IN VITRO MODEL OF VASCULO-ANGIOGENESIS: DEMONSTRATION THAT BONE MARROW DERIVED ENDOTHELIAL PROGENITOR CELLS FORM NEW HYBRID CAPILLARY BLOOD VESSELS JOINTLY WITH GASTRIC ENDOTHELIAL CELLS
INTRODUCTION
Neovascularization, the formation of new blood vessels, is essential for re-establishment of a microvascular network following injury and is therefore critical for the delivery of oxygen and nutrients to the healing site and tissue injury healing (1-7). The process of embryonic neovascularization has been thoroughly described (8, 9). During embryonic development blood vessels are formed from bone marrow-derived stem cells (BMD-SCs) and endothelial progenitor cells (BMD-EPCs) by the process of vasculogenesis that involves differentiation of angioblasts from mesoderm and the formation of primitive blood vessels by these cells (8, 9). This vascular plexus forms new capillaries by sprouting or non-sprouting angiogenesis through a series of morphogenic processes regulated by members of the fibroblast growth factor family (9) and vascular endothelial growth factor (VEGF) (10, 11).
Unlike embryonic vascularization, the process of postnatal vascularization is not fully understood and thus the concept is continuously evolving (12-14). Previously, the prevailing theory was that postnatal neovascularization in adult tissues occurs solely through angiogenesis - the sprouting of pre-existing endothelial cells (ECs) from the area bordering injury, which forms new blood vessels in regenerating tissues (1-3, 6, 7). Asahara et al. and others challenged this theory and showed that neovascularization in some tissues (e.g., infarcted heart or ischemic limbs) can be accomplished by postnatal vasculogenesis - the homing of BMD-EPCs to injured and/or diseased tissues resulting in the formation of new blood vessels (6, 7, 15-19). In some tissues, vasculogenesis is initiated by the action of stromal derived factor 1 (SDF-1) on BMD-EPCs through its CXCR4 receptor; SDF-1-CXCR4 signaling mediates activation, mobilization, homing and retention of BMD-EPCs at sites of injury (20-23). Under conditions of stress or injury, SDF-1 is locally released within the bone marrow and the elevated levels of SDF-1 in the circulation then facilitate the mobilization and homing of BMD-SCs and BMD-EPCs (20).
Despite recent progress, the potential role and contributions of BMD-EPCs to vascular regeneration and whether BMD-EPCs can form new capillary blood vessels either independently or jointly with existing ECs are not fully known. Our recent study using gastric ulcer (GU) tissue specimens from humans and rats demonstrated that BMD-EPCs are incorporated into capillary blood vessels of GU granulation tissue side by side with ECs, thus forming new blood vessels comprised of both cell types (24).
The aim of this study was to establish an in vitro model of vasculogenesis/angiogenesis by co-culture of BMD-EPCs and gastric endothelial cells (GECs) on Matrigel and examine in this model direct interactions of these cells and the mechanisms involved. Our previous studies established a technique for isolating GECs, which we used to examine the cellular and molecular events and mechanisms of angiogenesis and its regulation, e.g. inhibition by the nonsteroidal anti-inflammatory drugs (NSAIDs), effect of aging, VEGF, and nerve growth factor (NGF), and the role of hypoxia, nuclear transport by importin, serum response factor (SRF) etc. (25-30).
The main hypothesis of the present study was that BMD-EPCs can form new capillary blood vessels alone as well as jointly when co-cultured with GECs; and, that the resulting vasculogenesis/angiogenesis combination is an integral component of new vessel formation, which can be demonstrated in vitro. We determined whether co-culture of BMD-EPCs with GECs affects the formation of endothelial tubes and/or their characteristics (tube diameter, number of loops) compared to direct culture of either cell type alone. In addition, we examined the expression of stromal-derived growth factor-1 (SDF-1; a factor that mediates activation, mobilization, homing and retention of BMD-EPCs), and its receptor CXCR4 in BMD-EPCs and GECs.
MATERIALS AND METHODS
The use of rats for isolation of bone marrow-derived stem cells (BMD-SCs), BMD-EPCs and GECs was approved by the Institutional Animal Care and Use Committee (Subcommittee for Animal Studies) of the Tibor Rubin VA Medical Center (approval number 1002-945). Rats received humane care based on the National Institutes of Health recommendations outlined in the Guide for the Care and Use of Laboratory Animals.
