and both low COMT (associated with high catecholaminergic tone) and high COMT activity (low catecholaminergic tone) may cause problems (29, 30).
In the present study, we generated a novel genetically modified mouse strain that is selectively deficient in MB-COMT protein. Since the association of COMT polymorphism with schizophrenia in general is still a hot topic (31-33), we were interested in behavioral endophenotypes that have been proposed to model manifestations of schizophrenia, such as aggressive behavior (34), prepulse inhibition (35) and memory impairment (36), in mice. To get a more general view of their behavior, also general motor activity, pain sensitivity and depression endophenotype were assessed. We demonstrate how the genetic modification affects the levels of MB-COMT protein expression as well as COMT activity in the mouse brain and peripheral tissues. We also show how MB-COMT deficiency impacts extracellular dopamine and the behavioral phenotype of mice.
MATERIALS AND METHODS
Generation of MB-COMT deficient mouse strain
1. Targeting construct
The MB-COMT-deficient strain was constructed by two point mutations (ATGCTG to GAGCTC) of P2 promoter in exon 2 of the Comt gene (Fig. 1). The first mutation induces a change of methionine to glutamic acid, the second mutation does not affect coding (leucine remains leucine). Briefly, a BAC clone containing exons 2 to 4 and partial exon 5 of Comt gene was subcloned into a minimal vector, pACYC177. To generate point mutations in exon 2, DNA fragment containing exon 2, introns 1 and 2 amplified by PCR was inserted into pGEM-4Z vector digested with EcoRI and SmaI. The point mutations in exon 2 were introduced into the pGEM-4Z clone by site directed mutagenesis using QuikChange Site-Directed Mutagenesis kit (Stratagene, CA, USA). A neo-resistant gene flanked with two loxP sites was introduced into intron 2 with a loxP-PGK-Neo-loxP cassette (Gene Bridges, Heidelberg, Germany). Finally, a 3012-bp DNA fragment containing exon 2 with point mutations and Neo cassette was excised from pGEM-4Z with SmaI and XbaI and replaced wild type exon 2 region in pACYC177 backbone by Red/ET recombination.
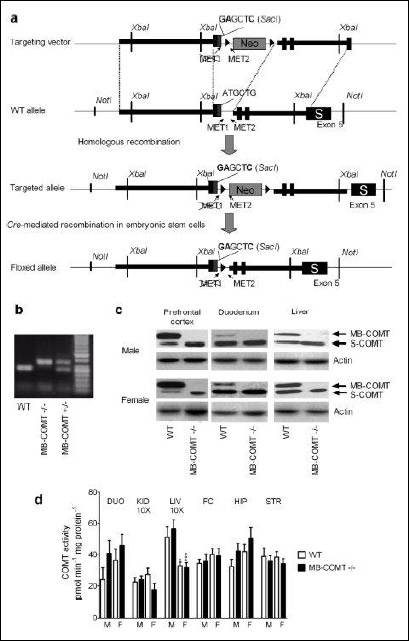 |
Fig. 1. (a): A general outline of the generation of MB-COMT deficient mice with site-directed knock-in mutagenesis. Point mutations of ATGCTG → GAGCTC of the MET1 site results in a change from methionine (MET, M) to glutamic acid (Glu, E). NotI, XbaI, SacI = restriction enzymes. Neo = neomycin resistance element. MET1, MET2 = initiation codons 1 and 2. (b): Sample agarose gel showing the bands for wild-type (WT) as well as heterozygous (MB-COMT+/–) and homozygous (MB-COMT-/-) MB-COMT deficient mice. The PCR amplified fragments were visualized with SYBR Green (Qiagen) staining under UV light after electrophoresis in 1.7% agarose gel. (c): Western blot analysis of COMT isoforms in wild-type and MB-COMT deficient mice in the prefrontal cortex, duodenum and liver. MB = MB-COMT, S = S-COMT. (d): Specific total COMT activities in the duodenum (DUO), kidney (KID), liver (LIV), frontal cortex (FC), hippocampus (HIP) and striatum (STR) of the wild-type and MB-COMT deficient mice. Kidney and liver values have been divided by ten to fit them in the same graph with other tissues. *P < 0.05, ***P < 0.001 versus corresponding male mice; n = 5 – 12, Student's t-test after two-way ANOVA. |
2. Gene targeting and screening of colonies in embryonic stem cells
G4 embryonic stem cells (ES, derived from mouse 129S6/C57BL/6Ncr) were cultured on neomycin-resistant primary embryonic fibroblast feeder layers, and 106 cells were electroporated with 30 µg of linearized targeting construct. After electroporation, the cells were plated on 100-mm culture dishes and exposed to G418 (300 µg/ml; Sigma). Colonies (288) were picked after 7 – 9 days selection, and grown on 96-well plates. In order to delete Neo cassette in the targeted ES cells, the cells were re-electroporated with the plasmid pCAGGS-Cre. After electroporation, the cells were plated on 100-mm culture dishes and colonies were picked after 3 – 5 days growth, and grown on 96-well plates. In order to detect targeted ES clones with Neo deletion, DNAs isolated from these colonies were screened by PCR with several different primer pairs. More than 20 correct clones were found from 96 clones. The right clones were further confirmed by sequencing.
3. Producing a MB-COMT deficient mouse colony
Positive ES clones were used for blastocyst injection. Chimeric males were mated with C57BL/6J females, and DNA from tissue samples taken from ear lobes of F1 pups was typed by PCR (see below). F1 heterozygous mice were mated, and F2 mice of all three genotypes were obtained. Heterozygous and homozygous MB-COMT deficient mice are healthy and viable, and they breed normally. This could be expected because the Comt gene disrupted knock-out mice (37, 38) as well as S-COMT deficient mutant mice (23, 39) are also seemingly normal.
Homozygous male and female mice of the MB-COMT strain as well as their wild-type (WT) littermates were used for the animal experiments. The mice were bred at the Laboratory Animal Centre of University of Helsinki, Finland, and the sixth to seventh generations of the heterozygous mating pairs were used for the experiments. The mice were regularly backcrossed to C57BL/6J females and less than 2% of 129S6 background was left in the 7th generation. The animals were weaned, sexed and earmarked at the age of three weeks. After weaning, they were group housed in clear polycarbonate individually ventilated filter-top cages under 12:12 light cycle at an ambient temperature of 22°C with drinking water and mouse chow available ad libitum.
The genotype of WT and MB-COMT deficient (either heterozygous or homozygous) mice was determined from ear biopsies obtained during ear marking using a PCR method. The PCR mix consisted of 3'HAUF2 (5'-GAAGTGGGTATGGCAGCGCTTATA-3') and 5'HADR2 (5'-AACACACATTCCTCTCATGCTCCT-3') primers (Oligomer, Helsinki, Finland) as well as FailSafe system premix B and PCR enzyme (Epicentre, Madison, WI, USA). The amplified fragments were visualized with SYBR Green (Qiagen, Venlo, The Netherlands) staining under UV-light after electrophoresis in 1.7% agarose gel. An example of the genotyping results is shown in Fig. 1B and the corresponding western blots in Fig. 1C. Animals were used for experiments when they were two to three months old. The phase of the estrus cycle was not determined.
Animal experiments were conducted according to the 3R principles of the EU directive governing the care and use of experimental animals (2010/63/EU), and following local laws and regulations (Finnish Act on the Protection of Animals Used for Scientific or Educational Purposes (497/2013), Government Decree on the Protection of Animals Used for Scientific or Educational Purposes (564/2013)). The protocols were authorized by the national Animal Experiment Board of Finland.
Total COMT activity assay
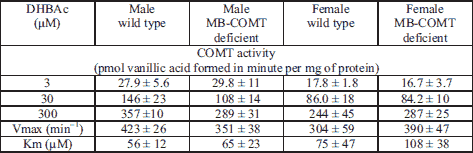
Brain and peripheral tissues for total COMT activity assay were collected from homozygous MB-COMT deficient and wild-type mice of both sexes. The tissues were frozen on dry ice and stored at –80°C before analysis. Detailed method of the COMT activity assay is given elsewhere (23). Activity data is given as picomoles of vanillic acid (and isovanillic acid for meta/para calculation) formed per minute per milligram of tissue. Besides a regular COMT assay with a high substrate concentration (400 µM of dihydroxy benzoic acid, DHBAc), liver COMT activities were measured also with graded concentrations of DHBAc (3, 30 and 300 µM) and enzyme kinetics (Km and Vmax) were calculated with GraphPad Prism 5.0. (San Diego, CA, USA). Hippocampal COMT activities were measured also with two lower DHBAc concentrations (30 and 300 µM).
