SPATIAL DIFFERENCES OF MATRIX METALLOPROTEINASE-2 AND MATRIX METALLOPROTEINASE-9 WITHIN ABDOMINAL AORTIC ANEURYSM WALL
AND INTRALUMINAL THROMBUS
INTRODUCTION
Formation of an abdominal aortic aneurysm (AAA) is a complex process involving aortic wall degradation (1). Many enzymes take part in this processes, including neutrophil elastase, serine proteases and matrix metalloproteinases (MMPs): mostly MMP-2 and MMP-9 (2). MMPs may contribute to structural changes and can degrade most components of the extracellular matrix of the vessel wall, leading to an imbalance between extracellular matrix (ECM) synthesis and degradation (3, 4). MMP-2 and MMP-9 have been detected, not only in AAA patient plasma, but also in the intraluminal thrombus (ILT), the aneurysmal wall and in the interface liquid between the thrombus and the wall (2, 5). Thompson et al. (6) have demonstrated increased activity of MMP-9 in aortic aneurysm tissue compared to normal or athero-occlusive aortas. Reilly et al. (7) and Katsuda et al. (8) provided evidence that MMP-9 is capable of degrading elastic lamellae in vitro. Increased expression of MMP-2 has also been observed in the AAA tissue (9, 10). Activity of MMPs in the wall of the vessel chiefly depends on the balance between their activators and the tissue inhibitors (TIMP-1 and TIMP-2) of metalloproteinases. An imbalance may result in uncontrolled changes in the extracellular matrix which may lead to a widening of the aneurysm’s vessel and possibly a future abdominal aortic aneurism rupture (11).
AAAs often contain ILT, speculated to be the main source of proteolytic compounds that influence aortic wall degradation (12-14). Although it has been suggested that the presence of an ILT is related to AAA rupture, the role of a parietal thrombus in the pathogenesis of an abdominal aortic aneurism is not clear. Recent studies have found that ILT thickness is associated with apoptosis of vascular smooth muscle cells, and elastin degradation correlates positively with MMP-2 and active MMP-9 in the adjacent AAA wall (15, 16). Proteolytic activity is significantly higher in the layer of the parietal thrombus that has immediate contact with blood flow, in comparison with the layer of thrombus at the AAA wall site (5, 17).
The morphology of AAAs is asymmetrical. An intraluminal thrombus increases evenly in the first stage of AAA formation, but over the course of time thrombi inside the AAA sac can be seen to have variable thickness. Presumably this is caused by amplification of the activity of coagulation processes and secondary fibrinolysis in thin parts of the thrombus. All of the above supports the possibility of the presence of regional variation in the AAA wall and ILT composition (18, 19). It has been suggested that the AAA wall strength varies not only between different individuals but also within the same AAA (20). Moreover Wiernicki et al. (21) found that elastase activity, active MMP-9 and MMP-9/TIMP-1 ratio were higher in the wall covered by a thin thrombus (< 10 mm) as compared to a thick thrombus (> 25 mm). However no proteolytic activity was measured within the intraluminal thrombus. It was suggested that a thicker ILT can act as a barrier for neutrophils and other inflammatory cells to prevent them reaching the wall of the AAA sac from the circulating blood; and thin mural thrombi (up to 1 cm) enable penetration of blood components to the wall. This is possible because of the presence of a tubule system connecting blood flow with consecutive layers of a parietal thrombus (22).
Early studies focused only on the characterization and localization of MMPs in the aneurysmal wall or ILT. They examined fragments of aneurysm wall covered with a thrombus with fragments of the wall directly adjacent to flowing blood; or noticed the presence or lack of MMPs in the aneurysm sac. There are only a few reports which have evaluated a parietal thrombus in terms of variation in parameters resulting from wall thickness in a sac of the same aneurysm. Studies have shown that an ILT occurring in an AAA often has variable thickness associated with variation in parameters which take part in proteolytic degradation (15, 17, 21). It has been suggested that ILT thickness is related to the risk of AAA rupture and may be a new prognostic factor.