Isolation of bone marrow-derived stem cells and differentiation into bone marrow-derived endothelial progenitor cells
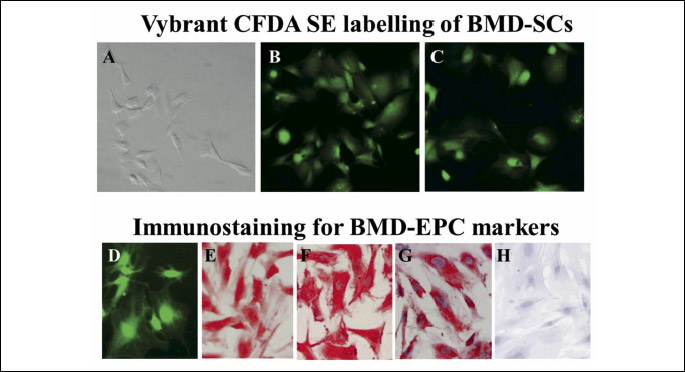
BMD-SCs were harvested from the bilateral femora and tibiae of five H. pylori and viral free Fisher F-344 rats 3 months of age (purchased from the National Institute on Aging, USA). Animals were euthanized and the hind limb femora and tibia were immediately removed. The bones were fractured using a bone cutter under sterile conditions and the marrow plugs were extracted by flushing the bone shaft with sterile Dulbecco’s modified Eagle’s medium (Invitrogen, USA) containing penicillin, streptomycin and amphotericin B (Invitrogen, USA) using a syringe. The marrow was disaggregated by passing it gently through a 21-gauge catheter and syringe followed by passage through a sterile 30 µm nylon mesh to create a single cell suspension. BMD-SCs were then cultured for 2 weeks in complete endothelial medium supplemented with EGF, bFGF, VEGF, hydrocortisone and 5% fetal bovine serum at 37ºC in a humidified incubator under 95% air/5% CO2 to induce differentiation into the BMD-EPC lineage similar to the method described in a previous study (31). Cells were then selected for CD34 using anti-CD34 coated microbeads (MACS, Miltenyi Biotec GmbH, Germany) and the midiMACS cell separator system (Miltenyi Biotec, Germany). The cells were characterized as BMD-EPCs by positive staining for the EPC markers - CD133 (ab19898; Abcam, USA), CD34 (sc-7324; Santa Cruz, USA) and VEGF-R2 (sc-504; Santa Cruz, USA) similar to a previous study (32). BMD-EPCs were labeled with Vybrant CFDA SE (Invitrogen, USA), a long-lasting ester that yields a fluorescent signal, which is retained by the cells, inherited by daughter cells after cell division, or cell fusion, and is not transferred to adjacent cells in a population (33).
Isolation of endothelial cells from gastric mucosa of rats
Gastric endothelial cells (GECs) were isolated from stomachs of Fisher F-344 rats ( H. pylori and viral free), 3 months of age (purchased from the National Institute on Aging, USA) using CD31 (PECAM-1) selection and magnetic bead separation as described previously (27). GECs were identified by positive staining for VWF/Factor VIII - related antigen (AB7356; Chemicon, USA), CD31 (MAB1393; Chemicon, USA) and VEGF-R2 (sc-504; Santa Cruz, USA); and, by absence of staining for the myofibroblast marker, smooth muscle α-actin. GECs were grown on collagen coated dishes in EC growth media containing FBS, heparin and endothelial cell growth supplements.
Studies in bone marrow-derived endothelial progenitor cells and gastric mucosa of rats
Vybrant CFDA SE labeled BMD-EPCs were visualized under an epifluorescence microscope as green fluorescing cells. Labeled BMD-EPCs were either directly seeded onto growth factor-reduced Matrigel or mixed with GECs (1:5 ratio) and seeded onto growth factor-reduced Matrigel. Tube formation was assessed after 24 hours under bright field and epifluorescence that allowed unequivocal identification of unlabeled GECs and Vybrant CFDA SE labeled BMD-EPCs.