Western blotting
For Western immunoblotting (WB), the tissue samples were collected from at least two male and two female mice and rinsed in physiological saline solution. Immediately after dissection, the tissues were placed in ice-cold centrifuge tubes on dry ice to minimize decomposition. All the tissue samples were frozen and stored in –80°C until analyzed. Tissues (prefrontal cortex, duodenum, liver) were lysed in 10 volumes of RIPA lysis buffer (20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA) containing protease and phosphatase inhibitors, homogenized manually, incubated for 20 min on ice and centrifuged (18,000 g for 15 min at 4°C). The supernatants were resolved by electrophoresis on a 4 – 20% precast gel (Mini Protean TGX Gel, Biorad, Hercules, CA, USA). In some cases, a prolonged running time was use for a better separation. Proteins were transferred onto Trans-Blot Turbo Nitrocellulose filter and transferred using the Trans-Blot Turbo Transfer system (Biorad, Hercules, CA, USA). The membranes were blocked with 0.1% (w/w) Tween-20/TBS containing 5% (w/w) non-fat dried milk at room temperature for 1 hour. After blocking, the membranes were incubated overnight with mouse anti-COMT antibody (1:8000, BD Biosciences, Franklin Lakes, NJ, USA) and mouse anti β-actin antibody (1:10000, clone AC-74, purified immunoglobulins, 107K4791, Sigma-Aldrich Inc., MO, USA) followed by incubation with goat anti-mouse secondary antibody, HRP conjugated (1:2000, Thermo Fisher Scientific, Waltham, MA, USA) for 1 hour at room temperature. The membranes were incubated with ECL detection reagent (Thermo Fisher Pierce, Rockford, IL, USA) for 5 min to visualize proteins, and then visualized using C-Digit blot scanner (Li-COR, Lincoln, NE, USA). Blots (Fig. 1C) and the relative optical density values of protein bands were analyzed using ImageJ (v.1.50i, NIH, Bethesda, MA) software. The reported optical density values were calculated relative to actin band, which was run parallel to each experiment.
Microdialysis
The method used has been described elsewhere in full detail (40). Briefly, the mice were implanted with guide cannulas (AT-4.7, AgnTho's AB, Lidingo, Sweden) under isoflurane anesthesia and buprenorphine analgesia (0.05 mg kg–1 s.c. b.i.d. for 24 hours). The guide cannula was aimed at the mPFC (A/P = +1.8, L/M = 0.5, D/V = –1.0) and striatum (A/P = +0.6, L/M = 1.8, D/V = –2.2) according to the mouse brain atlas (41). After the surgery, the animals were monitored for complications during recovery. Animal was sacrificed if substantial weight loss or signs of infection or illness were observed or if the guide cannula fell off.
After a 5 - 7-day recovery period, a microdialysis probe (AT-4.7.2, Agn Tho's AB, Lidingo, Sweden) perfused with artificial cerebrospinal fluid (147 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl and 1.0 mM MgCl2) was placed in the guide cannula at 7:30 AM. After a 120-min stabilization period, collection of samples at 20-min intervals commenced. After the collection of four baseline samples, the animals were administered with carbidopa (30 mg kg–1, i.p.) and L-DOPA (10 mg kg–1, i.p.). The drug doses were chosen to match the ones used in our previous L-DOPA studies (23, 42). After drug administration, sample collection continued for five hours. Probe placements were verified afterwards from brain sections mounted on glass slides. We aimed at having six animals per each group because earlier experience with microdialysis had indicated that this number would be large enough to reveal significant differences in dopamine outflow. In prefrontal cortex, there were 7 animals in male homozygous and wild-type groups and female homozygous group, and 6 animals in female wild-type group. In dorsal striatum, there were 6 animals in male and female homozygous groups, and 5 animals in male and female wild-type groups.
The concentrations of dopamine, DOPAC and HVA in the mPFC and dorsal striatal microdialysis samples were determined by HPLC with electrochemical detection (40). Detection limits for the system that was used for mPFC microdialysis samples was 15 pM for dopamine, 0.4 nM for DOPAC and 0.6 nM for HVA. Detection limits for the system that was used for dorsal striatal microdialysis were 0.15 nM for dopamine, 5 nM for DOPAC and 7 nM for HVA.
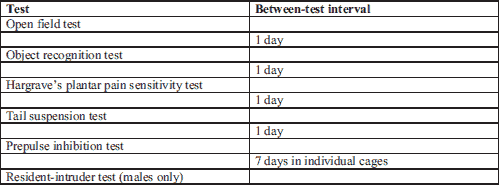
Behavioral tests
Owing to a limited number of animals, most of the behavioral tests were performed in the same animals with at least a one-day interval in an order from less stressful to more demanding tasks. The order of testing and between-test intervals are presented in Table 1.
1. Open field test for assessment of locomotor activity
Open field activity was determined between 9 AM and 2 PM. The open-field apparatus consisted of a four-sided 50 cm × 50 cm × 50 cm (L × W × H) wooden box. The floor of the box was divided into 16 squares. Each animal was tested for a 5-min period. Animals (n = 7 in each group) were placed in the center of the test cage and allowed to explore freely for 5 min. During the test time, the number of squares passed were counted. After each animal, the test apparatus was cleaned with a 10% ethanol solution and water to remove any olfactory cues.
2. Object recognition test
On the second day of testing, the animal was placed in the same cage as used in measuring locomotor activity with two identical objects A and A' (clear plastic measuring cups). Training took place by placing individual mice (n = 7 in each group) into the field for 5 min, into which the objects A and A' were positioned in two adjacent corners, 10 cm from the walls. The time spent exploring each object was registered. Two hours later the animal was placed in the cage with objects A and B. The object B (a red plastic pentagon) was novel and its shape and color differed from the object A. Again, time spent exploring the objects was registered. Difference in time spent exploring the novel object B versus the familiar object A gave an estimate of the function of short-term memory; mice with intact short-term memory would concentrate on exploring the novel object. Twenty-four hours later the animal was once more placed in the cage, this time with objects A and C. The object C (a green plastic pyramid) was novel and different than the objects A and B. Time spent exploring these objects gave an estimate of the function of long-term memory. The objects B and C were different from each other because mice with properly functioning long-term memory would assumedly remember the object B from the previous testing session.
The test is based on the fact that animals will not explore the familiar objects as often as they explore a novel object. A discrimination index for each object was expressed as a ratio of the amount of time spent exploring the new object (B or C) [Tnew / (TA + Tnew)] * 100, where TA and Tnew are the times spent exploring the familiar object A and the novel object, respectively. Between trials, the objects were cleaned with 5% ethanol solution. Exploration was defined as sniffing or touching the object with the nose and/or forepaws. The time spent exploring each object was videotaped and analyzed later.
3. Plantar test for pain sensitivity (Hargraves's method)
The plantar test was carried out using Ugo Basile SRL 7371 Plantar test apparatus (Varese, Italy). IR intensity was set to 60%. Animals (n = 6 – 7 in each group) were left to the chamber for 20 min habituation period, after which the IR beam was positioned under hind paw and withdrawal latency was measured. For each animal, the withdrawal latency was measured for both hind paws alternately three times. For analysis, the mean withdrawal latency was calculated. Two animals (one from each WT group) were excluded because they did not calm down within one hour to allow the measurement to be conducted, i.e. they did not stand still long enough that the experimenter could have placed the IR beam under the paw.
4. Tail suspension test (TST)
TST is an analogue to the Porsolt forced swim test and is based on the fact that mice suspended by the tail alternate periods of struggle and immobility. Mice (n = 7 in each group) were suspended by the tail, using adhesive tape, approximately one cm from the base of the tail to a wooden beam. The total duration of immobility during the 6-min test period was measured. Immobility was defined as complete lack of movements besides respiration.