Our aim was to investigate local differences in MMP-2, MMP-9 and TIMP-1 in thrombus segments with different thicknesses, and in adjacent segments of the aneurysm sac walls. We hypothesized that thin segments of ILT and adjacent AAA walls would differ in these parameters from thick segments. Comparison of selected metalloprotease concentrations with inhibitor concentration in parietal thrombi segments and in adjacent walls would allow evaluation of the role of thrombi in the process of aneurism rupture.
In the presented study the protein concentrations of MMP-2/-9 and TIMP-1 were measured and the MMP-9/TIMP-1 ratio calculated as an estimate of MMP-9 activity according to Wiernicki et al. (21). As suggested by the results of several studies, MMP-9/TIMP-1 ratios give a reasonable index of net MMP-9 activity because TIMP-1 is a major inhibitor of these enzymes. The MMP-9/TIMP-1 ratios can be used as a surrogate for an activity measurement of MMP-9 according to Goldberg et al. (23) and the MMP/TIMP balance reflects the net proteolytic activity present in several physiological processes.
MATERIALS AND METHODS
Study participants
Thirty six patients (27 men and 9 women with mean age of 71 years, ranging from 56 to 84 years) that underwent AAA repair at the Department of Vascular Surgery and Angiology at the Pomeranian Medical University in Szczecin, Poland, were entered into the study. Aneurysm wall and ILT tissues were harvested during surgery for analysis. Patients with co-existing renal dysfunction, liver dysfunction and haematological disorders were excluded. Inclusion criteria included a pre-surgery CT scan which revealed the presence of an intraluminal thrombus with a minimum width of 25 mm at the largest width and maximum width of 10 mm at the thinnest width and with an eccentric blood flow lumen.
The study conformed to the Declaration of Helsinki and was approved by the the Ethics Committee (KB-0080/18/09) of the Pomeranian Medical University. Informed consent for collection of samples was acquired prior to surgery.
Imaging
The largest AAA diameter in all patients was measured using computed tomography (CT). An abdominal aortic aneurysm was defined as an aorta with an infrarenal aortic diameter greater than 30 mm. Aortic lumina were measured at the level of maximal dilatation on the CT scan and the ILT thickness was calculated as the difference between maximal aortic lumen diameter and diameter of the lumen at the ILT.
Tissue samples
The collected aortic biopsies were from two different regions of the aneurysm, two segments of the aneurismal ILT were examined: a thick section with a thickness ł 25 mm (A1) and a thin section with a thickness of ≤ 10 mm (B1) (dimensions following 21); plus each of two sections of the wall: one section (A) lying directly under the thick part of the ILT and one section (B) under the thin part of the ILT (Fig. 1). Tissue samples were obtained from the middle third of the longitudinal dimension of the ILT, close to the spot of the AAA sac’s maximum dilatation at the same level. Biopsy samples were retrieved from the anterior or posterior-lateral position at the aneurysm wall, after the clamping of the aorta. A laser micrometer was used to measure the thickness of the thrombus from each site before excision of specimens. Due to potential risk accompanying the surgery, samples were not collected from the posterior wall. After harvesting, the samples were placed in ice and transported to the laboratory immediately. Samples were weighed, divided into relevant sections, and stored at –80°C until analysis.
