ANTI-ASTHMATIC EFFECTS OF VOLATILE ORGANIC COMPOUNDS FROM CHAMAECYPARIS OBTUSA, PINUS DENSIFLORA, PINUS KORAIENSIS AND LARIX KAEMPFERI WOOD PANELS
INTRODUCTION
The cause of asthma is unknown, but its relationship to heredity and environmental factors is important (1-3). Genetic susceptibility and environmental triggers may act synergistically in inducing asthma (1-3); environmental triggers include pollens, fungi, dust, mites, and insects (1, 4, 5). To elucidate the pathogenesis of asthma, it is essential to have knowledge of the activities within a normal immune system. In a normal humoral immune system, antigen presenting cells (APCs), comprised of B cells, dendritic cells, and macrophages, detect intruding pathogens and induce endocytosis (1, 6). Subsequently, the intruding pathogen is processed into small peptide molecules (7). On the surface of APCs, the pathogen’s degraded peptide molecules are presented along with major histocompatibility complex (MHC) I and II proteins for detection by T cells (7). The role of T cells in the immune response is important, and T cells are classified into killer (CD8+) and helper (CD4+) types. Killer T cells recognize MHC class I proteins, while helper T cells recognize MHC class II proteins (7). Helper T cells activated by MHC class II proteins secrete various cytokines and induce B cells to differentiate into memory and plasma B cells (6). When an antigen is encountered a second time, memory B cells detect the antigen and rapidly differentiate into plasma B cells (6); those plasma cells then produce large amounts of immunoglobulins (6).
Based on this background information on the normal immune system, allergic asthma can be considered a localized form of anaphylaxis that occurs when lung airways are repeatedly exposed to a specific allergen (1). When the same allergen re-enters the allergic patient’s airway, dendritic cells in the airway submucosa recognize it as an antigen. Dendritic cells then induce phagocytosis and antigen presentation, which is followed by the production and secretion of large quantities of allergen specific-IgE via activated plasma B cells (4, 6, 8). In this process, the activated mast cells are degranulated, releasing stored mediators such as histamine, serotonin, and protease contained in cytoplasmic granules as well as newly formed mediators such as prostaglandins and leukotrienes (8). These mediators cause localized vasodilation, bronchoconstriction, and mucus secretion or systemic anaphylaxis (8, 9). Such reactions can occur within minutes of allergen exposure (1, 5, 8, 9) or several hours later when other mediators such as cytokines and chemokines are secreted (1, 5, 8), which is referred to as a late-phase reaction. Late-phase reactions are characterized by mucus secretion and airway narrowing (1, 5, 8, 9). When an allergen is repeatedly exposed to an allergenic site, many innate and adaptive immune cells in the blood infiltrate the adjacent tissues (1). The hallmarks of asthmatic airway remodeling are an increased number of epithelial cells and an increase in the size and number of smooth muscle cells resulting from the combined interaction of a variety of inflammatory mediators such as interleukins (ILs) and tumor necrosis factor-αlpha (TNF-α) (1, 10). Various cytokines reported to be essential in allergic asthma are released from Th2 cells: IL-3, -4, -5, -9, -10, and -13 (4, 8, 9, 11-13). One locus on human chromosome 5q31.1 is comprised of a cytokine cluster that includes IL-3, -4, -5, -9, -13, and GM-CSF (9, 14). IL-4, -5, -9 and -13 are typical Th2 cytokines associated with allergic airway inflammation (11, 13, 15). IL-4 is secreted by activated helper
T cells and acts on Th2 and B cells, thymocytes, mast cells, and eosinophils to promote differentiation and proliferation (6, 13); moreover, IL-4 is critical for IgE synthesis and eosinophil chemotaxis (6). In addition, IL-4 induces class switching of IgE through a synergistic effect with IL-5. Furthermore, IL-4 is associated with airway eosinophilia, airway remodeling, mucus metaplasia, and pro-fibrotic effects (13). IL-5 secretion induces recruitment of inflammatory cells such as eosinophils and neutrophils and their exit via amoebic movement through small holes on the surface of blood vessels (6, 13, 16). In addition, IL-5 is essential for eosinophil production, maturation, and activation (17). It also contributes to airway epithelial fibrosis and mucus metaplasia (13). IL-9 increases IL-4 mediated production of IgE in human and murine B cells, and it induces eosinophils to mature through a synergistic effect with IL-5 (15). In addition, IL-9 promotes transcription of mucin, which is produced in respiratory endothelial cells (15, 16). IL-13 has biological activities similar to those of IL-4 (13), and induces IgE class switching, chemotaxis, activates fibroblasts, and stimulates mucus production (7, 11). It is also needed for the production of IgE (7).