5. Acoustic startle response and prepulse inhibition of acoustic startle
Acoustic startle response and prepulse inhibition of acoustic startle were assessed using two automated SRLab System chambers (San Diego Instruments, San Diego, CA, USA). A speaker located in the ceiling of the chamber provided background noise (65 dB) and acoustic stimuli. A piezoelectric sensor attached to the base transduced the startle response. Baseline acoustic startle response was recorded at 68, 82, 90, 100, and 120 dB.
After a 5-min acclimation period, four successive trials of 40 ms noise bursts at 120 dB were presented (not included in the analysis). Subjects (n = 7 in each group) were then exposed to five different types of acoustic stimuli in a randomized order: pulse alone (120 dB noise for 40 ms), no stimulus (background noise only), and three separate prepulse pulse combinations, with the prepulse (PPS) set at four sound levels of 68, 72, 76 and 80 dB for 20 ms followed by a 40 ms pulse at 120 dB. There were 100 ms between the prepulse and the pulse. A total number of 20 trials under each acoustic stimulus condition were presented with an average 20-s variable ITIs randomly ranging from 5 to 25 s. The startle amplitude for each trial was measured for 65 ms starting from the onset of the startle stimulus. Percent PPI of startle response was calculated as: PPI% = [1-(startle response to PPS + pulse trial / startle response to pulse-alone trial)] * 100.
6. Resident intruder test
Male mice (n = 7) were singly housed for a week. An intruder from the opposite genotype was introduced to the home cage of the resident. The number of threats, body sniffs and bites, and latency to attack was measured. The cut-off time was 180 s.
Statistical analysis
All data are given as mean ± S.E.M. AUC values were calculated employing the trapezoidal rule (calculated of time points between 40 and 300 min for microdialysis data). The microdialysis data was normalized to baseline (% of baseline) before the calculation of AUCs.
For COMT activity data, microdialysis data as well as behavioral data, two-way analysis of variance (ANOVA) with sex and genotype as independent variables was used as the statistical testing method. The statistical analysis of microdialysis results was performed on the AUC values. Repeated measures model was applied to acoustic startle response and prepulse inhibition of acoustic startle as well as COMT kinetic data. Since the initial two-way ANOVA for repeated measures showed a significant sex effect, the sexes were further analyzed separately with one-way repeated measures ANOVAs. Student's t-test was used for the two-group post hoc comparisons in all cases. Results from the resident-intruder test were analyzed with Student's t-test only. Statistical analysis was carried out using SPSS 22 software.
RESULTS
Generation and phenotype of MB-COMT deficient mice
The targeting vector used in the genetic engineering, WT target allele, targeted allele construct after homologous recombination as well as floxed allele after Cre recombination in embryonic stem cells are presented in Fig. 1A. A sample agarose gel blot of the PCR products of DNA extracted from ear biopsies obtained from WT as well as heterozygous and homozygous MB-COMT deficient mice is given in Fig. 1B. Representative Western blots from prefrontal cortex, duodenum and liver of male and female WT and homozygous MB-COMT deficient mice (Fig. 1C) show that the latter express S-COMT protein while no MB-COMT protein is present. When the available S-COMT and actin (loading control) bands were quantified, the S-COMT/actin ratios were similar in the tissues obtained from WT and MB-COMT deficient mice. For example, S-COMT/actin ratios were 1.20 ± 0.13 and 1.04 ± 0.11 in the liver and 0.64 ± 0.07 and 0.61 ± 0.06 in the striatum of WT and MB-COMT deficient mice, respectively, indicating that there was no evidence of compensatory upregulation of S-COMT protein expression. Since the number of blots per each tissue was limited, this should be considered as a preliminary finding.
COMT activity
The total COMT activities in the duodenum, kidney, liver, hippocampus, prefrontal cortex and striatum of MB-COMT mutant mice and their WT littermates are presented in Fig. 1D. Overall, a lack of MB-COMT did not induce significant changes in COMT activity. Female mice showed lower COMT activity in the liver than male mice (two-way ANOVA: sex effect F1, 45 = 24.74; P < 0.001). Genotype and sex did not have an effect on total COMT activity in the duodenum, kidney, hippocampus, prefrontal cortex, and striatum.
No sex or genotype effects were detected in meta/para ratios in any of the tissues studied. The values were always less than 15, which is typical for S-COMT activity (e.g. in the liver 6.8 ± 0.4 versus 6.9 ± 0.5 males and 6.2 ± 0.2 versus 6.1 ± 0.2 in females in the WT and MB-COMT deficient mice, respectively. In the PFC, the corresponding meta/para ratios were 8.5 ± 0.8 versus 8.3 ± 0.5 and 7.6 ± 0.6 versus 7.4 ± 0.5; mean ± S.E.M., n = 11). Therefore, sex and genotype did not have an effect on the ratio between 3-O- and 4-O-methylation, despite the lack of MB-COMT in MB-COMT mutant mice.
COMT enzyme kinetics
As expected, increased total COMT activity in the liver (Table 2) and hippocampus (Table 3) homogenates was observed with increasing concentrations of the substrate. There were no significant differences in the kinetic values between genotypes and sexes in the liver, or in COMT activity in the hippocampus. However, there was a sex difference in liver COMT activity across different substrate concentrations (two-way ANOVA for repeated measures: F2, 40 = 8.645, P < 0.01). Meta/para ratios were always less than 15, typical to S-COMT activity, even at the lowest substrate levels (data not shown).
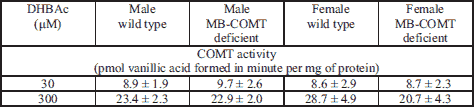
Microdialysis
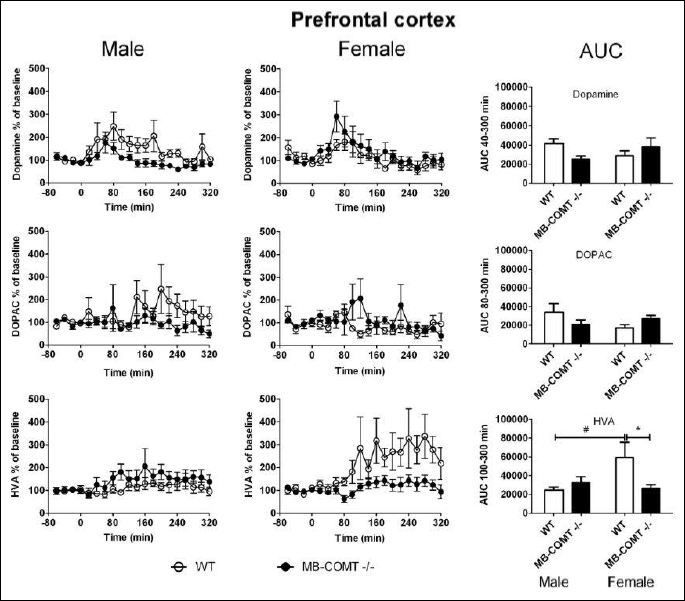
In the mPFC, neither genotype nor sex per se had a significant effect on extracellular dopamine, DOPAC or HVA levels. However, the effect of genotype on dopamine and HVA levels was sex-dependent (Two way ANOVA: dopamine: sex genotype interaction, F1, 22 = 4.527, P < 0.05; HVA: sex × genotype interaction F1, 22 = 5.579, P < 0.05) (Fig. 2). In males, the level of dopamine was lower in MB-COMT deficient animals than WT animals, whereas female MB-COMT–/– mice showed slightly higher dopamine levels than their WT counterparts. HVA levels, on the other hand, were lower in female MB-COMT deficient mice than WT animals. However, male MB-COMT–/– mice showed a minimal increase in HVA as compared to their WT littermates. Baseline levels uncorrected for probe recovery were approximately 10 fmol per 35 µl for dopamine, 0.2 pmol per 35 µl for DOPAC and 0.5 pmol per 35 µl for HVA.