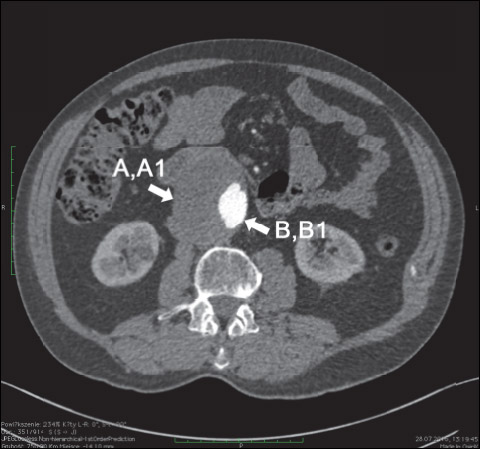 |
Fig. 1. Computed tomography image of representative abdominal aortic aneurysm with eccentric, perimural blood flow channel in the intraluminal thrombus. Arrows indicate areas of biopsy: A1 - a thick section with a thickness ≥ 25 mm, B1 - a thin section with a thickness of ≤ 10 mm plus each of two sections of the wall: one section (A) lying directly under the thick part of the ILT and one section (B) under the thin part of the ILT. |
Protein extraction
Wall or thrombus tissues (0.15 – 0.40 g wet mass) were homogenized to powder consistency with a metal homogenizer by immersing in liquid nitrogen and then homogenizing in lysis buffer containing 150 mM NaCl, 10 mmol/L Tris-HCL pH 7.4, 0.25% Triton X-100. Protease inhibitors (complete-mini EDTA-free; from ROCHE) were added to the buffer prior to protein extraction. Samples were subsequently centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was collected and stored at –80°C until use. Each extract was analyzed for total protein concentration (using the Quick StartTM Bradford Protein Assay; Bio-Rad), according to the manufacturer’s guidelines.
Measurements
Sandwich enzyme-linked immunosorbent assay (ELISA) kits were used for the quantitative determination of MMP-2, MMP-9 and TIMP-1 concentration in accordance with the manufacturer’s instructions (Quantikine® Human MMP-9 and Quantikine® Human MMP-2 kits were purchased from R&D System Europe, Lts, Germany; Human TIMP-1 ELISA Kit was obtained from Sigma-Aldrich). The Human MMP-9 immunoassay measured both the human pro-MMP-9 (92 kDa) and the active form of MMP-9 (82 kDa). The Human MMP-2 immunoassay measured human pro-MMP-2 (72 kDa); the TIMP-1 immunoassay measured human free-TIMP-1 protein. Absorption was read at 450 nm (EnVision absorbance reader). The final concentrations were expressed as nanogram of target protein per milligram of total protein extract. Dilutions were made for some samples using kit diluent. Assays were performed in duplicate i.e. two assays for each of the four different tissue pieces from each patient.
Statistical analysis
One way ANOVA with repeated measures was used to examine differences between groups, followed by Tukey’s post-hoc tests. The relationship between quantitative variables was analyzed using Pearson’s correlation and multiple regression. All tests were conducted using analysis software Statistica (StatSoft, Inc., version 10, www.statsoft.com). P value < 0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics in subjects with an abdominal aortic aneurysm (AAA) are presented in Table 1. The results of proteolytic parameters are shown graphically in Fig. 2. MMP-9 and the MMP-9/TIMP-1 ratio, but not MMP-2, were elevated in thin thrombus sections B1, compared with thick thrombus sections A1 (113 ± 118 versus 63.0 ± 61.2, P = 0.004; 18.9 ± 27.8 versus 9.1 ± 10.6, P = 0.017, respectively) or wall sections A (113 ± 118 versus 31.7 ± 30.0, P < 0.001; 18.9 ± 27.8 versus 2.5 ± 2.2, P < 0.001, respectively) or B (113 ± 118 versus 39.5 ± 41.5, P < 0.001; 18.9 ± 27.8 versus 3.6 ± 4.5, P < 0.001). MMP-2 and TIMP-1 were higher in wall segments A, underlying thick ILT, compared with thrombus sections A1 (18.4 ± 8.5 versus 7.2 ± 7.6, P < 0.001; 14.3 ± 5.9 versus 8.5 ± 5.4, P < .001, respectively) and B1 (18.4 ± 8.5 versus 5.2 ± 2.9, P < 0.001; 14.3 ± 5.9 versus 8.9 ± 4.9, P < 0.001, respectively) as well as in wall segments B, underlying thin ILT, compared with thrombus sections A1 (15.9 ± 7.3 versus 7.2 ± 7.6, P < 0.001; 13.0 ± 5.0 versus 8.5 ± 5.4, P < 0.001, respectively) and B1 (15.9 ± 7.3 versus 5.2 ± 2.9, P < 0.001; 13.0 ± 5.0 versus 8.9 ± 4.9, P = 0.003, respectively).