In this study, we investigated the effects of volatile organic compounds (VOCs) in conifer wood on airway tissues and on inflammatory cytokine expressions. VOCs extracted from wood are composed of fatty acids, sterols, terpenes, resin acids, phenols, and tannic acids (18). Among those compounds, terpene neutralizes ozone and repels insects (2). Moreover, terpene has anticancer, antimicrobial, antifungal, antiviral, and anti-inflammatory effects (2). Monoterpene has been reported to exert an analgesic effect by inhibiting pro-inflammatory mediators (19), while terpene has a therapeutic effect on bronchitis, sinusitis, steroid-dependent asthma, and respiratory infection (2). In this study, we investigated four types of wood obtained from Chamaecyparis obtusa, Pinus densiflora, Pinus koraiensis, and Larix kaempferi. All four species are conifers, and their woods are widely used as building and furniture materials. Essential oil of C. obtusa contains a monoterpene that has been reported to have an anti-inflammatory effect (19). Studies on essential oils extracted from leaves and twigs of C. obtusa have revealed that C. obtusa contains various terpenes, including γ-terpinene, β-thujaplicin, α-terpineol, β-phellandrene, β-myrcene, limonene, and bornyl acetate (19). Other studies have shown that elemol, a key component of a C. obtusa extract, has a potent effect on atopic dermatitis (20, 21). Also, it has been shown that C. obtusa induces anti-inflammatory effects on both local pulmonary inflammation and on systemic peripheral blood mononuclear cells via the COX-2 pathway (23). P. densiflora, P. koraiensis, and L. kaempferi are distributed in Japan, northeast China, and Korea (24), while C. obtusa is a native of Japan. It has been reported that an essential oil extracted from P. densiflora has bactericidal and anti-oxidative effects (24). VOCs from wood can have significant inhibitory effects on pro-inflammatory mediators, such as inducible nitric oxide synthase, IL-6, and IL-1β (25). In this study, the VOCs from C. obtusa, P. densiflora. P. koraiensis, and L. kaempferi wood panels were assessed to examine their effects on asthmatic symptoms.
MATERIALS AND METHODS
Animal preparations
Forty-nine ICR mice (5-weeks-old) were obtained from Koatech (Pyeongtaek, Gyeonggi, South Korea) and divided into seven groups (n = 7 per group). The 7 animals of each group were placed in one acrylic chamber (50 cm × 50 cm × 50 cm) and the animals were housed in poly-carbonated cages. Experiments were performed in a laboratory with a controlled environment animal room (temperature, 23 ± 2°C; relative humidity, 50% ± 10%; frequent ventilation).
Mouse asthma model
The vehicle group was administered saline via intraperitoneal (i.p.) and intranasal (i.n.) routes, whereas the negative control group was administered OVA i.p. and i.n. The positive control group was administered OVA i.p. and i.n. and budesonide i.n. as an antagonist. The vehicle group, OVA-induced asthma model group, budesonide treatment after asthma induction group, and each of the wood panel VOC-treated after asthma induction (C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi) groups were maintained separately in acrylic chambers. The size of each of the wood panels was 684 cm3.