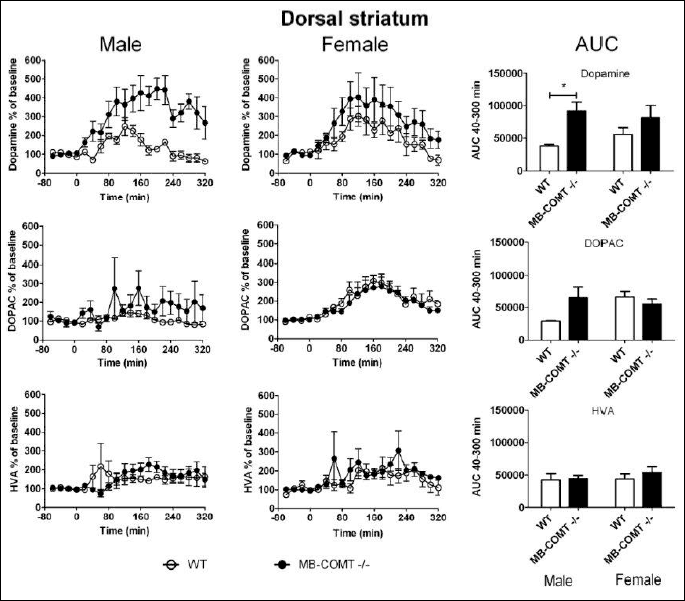
In the dorsal striatum, genotype had an effect on extracellular dopamine levels (Two-way ANOVA: genotype effect F1, 18 = 8.130, P < 0.05) (Fig. 3). MB-COMT deficient mice showed higher dopamine levels than their wild-type littermates. Neither genotype nor sex had an effect on dorsal striatal DOPAC and HVA levels. Baseline levels uncorrected for probe recovery were approximately 100 fmol per 35 µl for dopamine, 3 pmol per 35 µl for DOPAC and 1 pmol per 35 µl for HVA.
Behavior
1.Open field for assessment of locomotor activity
Assessment of locomotor activity did not show any differences between WT and MB-COMT deficient animals (Fig. 4A).
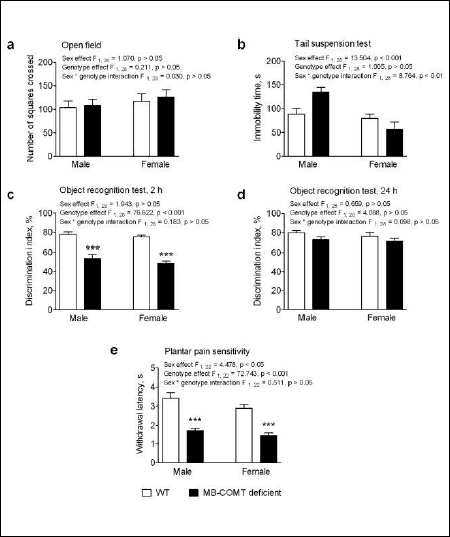 |
Fig. 4. (a): Number of squares crossed in the open field in male and female MB-COMT deficient mice (black columns) and their wild-type (WT) littermates (open columns). (b): Immobility time (s) of male and female MB-COMT deficient mice (black columns) and their wild-type (WT) littermates (open columns) in the tail suspension test. The mice were suspended by the tail using adhesive tape and allowed to hang for 6 min. The immobility time was recorded as a measure of helplessness. A discrimination index (%) of male and female MB-COMT deficient mice (black columns) and their wild-type (WT) littermates (open columns) in the object recognition test. (c): and (d): Training time was 5 min to familiarize two identical objects. The exploration time (5 min, to explore one familiar and one new different object) was tested after 2 hours (Fig. 2c) or 24 hours (Fig. 2d) delay. ***P < 0.001, Student's t-test after two-way ANOVA. (e): A plantar test for pain sensitivity was performed using infrared beam exposure (Hargrave's test) in male and female MB-COMT deficient mice (black columns) and their wild-type (WT) littermates (open columns). After 20 min of habituation, withdrawal latencies of the both hind paws were measured alternating three times and the mean values were calculated for each mouse. n = 6 – 7, mean ± S.E.M.; *** P < 0.001 versus corresponding WT mice, Student's t-test after two-way ANOVA. Two-way ANOVA F-values are shown in figures. |
2. Object recognition test
In object recognition test, short term memory of 2 h and long-term memory of 24 h were assessed. There was a significant difference between MB-COMT deficient and WT mice in short-term memory in male as well as in female mice where MB-COMT deficient animals from both sexes showed a significantly lower novel object discrimination index (two-way ANOVA: genotype effect F1, 28 = 76.622, P < 0.001) (Fig. 4C). On the other hand, no differences were observed in long-term memory between WT and MB-COMT deficient mice in the novel object discrimination indexes (Fig. 4D).
3. Plantar test for pain sensitivity
Pain sensitivity was assessed using Hargrave's plantar test method. Pain sensitivity was sex and genotype-dependent (two-way ANOVA: sex effect F1, 22 = 4.478, P < 0.05; genotype effect F1, 22 = 72.743, P < 0.001). Both male and female MB-COMT deficient mice were more sensitive to pain than WT animals as shown by shorter paw withdrawal latencies (Fig. 4E). The observed sex effect is caused by the shorter withdrawal latencies found in both genotypes of female vs. male mice.
4. Tail suspension test
The TST, which is mostly used to assess depressive-like behavior, revealed a sex difference in the immobility time (two-way ANOVA: sex effect F1, 28 = 13.504, P < 0.001). In addition, the effect of MB-COMT deficiency on immobility time was different between sexes (two-way ANOVA: genotype x sex interaction F1, 28 = 8.764). Male, but not female, MB-COMT deficient mice showed longer immobility time than corresponding WT mice (Fig. 4B).
5. Acoustic startle response and prepulse inhibition of acoustic startle
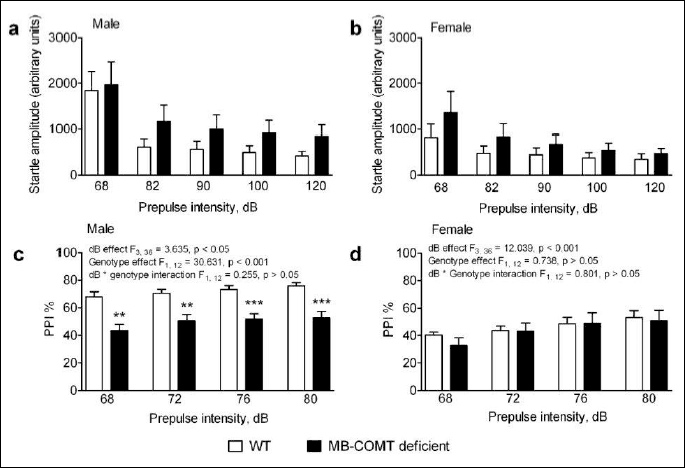
The acoustic startle response depended on the intensity of sound and was different between sexes across a range of sound intensities (68 – 120 dB) (two-way ANOVA: dB effect F4, 96 = 39.921, P < 0.001; dB × sex interaction F4, 96 = 4.325, P < 0.01) (Fig. 5A and 5B).
Prepulse inhibition, which is used to determine the sensorimotor gating function, was both sex and genotype-dependent (two-way ANOVA: sex effect F1, 24 = 14.691, P < 0.001; genotype effect F1, 24 = 9.159, P < 0.01; sex genotype interaction F1, 24 = 5.908, P < 0.05), and the level of inhibition was intensity-dependent (two-way ANOVA: dB effect F3, 72 = 14.315, P < 0.001; dB sex interaction F3, 72 = 1,226, P > 0.05; dB genotype interaction F3, 72 = 0.900, P > 0.05; dB sex genotype interaction F3, 72 = 0.148, P > 0.05). Prepulse inhibition was significantly reduced in MB-COMT deficient male mice compared to WT animals throughout all prepulse intensities (one-way ANOVA: males: genotype effect F1, 12 = 30.631, P < 0.001; dB effect F3, 36 = 3.635, P < 0.05; dB genotype interaction F3, 36 = 0.255, P > 0.05) (Fig. 5C). Conversely, no differences were seen between female WT and MB-COMT deficient animals (one-way ANOVA: females: genotype effect F1, 12 = 0.738, P > 0.05; dB effect F3, 36 = 12.039, P < 0.001; dB genotype interaction F3, 36 = 0.801, P > 0.05) (Fig. 5D).