Immunohistochemical staining for stromal derived factor 1 and CXCR4
We examined the expression of SDF-1 and CXCR4 in BMD-EPCs and GECs by immunostaining using methods similar to those described in our previous paper (30). Cells were cultured on collagen-coated coverslips, then fixed in paraformaldehyde and permeabilized with methanol. The expression of SDF-1 and CXCR4 in BMD-EPCs and GECs was examined by immunostaining using specific antibodies against SDF-1 (MAB350; R&D Systems, USA) and CXCR4 antibody (ab2074; Abcam, USA). Cells stained in absence of primary antibody were used as negative controls.
Statistical analysis
Data are presented as mean ± S.D. Statistical significance was analysed by either Student’s t-test to compare data between 2 groups using Prism (GraphPad Software Inc., La Jolla, CA). A P value of < 0.05 was considered statistically significant.
RESULTS
BMD-SCs isolated from hind limb bilateral femora and tibia of rats were labeled with Vybrant CFDA SE and were maintained in culture for at least 14 days (Fig. 1A-1C).
CD34(+) BMD-EPCs were selected from the BMD-SCs after a 14-day culture in EC media using anti-CD34 coated microbeads and the midiMACS cell separator system. BMD-EPCs and expressed the progenitor cell markers, CD34 and CD133 and VEGF-R2 (Fig. 1D-1H).
BMD-EPCs when seeded on Matrigel formed capillary like tubes (Fig. 2A and 2B) at 24 hours, and, when co-cultured with GECs, formed a robust network of hybrid capillary-like tubes composed of both BMD-EPCs and GECs (Fig. 2C and 2D) at 24 hours. These ‘hybrid’ tubes were formed by individual BMD-EPCs (displaying green fluorescence) nested between 4 to 5 GECs (corresponding to the seeding ratio 1 to 5 of BMD-EPCs to GEC). The hybrid tubes at the junctions of BMD-EPCs and GECs were 1.5-fold wider in diameter (P < 0.001) versus tubes formed by GECs alone. Moreover, more extensive and more numerous (5.1-fold increase) loops (P < 0.01) were formed at the junctions of BMD-EPCs and GECs when compared to tube segments formed by GECs alone (Fig. 2A and 2B).
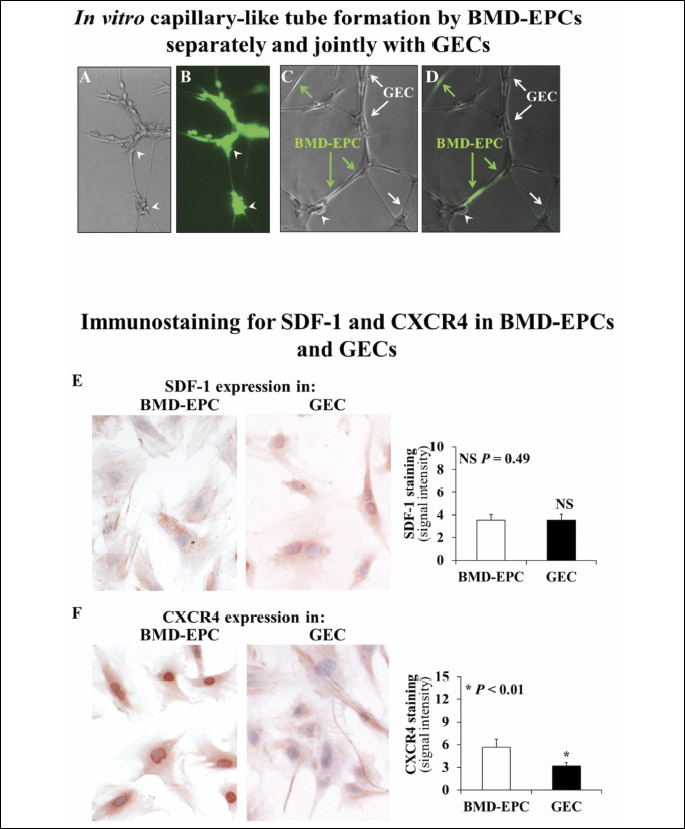
Since SDF-1 can trigger homing of BMD-EPCs to tissues (20) and can initiate vasculogenesis in some tissues through its CXCR4 receptor on BMD-EPCs (20-23), we examined the expression of SDF-1 and its receptor, CXCR4 in both BMD-EPCs and GECs (Fig. 2E and 2F). Both BMD-EPCs and GECs expressed SDF-1 (Fig. 2E) and CXCR4 (Fig. 2F). In BMD-EPCs, SDF-1 expression was localized to the cell membrane while in GECs SDF-1 expression localized to both cell membranes and nuclei. In BMD-EPCs, CXCR4 expression was present on cell membranes but was stronger in nuclei. In GECs, CXCR4 expression was mostly limited to the cell membrane and cytoplasm.