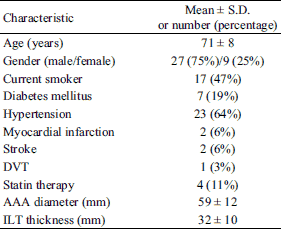
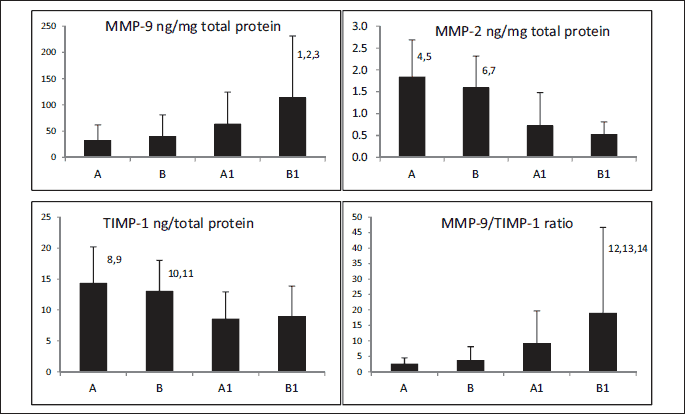
Correlations between pairs of parameters measured at sites A, B, A1 and B1 are shown in Table 2. A strong correlation between MMP-9 and TIMP-1 was observed only in thick thrombus sections (r = 0.35, P = 0.039). This contrasts with the correlation between MMP-2 and TIMP-1 which was found at all sites (r1 = 0.52, P = 0.001, r2 = 0.65, P < 0.001, r3 = 0.55, P < 0.001, r4 = 0.54, P < 0.001, respectively for A, B, A1, B1).
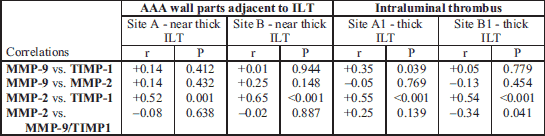
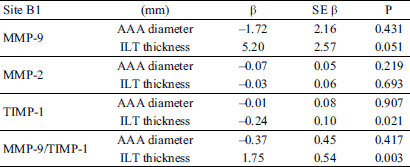
Multivariate regression of proteolytic parameters with AAA diameter and ILT thickness as independent variables showed association only in the thin ILT (B1) (Table 3). Other sites showed no statistically significant correlations. Thin ILT thickness correlated significantly with TIMP-1 (β = –0.24, P = 0.021) and MMP-9/TIMP-1 ratio (β = 1.75, P = 0.003).
DISCUSSION
Our study showed that concentrations of MMP-9 and MMP-9/total TIMP-1 ratio (a relative index of proteolytic state) were strongly raised within the thin thrombus segments compared to samples from thick thrombus segments and underlying walls of the AAA. We did not find a difference in TIMP-1 concentrations between thin and thick ILT sections but aneurysmal walls showed higher TIMP-1 levels than in the ILT. These observations are in agreement with a study by Wiernicki et al. (21) who studied sites of the adjacent walls (but not sites from the thrombus itself). So far there have not been any publications regarding the differences in concentration or activity of MMPs at different sites of the thrombus with different thicknesses (plus adjacent aortic walls) in the sac of one aneurysm. In another study Wiernicki et al. (24) have also shown that plasma concentrations of MMP-9 were relevantly higher in patients with ILTs with maximum thickness < 9 mm compared with patients with thicker ILTs. This may suggest that the presence of thin parietal thrombus enhances degradation of the aortic wall and subsequently leads to release of higher amount of proteolytic and inflammation markers into the blood stream. In our previous study we have shown higher plasma concentrations of tissue plasminogen activator (t-PA) and D-dimers in patients with AAAs and a strong negative correlation between t-PA plasma level and ILT thickness. This suggested that thrombotic/fibrinolysis imbalance may also favour accelerated formation of intraluminal thrombi and possibly aneurysm progression (25). This implies that a segment of thin ILT is probably more permeable to proteolytic markers than a thick segment. This cellular penetration is thought to be limited to one centimetre from the luminal surface (22). In our study sections of thrombus recognised as thin were subjected to protein assay if they did not exceed 1 cm in thickness, and ILT segments were described as thick with a thickness greater than 2.5 cm thick (21): levels of MMP-9 were found to be significantly different from the thin and thick sections of ILT. Presumably intensified proteolytic degradation as well as a higher activity of coagulation and secondary fibrinolysis occurred in the thin segments of parietal thrombi. These processes may possibly be induced by factors originating from serum, aneurysm sac wall or parietal thrombus and they are limited by ILT thickness. On the other hand Kazi et al. (26) and Vorp et al. (27) found that the thrombus-covered wall is thinner and thick ILT could be the source of proteolytic enzymes influencing the wall beneath. However histology demonstrated a preferential accumulation of neutrophils and other cells responsible for MMP production at the luminal pole of the mural thrombus and their colocalization with MMP-9 staining. Therefore, previous data (22, 28, 29) suggest that MMP-9 could be stored and released