6. Resident-intruder test
Aggressive behavior was assessed with the resident-intruder test in male WT and MB-COMT deficient mice. Analysis revealed a shorter latency to attack in MB-COMT deficient mice compared to WT mice (Student's t-test: t = 5.595, P < 0.001, n = 7), and these animals also delivered more bites to the opposing mice (Student's t-test: t = 2.954, P < 0.05, n = 7). In WT animals, on the other hand, the number of body sniffs was higher (Student's t-test: t = 5.000, P < 0.001, n = 7), indicating that WT animals were more social than MB-COMT mice in whom aggressiveness was prominent (Fig. 6).
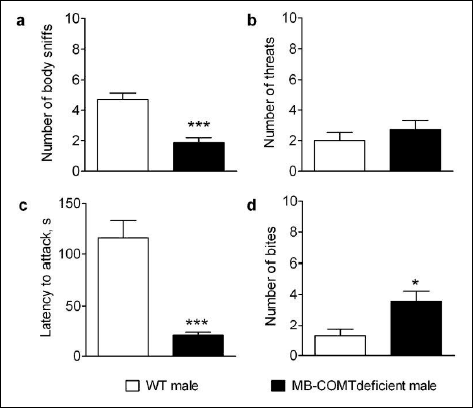 |
Fig. 6. Resident intruder test in male MB-COMT deficient mice (black columns) and their wild-type (WT) littermates reflecting aggressive behavior. The mice were housed individually for one week. An intruder of the opposite genotype was presented into the home cage of the resident for a maximum of 180 s and the following parameters were counted: number of body sniffs, number of threats, latency to attacks (min), and number of bites. Females did not show any aggressive behavior. n = 7, mean ± S.E.M. * P < 0.05, *** P < 0.001 versus corresponding WT mice, Student's t-test. |
DISCUSSION
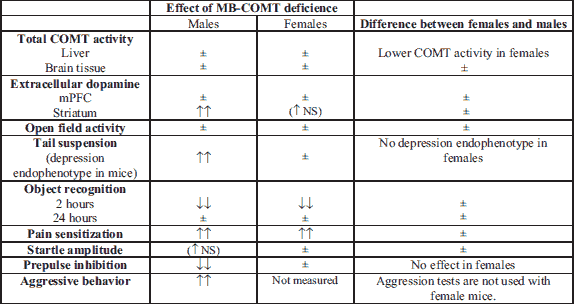
This novel mouse line specifically lacking MB-COMT protein offers for the first time the possibility of revealing the functional importance of MB-COMT. Both sexes of MB-COMT deficient mice showed increased pain sensitivity and disturbed short-term memory while their ambulatory activity remained intact. However, the main finding of our behavioral study is that exclusively MB-COMT deficient male mice exhibited behaviors that can be considered as schizophrenia endophenotypes in animal models such as aggressive behavior (34) and reduced prepulse inhibition (36). Prolonged immobility in the tail suspension test that was exclusively observed in male mice is considered to be depression-like behavior. Depression can also be associated with schizophrenia in humans (43). These behavioral changes were present while the gross COMT activity remained normal, even at low substrate concentrations. The results of microdialysis experiments also show alterations in dopamine metabolism in the dorsal striatum, a brain area that is affected in schizophrenia (44): homozygous MB-COMT deficient male mice showed higher dorsal striatal extracellular dopamine levels than their WT littermates after L-DOPA-carbidopa treatment. A summary of all major findings is shown in Table 4.
Employing a behavioral test battery permits the use of reduced number of mice per set of experiments. Nevertheless, there is no doubt that many of the behavioral tests are stressful and repeated stress may affect the results. Although both wild-type and MB-COMT deficient mice were exposed to stress similarly, it is possible that the latter are more sensitive to stress than the former, which may have rendered them more vulnerable to the behavioral manifestations that we observed. However, we think that this limited behavioral test battery demonstrates the main point: particularly the male MB-COMT deficient mice show a distinct behavioral phenotype. Sex differences in COMT-related neurochemistry and behaviors have been regularly reported (2, 37). We are aware of the importance of sex hormones on COMT biology, and our previous investigations have shown that their effects are complex and tissue dependent (45). It was not practically feasible to synchronize the estrous cycle in the present sets of experiments, which may have increased variability in the results of behavioral and microdialysis studies in female mice. Pain sensitization, 2-hour object recognition and general motility data were, however, similar in both sexes. It is unlikely that behavioral effects that were prominent in male mice would have been completely absent in females simply because of the estrus cycle induced variation. Therefore, we present the behavioral and microdialysis data obtained from female mice as well. However, we point out that these results should be judged with caution.
Unaltered total COMT activity in the mice lacking MB-COMT could be explained by a significant differences of enzyme kinetic properties of the two COMT isoforms. As mentioned in Introduction (1, 9-13), Km value of MB-COMT is less than one hundredth of the corresponding value of S-COMT. In addition, the meta/para ratio suggests dominance of O-methylation by S-COMT even in WT mice (less than 15, typically around 8 in our studies) and this ratio did not differ between the WT and MB-COMT deficient mice in any tissue studied. All in all, these findings suggest that S-COMT is the dominating enzyme of overall COMT activity in the body. Compared to S-COMT, the enzymatic capacity of MB-COMT is limited and even the complete lack of it is not detectable in the gross COMT activity or in the meta/para ratios. It is well known that COMT activity is easily down regulated, e.g. by estrogens, but we are not aware of any convincing evidence of up regulation of either COMT isoforms (2). Even in the present study, our preliminary findings do not support an increase in the amount of S-COMT protein in the MB-COMT deficient mice. It is interesting to note that in the S-COMT deficient mice (23), MB-COMT was able to maintain about 70% of the total COMT activity, suggesting that under extreme conditions it can compensate for missing S-COMT protein. MB-COMT certainly has a function under normal conditions as well, and our behavioral results in MB-COMT deficient mice give hints of its functional role in some brain areas.
We carried out microdialysis experiments in the mPFC and dorsal striatum to find out how MB-COMT deficiency affects carbidopa-L-DOPA induced dopamine efflux (Figs 2 and 3). The mPFC was one of our areas of choice because of extremely low dopamine transporter levels (46-48) and, hence, accentuated importance of COMT-mediated O-methylation in dopamine metabolism in this brain region (40, 49). However, in the mPFC, MB-COMT deficiency did not significantly alter dopamine levels induced by L-DOPA-carbidopa treatment. This may be due to the fact that MB-COMT deficient mice showed unaltered COMT activity also in the mPFC. However, in the dorsal striatum, the extracellular levels of dopamine were higher in MB-COMT deficient mice than in WT animals. This was unexpected since neither full Comt knock-out mice nor S-COMT deficient mice showed an increase in striatal dopamine in response to carbidopa-L-DOPA treatment (23, 42). Full Comt knock-outs may have developed compensatory mechanisms that prevent the accumulation of these catechols. Such compensation was apparently not needed in the selective absence of MB-COMT since total COMT activity was not decreased. These findings may be more understandable in the light of a close interaction of the mPFC and striatum described by Simpson et al. (44, 50). They found that local overexpression of COMT in the PFC caused enhanced dopamine release in the striatum. In the present study, COMT activity in the mPFC was intact while L-DOPA-induced striatal dopamine release was potentiated in MB-COMT deficient mice. Enhanced striatal dopaminergic activity is one of the hallmark neurochemical manifestations of schizophrenia (44).
MB-COMT deficient mice behaved and moved normally under resting conditions. In this sense, they resembled Comt knock-out mice, both homozygous and heterozygous, whose diurnal activity and general behavior are mostly undisturbed (38). Papaleo et al. (51) showed that altered COMT activity, either increased or decreased, did not affect the general health or physical abilities of mice. Recent studies by Risbrough et al. (52) produced similar findings in humanized mice carrying Val/Met alleles of the human COMT Val158Met polymorphism. Met/Met females expressing low COMT activity were rearing more than Val/Val females (52). Conversely, Babovic et al. (53) found in elaborate ethogram observations that, during the initial 60 minutes of testing, heterozygous Comt knock-out mice of both sexes exhibited decreased rearing and increased sifting and chewing while the behavior of homozygous Comt knock-out animals did not differ from the WT mice. S-COMT deficient mice generally behaved like WT mice except that females travelled shorter distances in the elevated plus maze and open field (39). Mice over-expressing of COMT protein showed marginally decreased (54) or no effect (50) on locomotor activity.