DISCUSSION
Our previous study using specimens of human and rat gastric ulcers demonstrated that BMD-EPCs incorporate into capillary blood vessels of granulation tissue, side by side with GEC, thus forming hybrid vessels (24). Our present study showed that BMD-EPCs when seeded on Matrigel can form capillary-like tubes autonomously consisting of only BMD-EPCs alone; and, when co-cultured with GECs, form extensive hybrid capillary-like-tubes jointly with GECs. These hybrid tubes are wider with significantly higher number of loops than in tube segments formed by ECs alone. A previous study showed that co-culture of adipose-derived stem cells (ASCs) and endothelial progenitor cells (EPCs) forms spheroids that induce superior vascularization of fibrin constructs compared to the corresponding monocultures (34). That study however, did not examine the effect of co-culturing EPCs with fully differentiated ECs.
Purified CD34+ hematopoietic progenitor cells from adult bone marrow (BMD-EPCs) can differentiate ex vivo into cells having an endothelial phenotype (15, 35). in vivo, these lineage-committed progenitor cells express various endothelial markers and potentially can incorporate into new capillary vessels at sites of ischemia and injury. Studies utilizing uncommitted, immature stem cells have demonstrated that BMD-SCs can differentiate into ECs (31, 36-38). A recent study examined the effect of culturing adipose tissue-derived stem cells (ASC) for over 30 passages and showed that after long-term expansion in vitro these ASCs maintained their prototypical immunophenotype, despite significant changes in morphology (39).
While the major questions of whether BMD-EPCs can form new vessels independently or can incorporate into newly formed vessels jointly with mature ECs have been controversial, our present study clearly answered these questions. In addition to incorporating directly into newly formed blood vessels, BMD-EPCs homing to sites of injury may provide a paracrine source of pro-angiogenic growth/survival factor levels that act on existing ECs to stimulate angiogenesis. BMD-EPCs can assist the angiogenic sprouts of ECs in joining, forming a cell cord and differentiating to ECs in situ thus establishing vessel segments between neighboring sprouts (40).
Our present study also showed that GECs express SDF-1, which is known to trigger homing and likely attracts BMD-EPCs to GECs facilitating the formation of hybrid tubes. Moreover, GECs also express CXCR4, which indicates the possibility of direct paracrine interactions and an interplay between the BMD-EPCs and GECs that are incorporated into hybrid capillary-like tubes.
A potential evolutionary advantage of postnatal vasculogenesis, in combination with angiogenesis (vasculogenesis/angiogenesis), versus angiogenesis alone would be the regeneration and formation of a dense, more complete, and elaborate microvasculature that would provide a better delivery of oxygen and nutrients to the regenerated mucosa and a more efficient removal of toxic metabolites.
A. Ahluwalia and M.K. Jones contributed equally to the paper.
Conflict of interests: None declared.
REFERENCES
- Cotran R, Kumar V, Robbins S. Gastric ulceration. In: Robbins Pathologic Basis of Disease. Cotran R, Kumar V, Robbins S. (eds.). Philadelphia, W.B. Saunders 1994, 298-299, 773-777.
- Tarnawski A, Hollander D, Stachura J, et al. Role of angiogenesis in healing of experimental gastric ulcer. In: Mechanisms of Peptic Ulcer Healing. Halter F, Garner A, Tytgat G, (eds). Dordrecht/Boston/London, Kluwer 1991, pp. 165-171.
- Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 2005; 50 (Suppl. 1): S24-S33.
- Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol 2014; 29 (Suppl. 4): 112-123.
- Tarnawski AS, Ahluwalia A. Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr Med Chem 2012; 19: 16-27.
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer 2010; 10: 505-514.
- Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653-660.
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995; 11: 73-91.