by polymorphonuclear leukocytes (PMNs) entrapped in the thrombus on its luminal side. Adolph et al. (22) have shown that the aneurysmal thrombus is active. This activity is associated with a leukocyte gradient from the luminal to the abluminal pole of the thrombus. Folkesson et al. (17) have also shown that, though thick multilayer thrombi contained significant amounts of proteases, the enzymatic activity was shown to be absent or very low at the abluminal face of the ILT. But they examined in their study only thick ILT with multiple layers and their findings indicated that the thick multilayer thrombi could not directly affect the wall beneath. The results of Folkesson et al. (17) are compatible with our results, especially considering that here observations concerning the thin ILT are of importance.
Activity of TIMP-1 has previously been associated with pro-MMP-9 activation blocking, whereas TIMP-2 suppresses pro-MMP-2 activation. It has been observed that homeostatic imbalance between MMP-9 activity and its tissue inhibitor (TIMP-1) occurs in abdominal aortic aneurysms (30, 31). Increased MMP-9/TIMP-1 ratio has been observed in aneurysm tissues in comparison to control group tissues (32) and increases in this ratio in wall segments covered with thin thrombus in comparison to the wall segments covered with thick thrombus (21), and this was also shown by our study. The inhibition of MMP-9 by TIMP-1 plays an important role in AAA pathogenesis (33). Furthermore, in the present study there was a significant correlation between the level of MMP-9 and TIMP-1 only in thick sections of ILT. In contrast the levels of MMP-9 and TIMP-1 showed no correlation in the remaining fragments of AAA, which possibly suggests an imbalance between MMP-9 and its naturally occurring inhibitor in the thin segments of an AAA. TIMPs appear to be distributed widely in tissues and are expressed by many different non-inflammatory, transformed, and inflammatory cells. Additionally, alpha granules from human platelets, which are released into circulation after platelet activation, are a rich source of this inhibitor. An ILT is a rich source of platelets and therefore is a potentially rich source of TIMP production. Possibly MMP-9 and TIMP-1 move freely from thin thrombus to the wall, but the amount of inhibitor is not sufficient to inhibit MMP-9 activity, which is highest in thin ILT segments. Additionally the expression of MMPs has been associated with cardiovascular disease via the activation of the extracellular MMP inducer (EMMPRIN, CD147), which regulates the expression of several MMPs, including MMP-2, MMP-9. Huet et al. (34) recently reported that EMMPRIN gene silencing gave altered gene expression of MMPs and their inhibitors, leading to aberrant tissue extracellular matrix remodelling in mouse and rat myocardium during aging. EMMPRIN also plays a key role in the pathogenesis of aortic aneurysmal diseases. The up-regulation of EMMPRIN as well as TIMPs in aortic aneurysms may have consequences via their effect on MMPs production. It is suggested that EMMPRIN inhibition might reduce the tendency of the aorta to rupture, but the specific role of this inducer in AAA needs to be explored further. In the present study significantly higher concentration of MMP-2 in the AAA walls, in comparison to thrombi, was observed, probably because of the site of production of this metalloprotease. However, there were no significant differences in concentration between the thin and the thick fragments of the thrombus and the adjacent walls. In the wall of an AAA, smooth muscle cells, rather than the inflammatory cells, are the main source of MMP-2. In analysed AAAs, levels of MMP-2 have been found to be lower than the content of MMP-9, which is the most abundant protease in the AAA tissue (35, 36), which we have confirmed in this study.