One of the most robust findings in COMT related behaviors is the alteration of pain sensitivity. We found increased sensitivity to thermal pain in both sexes of MB-COMT deficient mice in the plantar withdrawal test, which is in line with the shortened tail-flick time observed in the Comt knock-out mice (55). These results are in contrast to our findings in S-COMT deficient male mice in which baseline latency in the tail-flick test was prolonged (39). Collectively, these findings point to a critical role of MB-COMT isoform in nociception and suggest that low COMT activity may sensitize to acute pain. In addition, several studies have shown that administration of selective COMT inhibitors increases thermal, mechanic and inflammatory pain in rodents (55, 56). Findings in humans concerning the link between the Val158Met polymorphism and pain conditions partially and moderately support the results from animal studies. As shown by our meta-analyses (57), the low COMT activity allelic variant Met158 was associated with chronic pain, though only in patients with fibromyalgia or certain related widespread pain conditions. Although the Met158 allele in humans and COMT inhibitors in acute rodent pain models are pronociceptive, repeated administration of nitecapone produces an antiallodynic effect in neuropathic pain models (58, 59). The complex interplay between enhanced adrenergic and dopaminergic activity in different parts of the nociceptive system probably explains the complex effects of low COMT activity on pain sensations; this enigma has been described and discussed in recent reviews (57, 60).
Prolonged immobility in the tail suspension test or forced swimming test is considered to reflect hopelessness and depression. In our study, immobility time was significantly prolonged only in male MB-COMT deficient mice. In homozygous Comt knock-out mice, we did not see any change in the forced swimming time in male mice (39), and over-expression of the 22q11.2 segment, causing a doubling of COMT activity, did not alter the immobility time in the tail suspension test (54). Results with selective COMT inhibitors in combination with L-DOPA (61) have shown shortened immobility times. Similarly, in a long-term tolcapone study, anhedonia was effectively reversed (62). Tolcapone has shown antidepressant properties even in human patients (63). Numerous studies have explored the link between the Val158Met polymorphism and depression (64, 65) as well as the correlation between the Val158Met polymorphism and the efficacy of various antidepressants (65) with highly variable results. Collectively, the existing evidence from human studies implies that Met carriers with relatively low COMT activity may be more vulnerable to depression than individuals with the double Val allele, and this predisposition may be due to an altered stress response.
Regarding cognition-related behaviors, both male and female MB-COMT deficient mice had a lower object recognition index (34% and 38% decrease in males and females, respectively) than their WT littermates at 2 h after training. At 24 h, the effect was no longer present, suggesting that short-term memory was compromised in the MB-COMT deficient animals while long-term memory was intact. Babovic et al. (66) observed a similar deterioration in a novel object recognition test, but only in heterozygous Comt knock-out mice; this was observed more robustly in males than females. Homozygous Comt knock-out mice performed normally in the test. We have also studied the effect of selective COMT inhibitors on single-trial passive-avoidance retention in male rats (67). Contrary to the present findings, fairly high doses of entacapone and tolcapone improved several phases of learning and memory that were impaired either by scopolamine or brain lesions. Similarly, tolcapone treatment modestly improves the accuracy of working memory in abstinent smokers (68). However, tolcapone studies have generally yielded less convincing results (cited in 68). Effect of the human Val/Met polymorphism on various cognitive functions has been amply studied, but the results have not been quite uniform (69). These actions seem to be present only in males and postmenopausal females, both healthy and schizophrenic, suggesting that hormonal environment is a reasonable cause for variability (70). Supporting our results from the rat studies (67), it appears that individuals with a low COMT activity, carrying the Met allele, perform best in tests of declarative memory (71), whereas Val-allele carriers perform worse in tests of recognition memory (28). High (or normal) and low COMT activity mouse models mimicking the human Val158Met polymorphism have been created by introducing a Val allele (51) or either a Val or Met allele (52) into the mouse genome. Moreover, to decrease or increase COMT activity, respectively, the chromosome segment 22q11 has been hemizygously deleted (72) or the human chromosome segment 22q11.2 has been overexpressed (54) in mice. Simpson et al. (50) overexpressed COMT selectively in forebrain neurons using a double transgenic technique. Most but not all of these studies have found such alterations in cognition-related parameters that favor the view of high COMT activity being harmful and low activity being beneficial for working memory. As a whole, based on both mouse and human studies, altered COMT activity and the subsequent change in dopaminergic tone in COMT-dependent brain areas, such as the prefrontal cortex, are associated with cognitive functions. Based on our results, MB-COMT seems to be the critical COMT isoform for cognitive functioning. A shortage of COMT produces significantly increased thickness and neuronal density in the brain cortex but only in males, giving an anatomical background for sex differences that have been observed in several behaviors (73).
The Val158Met polymorphism is associated with schizophrenia as well as aggression, impulsive behavior and working memory deficits (31-33). However, based on large meta-analyses, the COMT polymorphism is not directly associated with schizophrenia in general (74, 75) but rather with the aggressive form of schizophrenia (34, 76, 77). Our present results showing highly increased aggressiveness and reduced prepulse inhibition in male mice deficient of MB-COMT fit very well with these human findings. Increased aggressive behavior has been reported also in heterozygous, but not in homozygous, Comt knock-out mice (37). However, increased impulsiveness was reported even in homozygous Comt knock-out mice by Papaleo et al. (78), who also observed that the acute startle response was enhanced in these mice while prepulse inhibition remained normal. In MB-COMT deficient mice, startle amplitude was not altered in either sex but prepulse inhibition was reduced in male mice. Increased COMT activity induced by different genetic manipulations does generally not affect prepulse inhibition (50, 54), but male Val/Val mice with high COMT activity had lower prepulse inhibition than Met/Met males with low COMT activity, while an opposite correlation was observed in female mice. Interestingly enough, in the resident intruder test in mice, intruders had a significant down-regulation of COMT expression in the hippocampus while residents had higher COMT expression (79).
Studies in various human populations have shown that polymorphisms found in COMT promoter regions are associated with risk of schizophrenia. Nicodemus et al. (80) showed in Caucasians that a haplotype of three COMT SNPs [rs4680 (Val158Met), rs2097603 (located in promoter P2), and rs165599] is associated with risk of schizophrenia. Honea and coworkers (81) found an association between a haplotype of rs4680 and rs2097603 and grey matter volume in hippocampus and dorsolateral prefrontal cortex in mentally and neurologically healthy Caucasians, highlighting the impact of these SNPs on the structure of brain areas relevant for schizophrenia. Wright et al. (82) in turn reported that in South African Xhora population two P2 region SNPs, rs2020917 and rs737865, were associated with risk of schizophrenia as well as increased reporter gene expression. Furthermore, a haplotype comprising the P2 region was found to be associated with the severity of negative symptoms of schizophrenia. Most recently, a copy number variation polymorphism in promoter P1 was suggested to be associated with schizophrenia in a Japanese population (83).
Although a mutation of the initiation codon as such is specific, we want to point out that the methylation status of the CpG (cytosine-phosphate-guanosine) islands in the COMT promoter areas seems to be associated with the COMT Val/Met polymorphism (84, 85). DNA methylation can affect gene transcription via interactions with chromatin proteins and binding of enhancers or transcription factors (85). Altered DNA methylation, either inherited or dictated by epigenetic factors, has been suggested to be associated with schizophrenia (86), drug abuse (87), other mental disorders (88, 89) and even cancers (90). This effects can be synergistic or independent of known genetic polymorphism. Low DNA methylation induces increased expression of both isoforms of COMT protein (86).
Generally, the behavior of MB-COMT deficient mice resembles to some extent that of heterozygous Comt knock-out mice, both of which have enough COMT activity to minimize the need to develop compensatory mechanisms. Notably, the behavioral findings in both types of mice are much more versatile and robust than those reported in homozygous Comt knock-out mice (37, 39, 53, 55, 91-93) or selectively S-COMT deficient mice (39). It remains open why selective lack of MB-COMT has multiple behavioral consequences. Our microdialysis findings indicate that these mice have increased striatal but decreased prefrontal cortical extracellular fluid dopamine levels pointing to the site-specific alterations in dopamine handling that are characteristic to cognitive symptoms in schizophrenia (44).