- Risau W. Mechanisms of angiogenesis. Nature 1997; 386: 671-674.
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435-439.
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439-442.
- Zaccone V, Flore R, Santoro L, et al. Focus on biological identity of endothelial progenitors cells. Eur Rev Med Pharmacol Sci 2015; 19: 4047-4063.
- Gu W, Hong X, Potter C, A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation 2017; Jan 24. doi: 10.1111/micc.12324.
- Zhang L, Xu Q. Stem/progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol 2014; 34: 1114-1119.
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964-967.
- Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond) 2011; 120: 263-283.
- Jain RK. Molecular regulation of vessel maturation. Nat Med 2003; 9: 685-693.
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95: 343-353.
- Deng X, Szabo S, Chen L, et al. New cell therapy using bone marrow-derived stem cells/endothelial progenitor cells to accelerate neovascularization in healing of experimental ulcerative colitis. Curr Pharm Des 2011; 17: 1643-1651.
- Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 2011; 11: 189-197.
- Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 2004; 35: 233-245.
- Takahashi M. Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J 2010; 74: 418-423.
- Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 2005; 15: 57-63.
- Ahluwalia A, Brzozowski T, Jones MK, Ichikawa Y, Tarnawski AS. Formation of new blood vessels during gastric ulcer healing. Role of bone marrow derived endothelial progenitor cells. J Physiol Pharmacol 2017; 68: 585-589.
- Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999; 5: 1418-1423.
- Jones MK, Szabo IL, Kawanaka H, Husain SS, Tarnawski AS. von Hippel Lindau tumor suppressor and HIF-1alpha: new targets of NSAIDs inhibition of hypoxia-induced angiogenesis. FASEB J 2002; 16: 264-266.
- Ahluwalia A, Jones MK, Tarnawski AS. Key role of endothelial importin-alpha in VEGF expression and gastric angiogenesis: novel insight into aging gastropathy. Am J Physiol Gastrointest Liver Physiol 2014; 306: G338-G345.
- Ahluwalia A, Jones MK, Brzozowski T, Tarnawski AS. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am J Physiol Gastrointest Liver Physiol 2016; 311: G981-G987.
- Jones MK, Wang H, Tomikawa M, et al. Isolation and characterization of rat gastric microvascular endothelial cells as a model for studying gastric angiogenesis in vitro. J Physiol Pharmacol 2000; 51: 813-820.
- Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J 2004; 18: 1264-1266.
- Pankajakshan D, Kansal V, Agrawal DK. in vitro differentiation of bone marrow derived porcine mesenchymal stem cells to endothelial cells. J Tissue Eng Regen Med 2013; 7: 911-920.
- Segal MS, Bihorac A, Koc M. Circulating endothelial cells: tea leaves for renal disease. Am J Physiol Renal Physiol 2002; 283: F11-F19.
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods 1994; 171: 131-7.
- Strassburg S, Nienhueser H, Bjorn Stark G, Finkenzeller G, Torio-Padron N. Co-culture of adipose-derived stem cells and endothelial cells in fibrin induces angiogenesis and vasculogenesis in a chorioallantoic membrane model. J Tissue Eng Regen Med 2016; 10: 496-506.
- Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92: 362-367.
- Allameh A, Jazayeri M, Adelipour M. in vivo vascularization of endothelial cells derived from bone marrow mesenchymal stem cells in SCID mouse model. Cell J 2016; 18: 179-88.
- Li Q, Xia S, Fang H, Pan J, Jia Y, Deng B. VEGF treatment promotes bone marrow-derived CXCR4+ mesenchymal stromal stem cell differentiation into vessel endothelial cells. Exp Ther Med 2017; 13: 449-454.
- Xu J, Wu D, Yang Y, Li K, Gao P. Endothelial-like cells differentiated from mesenchymal stem cells attenuate neointimal hyperplasia after vascular injury. Mol Med Rep 2016; 14: 4830-4836.
- Danisovic L, Oravcova L, Krajciova L, et al. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem Cells. J Physiol Pharmacol 2017; 68: 149-158.
- Zhan K, Bai L, Xu J. Role of vascular endothelial progenitor cells in construction of new vascular loop. Microvasc Res 2013; 90: 1-11.
A c c e p t e d : November 20, 2017