Interestingly, statistically significant correlations of AAA diameter or the ILT thickness with parameters levels were found only in thin thrombus sections in our study. In the presented work there was a significant positive correlation between MMP-9/TIMP-1 ratio and ILT thickness and a significant negative correlation between TIMP-1 and ILT thickness, all measured in thin ILT sections. These results are in agreement with Khan et al. (15), who observed a positive correlation between the level of the active form of MMP-9 and the thickness of the parietal thrombus. This may be because of the presence and activity of neutrophil gelatinase-associated lipocalin (NGAL) within ILTs, which has been suggested to influence the activity of MMP-9 by binding covalently to it (15, 37). It has also been suggested that a better indicator of possible abdominal aortic aneurysm rupture could be the increase in ILT thickness rather than AAA maximal diameter. In our study the thin segments of the ILTs showed the largest concentration of MMP-9 and in the wall adjacent to thin ILT segments the MMP-9 concentration was higher than in the wall beneath thick thrombus segments (but not statistically significantly). Nowadays, the risk of AAA rupture is estimated not only by using its diameter and yearly growth (38), but also by the vessel walls’ mechanical properties, and the presence of a thrombus inside the aneurysm sac is also taken into consideration (39). It has been observed that the speed of growth of the thrombus may be a better prodrome of AAA rupture than the growth of the aneurysm’s maximal diameter and the risk of an aneurysm’s rupture may also depend on the morphology of the thrombus filling it. Moreover, aneurysm diameter positively correlates with increase in the volume of the thrombus, as we have shown in our earlier work (25), and inside ruptured aneurysms thrombi of a larger volume were observed in comparison to those inside unruptured aneurysms (40). Possibly the ILT widens inside the aneurism’s wall simultaneously with the AAA’s growth in diameter. On the other hand, it has been shown that ruptured and unruptured aneurysms, selected according to an identical diameter, do not show significant differences in thrombus size (41). Despite these inconsistent observations, the adverse influence of the thrombus is undeniable and well documented in the literature, and AAA walls covered with a thrombus show significant differences from the free walls. However, an ILT is an active formation and therefore, because of the dynamics taking place, it is suggested that its role is more complex than providing a simple correlation between ILT thickness and the diameter of aneurysm. In addition, aneurysm rupture is a localized process, predominantly occurring in the posterolateral part of the AAA, as reported by autopsy studies (42).
Taken together, the above data shows that thick ILT could not directly affect the AAA wall by providing active proteases. However, the presence of thin thrombus segments in the AAA sac was characterised by a higher proteolytic activity. In the light of current knowledge this possibly could be used as a potential indicator of a rupture site. A higher activity of MMP-9 and MMP-8 has been observed in material collected from the site of an aneurysm’s rupture and therefore it seems probable that processes occurring in thin thrombus segments have a negative influence on the underlying wall (16, 28). Wiernicki et al. (21) suggested that the thickness of the parietal thrombus is a significant factor influencing processes directly connected with the growth and earlier rupture of the wall adjacent to thin ILT. Accordingly, the evaluation of proteolytic activity inside the AAA wall could be a good prognostic factor indicating the wall’s susceptibility to rupture.
Limitations of the study
It should be noted that methods of thrombus size measurement vary across studies. In the study presented here, thrombus load was represented by the thickness of ILT taken at the level of maximal aortic dilatation on a 2D computed tomography scan. It is quite possible that the thrombus thickness at the level of maximum AAA diameter may not correlate with total volume of mural thrombus.