There are also other factors that regulate dopaminergic tone in the brain. One is the intensity of the retrograde transport of dopamine from the cavernous sinus to dopamine-rich brain areas, adding a peripheral component to dopamine balance that is critical, among others, for schizophrenia, Parkinson's disease and dementia (94). Another, commonly missed regulatory link may be the kynurenic pathway of L-tryptophan metabolism. This pathway may be seriously down-regulated by epigenetic factors like poor diet, lack of group B vitamins or excessive exercise indirectly modulating glutamatergic and dopaminergic functions in the brain. Also, altering levels of 5-hydroxytryptamine, interfering with actions of dopamine, and toxic metabolites of L-tryptophan may play a role in psychiatric disorders (95).
In conclusion, the main finding of our study is that exclusively MB-COMT deficient male mice exhibit schizophrenia-associated behavioral abnormalities such as aggressive behavior and reduced prepulse inhibition. Both male and female mice exhibited normal motor activity, but they were sensitized to acute pain and showed impaired short-term memory, suggesting that the behavioral phenotype was limited neither to schizophrenia-related behavioral endophenotypes nor sex (Table 4). Our findings indicate that, of the two COMT isoforms, MB-COMT is critical for normal behavior and its function in COMT-dependent brain areas cannot be entirely substituted by S-COMT. This is in contrast with S-COMT, the lack of which can be effectively compensated for by MB-COMT even in the peripheral tissues, as shown by our earlier studies on S-COMT deficient mice.
Abbreviations: ANOVA, analysis of variance; COMT, catechol-O-methyltransferase; DHBAc, dihydroxybenzoic acid; L-DOPA, L-3,4-dihydroxyphenylalanine; MB-COMT, membrane-bound catechol-O-methyltransferase; PPI, prepulse inhibition; SAM, S-adenosyl-L-methionine; S-COMT, soluble catechol-O-methyltransferase; S.E.M., standard error of mean; TST, tail suspension test; WT, wild-type
Acknowledgements: The authors warmly thank Ms. Liisa Lappalainen, M.Sc. and Ms. Kati Rautio for their excellent technical assistance.
This study was supported by grants from the Academy of Finland (AT, decision # 257339; PTM, decision # 257898) and the Sigrid Juselius Foundation (PTM).
Conflict of interests: None declared.
REFERENCES
- Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev 1975; 27: 135-206.
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 1999; 51: 593-628.
- Lundstrom K, Salminen M, Jalanko A, Savolainen R, Ulmanen I. Cloning and characterization of human placental catechol-O-methyltransferase cDNA. DNA Cell Biol 1991; 10: 181-189.
- Salminen M, Lundstrom K, Tilgmann C, Savolainen R, Kalkkinen N, Ulmanen I. Molecular cloning and characterization of rat liver catechol-O-methyltransferase. Gene 1990; 93: 241-247.
- Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci USA 1991; 88: 1416-1420.
- Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem 1994; 223: 1049-1059.
- Tenhunen J, Ulmanen I. Production of rat soluble and membrane-bound catechol O-methyltransferase forms from bifunctional mRNAs. Biochem J 1993; 296: 595-600.
- Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I. Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol 1993; 12: 253-263.
- Roth JA. Membrane-bound catechol-O-methyltransferase: a reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol 1992; 120: 1-29.
- Malherbe P, Bertocci B, Caspers P, Zurcher G, Da Prada M. Expression of functional membrane-bound and soluble catechol-O-methyltransferase in Escherichia coli and a mammalian cell line. J Neurochem 1992; 58: 1782-1789.
- Bai HW, Shim JY, Yu J, Zhu BT. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem Res Toxicol 2007; 20: 1409-1425.
- Masuda M, Tsunoda M, Imai K. Low catechol-O-methyltransferase activity in the brain and blood pressure regulation. Biol Pharm Bull 2006; 29: 202-205.
- Myohanen TT, Mannisto PT. Distribution and functions of catechol-O-methyltransferase proteins: do recent findings change the picture? Int Rev Neurobiol 2010; 95: 29-47.
- Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995; 34: 4202-4210.
- Karhunen T, Tilgmann C, Ulmanen I, Panula P. Catechol-O-methyltransferase (COMT) in rat brain: immunoelectron microscopic study with an antiserum against rat recombinant COMT protein. Neurosci Lett 1995; 187: 57-60.
- Ulmanen I, Peranen J, Tenhunen J, et al. Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur J Biochem 1997; 243: 452-459.
- Myohanen TT, Schendzielorz N, Mannisto PT. Distribution of catechol-O-methyltransferase (COMT) proteins and enzymatic activities in wild-type and soluble COMT deficient mice. J Neurochem 2010; 113: 1632-1643.
- Schott BH, Frischknecht R, Debska-Vielhaber G, et al. Membrane-bound catechol-O-methyl transferase in cortical neurons and glial cells is intracellularly oriented. Front Psychiatry 2010; 1: 142.
- Chen J, Song J, Yuan P, et al. Orientation and cellular distribution of membrane-bound catechol-O-methyltransferase in cortical neurons: implications for drug development. J Biol Chem 2011; 286: 34752-34760.
- Nissinen E, Tuominen R, Perhoniemi V, Kaakkola S. Catechol-O-methyltransferase activity in human and rat small intestine. Life Sci 1988; 42: 2609-2614.
- GTEx Analysis Release V6p (dbGaP Accession phs000424.v6.p1). Gene expression of COMT.
- Lundstrom K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I. Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta 1995; 1251: 1-10.
- Kaenmaki M, Tammimaki A, Garcia-Horsman JA, et al. Importance of membrane-bound catechol-O-methyltransferase in L-DOPA metabolism: a pharmacokinetic study in two types of Comt gene modified mice. Br J Pharmacol 2009; 158: 1884-1894.
- Ellingson T, Duddempudi S, Greenberg BD, Hooper D, Eisenhofer G. Determination of differential activities of soluble and membrane-bound catechol-O-methyltransferase in tissues and erythrocytes. J Chromatogr B Biomed Sci Appl 1999; 729: 347-353.
- Aberg E, Fandino-Losada A, Sjoholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord 2011; 129: 158-166.
- Fan JB, Zhang CS, Gu NF, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry 2005; 57: 139-144.
- Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 2006; 314: 1930-1933.
- Bertolino A, Blasi G, Latorre V, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci 2006; 26: 3918-3922.
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 2004; 29: 1943-1961.
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 2006; 60: 141-151.
- Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry 2005; 58: 901-907.
- Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98: 6917-6922.
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 2004; 161: 359-361.
- Bhakta SG, Zhang JP, Malhotra AK. The COMT Met158 allele and violence in schizophrenia: a meta-analysis. Schizophr Res 2012; 140: 192-197.
- Popova NK, Naumenko VS, Tibeikina MA, Kulikov AV. Serotonin transporter, 5-HT1A receptor, and behavior in DBA/2J mice in comparison with four inbred mouse strains. J Neurosci Res 2009; 87: 3649-3657.
- Van Snellenberg JX, Girgis RR, Horga G, et al. mechanisms of working memory impairment in schizophrenia. Biol Psychiatry 2016; 80: 617-126.
- Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95: 9991-9996.
- Haasio K, Huotari M, Nissinen E, Mannisto PT. Tissue histopathology, clinical chemistry and behaviour of adult Comt-gene-disrupted mice. J Appl Toxicol 2003; 23: 213-219.
- Tammimaki A, Kaenmaki M, Kambur O, et al. Effect of S-COMT deficiency on behavior and extracellular brain dopamine concentrations in mice. Psychopharmacology 2010; 211: 389-401.
- Kaenmaki M, Tammimaki A, Myohanen T, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem 2010; 114: 1745-1755.
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press, 1997.
- Huotari M, Gogos JA, Karayiorgou M, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci 2002; 15: 246-256.
- Cotton SM, Lambert M, Schimmelmann BG, et al. Depressive symptoms in first episode schizophrenia spectrum disorder. Schizophr Res 2012; 134: 20-26.
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 2010; 65: 585-596.