Another limitation results from the fact that the 92 kDa Pro- and 82 kDa active form of MMP-9 were measured together, rather than only its active form, and concentration, rather than activity. According to Goldberg et al. (23) the MMP-9/TIMP-1 ratio can be used as a surrogate for the activity of MMP-9, but even so direct activity measurements might be possible. Moreover, studies should be expanded with an evaluation of MMP concentration in the plasma and the wall without adjacent thrombus and this would allow a deeper insight into processes of proteolytic degradation and AAA formation.
Lastly, results from small studies using human specimens need to be interpreted carefully because of possibly marked regional differences. In particular, the smoking pack-year data need to be collected for future studies, as smoking is one of the most important risk factors for AAA, and could be used as a covariate.
In conclusion, our study has demonstrated that the part of an aneurysm associated with thin thrombus segment is characterized by a higher concentration of MMP-9 and a higher MMP-9/TIMP-1 ratio in comparison to parts associated with thick thrombus segments. This may imply that collection of MMP-9 in these areas is on the one hand probably connected to its source, neutrophils and macrophages, and on the other hand thin thrombus enables MMP-9 and morphotic blood elements and plasma factors to move from the aneurysm lumen into and through the thrombus and to the adjacent wall and this site may be considered as potentially exposed to rupture. The increase in MMP-9 concentration in the thin thrombus segments may confirm the previously suggested dependence of aneurysm rupture on thrombus thickness. Furthermore, the sites used for collecting specimen samples for research is extremely important, because there can be significant differences in metalloprotease concentration depending on the thrombus thickness and the wall it covers. If specimens are taken from the ventral AAA wall for examination, these samples do not necessarily represent the entire AAA wall.
Acknowledgements: This work was supported by FSN-465-06/15 Pomeranian Medical University.
Conflict of interests: None declared.
REFERENCES
- Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation 1998; 98: 193-195.
- Fontaine V, Jacob MP, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol 2002; 161: 1701-1710.
- Ziora D, Dworniczak S, Kozielski J. Induced sputum metalloproteinases and their inhibitors in relation to exhaled nitrogen oxide and sputum nitric oxides and other inflammatory cytokines in patients with chronic obstructive pulmonary disease. J Physiol Pharmacol 2008; 59 (Suppl. 6): 809-817.
- Petersen E, Wagberg F, Angquist KA. Proteolysis of the abdominal aortic aneurysm wall and the association with rupture. Eur J Vasc Endovasc Surg 2002; 23: 153-157.
- Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapiere CM. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg 1996; 24: 127-133.
- Thompson RW, Holmes DR, Mertens RA et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 1995; 96: 318-326.
- Reilly JM, Brophy CM, Tilson MD. Characterization of an elastase from aneurysmal aorta which degrades intact aortic elastin. Ann Vasc Surg 1992; 6: 499-502.
- Katsuda S, Okada Y, Okada Y, Imai K, Nakanishi I. Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am J Pathol 1994; 145: 1208-1218.
- Davis V, Persidskaia R, Baca-Regen L et al. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 1998; 18: 1625-1633.
- Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001; 104: 304-309.
- Wilson WR, Anderton M, Schwalbe EC et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation 2006; 113: 438-445.
- Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann NY Acad Sci 2006; 1085: 133-138.
- Ailawadi G, Eliason JL, Upchurch GR. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg 2003; 38: 584-588.
- Coutard M, Touat Z, Houard X, Leclercq A, Michel JB. Thrombus versus wall biological activities in experimental aortic aneurysms. J Vasc Res 2010; 47: 355-366.
- Khan JA, Abdul Rahman MN, Mazari FA, et al. Intraluminal thrombus has a selective influence on matrix metalloproteinases and their inhibitors (tissue inhibitors of matrix metalloproteinases) in the wall of abdominal aortic aneurysms. Ann Vasc Surg 2012; 26: 322-329.
- Koole D, Zandvoort HJ, Schoneveld A, et al. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J Vasc Surg 2013; 57: 77-83.
- Folkesson M, Silveira A, Eriksson P, Swedenborg J. Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis 2011; 218: 294-299.
- Reeps C, Pelisek J, Seidl S, et al. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology 2009; 76: 243-252.
- Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg 2008; 48: 417-423.
- McGloughlin TM, Doyle BJ. New approaches to abdominal aortic aneurysm rupture risk assessment: engineering insights with clinical gain. Arterioscler Thromb Vasc Biol 2010; 30: 1687-1694.
- Wiernicki I, Stachowska E, Safranow K, et al. Enhanced matrix-degrading proteolytic activity within the thin thrombus-covered wall of human abdominal aortic aneurysms. Atherosclerosis 2010; 212: 161-165.
- Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg 1997; 25: 916-926.
- Goldberg GI, Strongin A, Collier IE, et al. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of the metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem 1992; 267: 4583-4591.
- Wiernicki I, Millo B, Safranow K, Gorecka-Szyld B, Gutowski P. MMP-9, homocysteine and CRP circulating levels are associated with intraluminal thrombus thickness of abdominal aortic aneurysms: new implication of the old biomarkers. Dis Markers 2011; 31: 67-74.
- Siennicka A, Drozdzynska M, Chelstowski K, Cnotliwy M, Jastrzebska M. Haemostatic factors and intraluminal thrombus thickness in abdominal aortic aneurysm. Is secondary fibrinolysis relevant? J Physiol Pharmacol 2013; 64: 321-330.
- Kazi M, Thyberg J, Religa P, et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg 2003; 38: 1283-1292.
- Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol 2005; 25: 1558-1566.
- Vorp DA, Lee PC, Wang DH, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 2001; 34: 291-299.
- Houard X, Touat Z, Ollivier V, et al. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc Res 2009; 82: 532-541.
- Annabi B, Shedid D, Ghosn P, et al. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg 2002; 35: 539-546.
- van Laake LW, Vainas T, Dammers R, Kitslaar PJ, Hoeks AP, Schurink GW. Systemic dilation diathesis in patients with abdominal aortic aneurysms: a role for matrix metalloproteinase-9. Eur J Vasc Endovasc Surg 2005; 29: 371-377.
- Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2004; 78: 2106-2111.
- Nishimura K, Ikebuchi M, Kanaoka Y, et al. Relationships between matrix metalloproteinases and tissue inhibitor of metalloproteinases in the wall of abdominal aortic aneurysms. Int Angiol 2003; 22: 229-238.
- Huet E, Gabison E, Vallee B, et al. Deletion of extracellular matrix metalloproteinase inducer/Cd147 induces altered cardiac extracellular matrix remodeling in aging mice. J Physiol Pharmacol 2015; 66: 355-366.
- McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation 1997; 96: 2228-2232.
- Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann NY Acad Sci 1996; 800: 157-174.
- Kiczak L, Tomaszek A, Bania J, et al. Matrix metalloproteinase 9/neutrophil gelatinase associated lipocalin/tissue inhibitor of metallopteinases type 1 complexes are localized within cardiomyocytes and serve as a reservoir of active metalloproteinase in porcine female myocardium. J Physiol Pharmacol 2014; 65: 365-375.
- Hans SS, Jareunpoon O, Balasubramaniam M, Zelenock GB. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J Vasc Surg 2005; 41: 584-588.
- Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 2003; 37: 724-732.
- Raghavan ML, Vorp DA. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech 2000; 33: 475-482.
- Golledge J, Iyer V, Jenkins J, Bradshaw B, Cronin O, Walker PJ. Thrombus volume is similar in patients with ruptured and intact abdominal aortic aneurysms. J Vasc Surg 2014; 59: 315-320.
- Simao da Silva E, Rodrigues AJ, Magalhaes Castro de Tolosa E, Rodrigues CJ, Villas Boas do Prado G, Nakamoto JC. Morphology and diameter of infrarenal aortic aneurysms: a prospective autopsy study. Cardiovasc Surg 2000; 8: 526-532.
A c c e p t e d : December 27, 2016