- Schendzielorz N. Rysa A, Reenila I, Raasmaja A, Mannisto PT. Complex estrogenic regulation of catechol-O-methyltransferase (COMT) in rats. J Physiol Pharmacol 2011; 62: 483-490.
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 2002; 936: 58-67.
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 2002; 22: 389-395.
- Sesack SR, Hawrylack VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 1998; 18: 2697-2708.
- Yavich L, Forsberg M, Gogos JA, Karayiorgou M, Mannisto PT. Site specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci 2007; 27: 10196-10202.
- Simpson EH, Morud J, Winiger V, et al. Genetic variation in COMT activity impacts learning and dopamine release capacity in the striatum. Learn Mem 2014; 21: 205-214.
- Papaleo F, Crawley JN, Song J, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci 2008; 28: 8709-8723.
- Risbrough V, Ji B, Hauger R, Zhou X. Generation and characterization of humanized mice carrying COMT158 Met/Val alleles. Neuropsychopharmacology 2014; 39: 1823-1832.
- Babovic D, O'Tuathaigh CM, O'Sullivan GJ, et al. Exploratory and habituation phenotype of heterozygous and homozygous COMT knockout mice. Behav Brain Res 2007; 147: 18-27.
- Suzuki G, Harper KM, Hiramoto T, et al. Over-expression of a human chromosome 22q11.2 segment including TXNRD2, COMT and ARVCF developmentally affects incentive learning and working memory in mice. Hum Mol Genet 2009; 18: 3914-3925.
- Kambur O, Talka R, Ansah OB, et al. Inhibitors of catechol-O-methyltransferase sensitize mice to pain. Br J Pharmacol 2010; 161: 1553-1565.
- Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta(2)- and beta(3)-adrenergic receptors. Pain 2007; 128: 199-208.
- Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics 2012; 22: 673-691.
- Kambur O, Mannisto PT, Pusa AM, Kaenmaki M, Kalso EA, Kontinen VK. Nitecapone reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats. Eur J Pain 2011; 15: 732-740.
- Pertovaara A, Wei H, Kalmari J, Ruotsalainen M. Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in the rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp Neurol 2001; 167: 425-434.
- Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs. nociceptive pain modalities. CNS Neurol Disord Drug Targets 2012; 11: 222-235.
- Mannisto PT, Lang A, Rauhala P, Vasar E. Beneficial effects of co-administration of catechol-O-methyltransferase inhibitors and L-dihydroxyphenylalanine in rat models of depression. Eur J Pharmacol 1995; 274: 229-233.
- Moreau JL, Borgulya J, Jenck F, Martin JR. Tolcapone: a potential new antidepressant detected in a novel animal model of depression. Behav Pharmacol 1994; 5: 344-350.
- Fava M, Rosenbaum JF, Kolsky AR, et al. Open study of the catechol-O-methyltransferase inhibitor tolcapone in major depressive disorder. J Clin Psychopharmacol 1999; 19: 329-335.
- Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev 2013; 37: 1597-1610.
- Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog Neurobiol 2010; 92: 112-133.
- Babovic D, O'Tuathaigh CM, O'Connor AM, et al. Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience 2008; 155: 1021-1029.
- Khromova I, Voronina T, Kraineva VA, Zolotov N, Mannisto PT. Effects of selective catechol-O-methyltransferase inhibitors on single-trial passive avoidance retention in male rats. Behav Brain Res 1997; 86: 49-57.
- Ashare RL, Wileyto EP, Ruparel K, et al. Effects of tolcapone on working memory and brain activity in abstinent smokers: a proof-of-concept study. Drug Alcohol Depend 2013; 133: 852-856.
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry 2008; 64: 137-144.
- Papaleo F, Sannino S, Piras F, Spalletta G. Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. Eur Neuropsychopharmacol 2015; 25: 2349-2363.
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet 2004; 34: 533-539.
- Kimoto S, Muraki K, Toritsuka M, et al. Selective overexpression of Comt in prefrontal cortex rescues schizophrenia-like phenotypes in a mouse model of 22q11 deletion syndrome. Transl Psychiatry 2012; 2: e146. doi: 10.1038/tp.2012.70
- Sannino S, Gozzi A, Cerasa A, et al. COMT genetic reduction produces sexually divergent effects on cortical anatomy and working memory in mice and humans. Cereb Cortex 2015; 25: 2529-2541.
- Ira E, Zanoni M, Ruggeri M, Dazzan P, Tosato S. COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J Psychiatry Neurosci 2013; 38: 366-380.
- Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry 2005; 10: 765-770.
- Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull 2011; 37: 913-920.
- Volavka J, Bilder R, Nolan K. Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann NY Acad Sci 2004; 1036: 393-398.
- Papaleo F, Erickson L, Liu G, Chen J, Weinberger DR. Effects of sex and COMT genotype on environmentally modulated cognitive control in mice. Proc Natl Acad Sci USA 2012; 109: 20160-20165.
- Ginsberg SD, Che S, Hashim A, et al. Differential regulation of catechol-O-methyltransferase expression in a mouse model of aggression. Brain Struct Funct 2011; 216: 347-356.
- Nicodemus KK, Kolachana BS, Vakkalanka R, et al. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet 2007; 120: 889-906.
- Honea R, Verchinski BA, Pezawas L, et al. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage 2009; 45: 44-51.
- Wright GE, Niehaus DJ, van der Merwe L, et al. Association of MB-COMT polymorphisms with schizophrenia-susceptibility and symptom severity in an African cohort. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 163-169.
- Higashiyama R, Ohnuma T, Takebayashi Y, et al. Association of copy number polymorphisms at the promoter and translated region of COMT with Japanese patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2016; 171B: 447-457.
- Swift-Scanlan T, Smith CT, Bardowell SA, Boettiger CA. Comprehensive interrogation of CpG island methylation in the gene encoding COMT, a key estrogen and catecholamine regulator. BMC Med Genomics 2014; 7: 5.
- Thomson JP, Skene PJ, Selfridge J, et al. CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 2010; 464: 1082-1086.
- Nohesara S, Ghadirivasfi M, Mostafavi S, et al. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J Psychiatr Res 2011; 45: 1432-1438.
- van der Knaap LJ, Schaefer JM, Franken IH, Verhulst FC, Oort FV, Riese H. Catechol-O-methyltransferase gene methylation and substance use in adolescents: the TRAILS study. Genes Brain Behav 2014; 13: 618-625.
- Hatzimanolis A, Vitoratou S, Mandelli L, et al. Potential role of membrane-bound COMT gene polymorphisms in female depression vulnerability. J Affect Disord 2013; 148: 316-322.
- Norrholm SD, Jovanovic T, Smith AK, et al. Differential genetic and epigenetic regulation of catechol-O-methyltransferase is associated with impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci 2013; 7: 30. doi: 10.3389/fnbeh.2013.00030
- Sasaki M, Kaneuchi M, Sakuragi N, Dahiya R. Multiple promoters of catechol-O-methyltransferase gene are selectively inactivated by CpG hypermethylation in endometrial cancer. Cancer Res 2003; 63: 3101-3106.
- Desbonnet L, Tighe O, Karayiorgou M, Gogos JA, Waddington JL, O'Tuathaigh CM. Physiological and behavioural responsivity to stress and anxiogenic stimuli in COMT-deficient mice. Behav Brain Res 2012; 228: 351-358.
- Kambur O, Mannisto PT, Viljakka K, et al. Stress-induced analgesia and morphine responses are changed in catechol-O-methyltransferase-deficient male mice. Basic Clin Pharmacol Toxicol 2008; 103: 367-373.
- O'Tuathaigh CM, Babovic D, O'Sullivan GJ, et al. Phenotypic characterization of spatial cognition and social behavior in mice with 'knockout' of the schizophrenia risk gene neuregulin 1. Neuroscience 2007; 147: 18-27.
- Krzymowski T, Stefanczyk-Krzymowska S. New facts and the concept of physiological regulation of the dopaminergic system function and its disorders. J Physiol Pharmacol 2015; 66: 331-341.
- Majewski M, Kozlowska A, Thoene M, Lepiarczyk E, Grzegorzewski WJ. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J Physiol Pharmacol 2016; 67: 3-19.
A c c e p t e d : December 30, 2016