The experimental groups were acclimated to their environment for one week before starting the experiments and were then administered an albumin + alum i.p. injection on day 7 and 21, OVA (100 µg/mouse) and alum (2 mg/mouse) were mixed for 30 minutes with 0.1 ml PBS. The solution was injected into the mouse’s intraperitoneal cavity. From day 21, 1.5 mg of OVA was dissolved with 50 µL of PBS individually, then introduced into the nasal cavity and this was repeated daily for 5 consecutive days. Zoletil 100® (Virbac, Carros, France) 0.024 mL and xylazine 0.006 mL were mixed and injected i.p. 15 minutes before the i.n. administration of albumin. On day 27, animals were sacrificed using CO2 in a fume hood. Two mice in each group were perfused with saline and then fixed with formalin for tissue sections. Five mice in each group were sacrificed for blood sampling via the abdominal aorta. Bronchoalveolar lavage (BAL) fluids were obtained from the trachea. Lung tissues were sampled and used for cDNA synthesis. Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University approved all experimental procedures (Approval n. CBNUA699-17-07).
Tissue preparation
Lungs and trachea tissues were fixed with formalin, prepared as paraffin tissue blocks, and microtome sections were prepared. The structural change of the tissue was identified by examination after applying H&E stain.
Staining
Inflammatory cells were identified by applying Diff-Quik® stain to BAL fluid. First, BAL fluid samples were smeared on a glass slide and dried. The slides were then dipped in fixative solution five times. After drying the slides, the slides were then dipped 5 times in stain solution 1 and dried again. The slides were then dipped 5 times in stain solution 2 and dried. All slides were then washed in distilled water, dried in air, and observed under a microscope. The lung tissue was fixed by 10% formalin. The tissues were embedded in paraffin, cut into sections (5 microns) and stained with hematoxylin and eosin (H&E). To investigate the thickening of bronchiolar wall and trachea, all tissue samples were examined with and Periodic- Periodic Acid-Schiff (PAS) staining captured with light microscopy (BX51; Olympus, Tokyo, Japan) and Olympus DP controller (DP21; Olympus, Japan) and manager at × 200 magnification.
ELISA
Blood samples were kept at room temperature for 30 minutes and then centrifuged at 8000 r/min. The supernatant was then transferred to a new tube. The serum TNF-α and IL-4 levels were measured by using commercial ELISA kits (TNF-α ELISA kit and IL-4 ELISA kit, Cloud-clone Corp., MD, USA). The assay protocols in the manufacturer’s manuals were followed.
RNA extraction and cDNA synthesis
The TRI reagent™ solution (Invitrogen by Thermo Fisher Scientific, MA, USA) was used to extract total RNA from mouse lung. The RNA concentration was measured at an absorbance of 260 nm by using an Epoch micro-plate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The RNA quality check was performed by performing electrophoresis in 1.5% agarose gels at 100 V for 20 minutes.
The quantified RNA (1µg) was reverse transcribed into cDNA by using Moloney murine leukemia virus reverse transcriptase (M-MLV; Invitrogen) with a random primer (9-mer; Takara Bio Inc., Shiga, Japan) following the manufacturer’s protocol.
qRT-PCR
Thirteen primers were used to identify the presence of IL-4, -5, -9, and -13 in the cDNA by performing qRT-PCR according to the inflammatory response. Each cDNA sample (2 µL) was mixed with 6.25 µL 2 × prime Q-Mastermix SYBR (Genetbio), 0.25 µL 50 × ROX dye, and 0.5 µL for each primer. The sequences of the primers used in the qRT-PCR are as follows. 5’-GAAGGATGCTTCTGCACTTGAG-3’ (forward) and 5’-AGCCTCATCGTCTCATTGCT-3’ (reverse) for IL-5 (product size; 148 bp); 5’- ACATCACACAAGACCAGACTC-3’ (forward) and 5’- GGTTACAGAGGCCATGCAAT-3’ (reverse) for IL-13 (product size; 159 bp); and 5’-CTCAACACGGGAAACCTCAC-3’ (forward) and 5’-CGCTCCACCAACTAAGAACG-3’ (reverse) for RN18s (product size, 110 bp). The cDNA was amplified by using a 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA) and proceeded as follows: cDNA denatured at 95°C for 10 minutes followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, and elongation at 72°C for 30 seconds. The relative expression levels of IL-5 and IL-13 in the 18s cDNA sample were determined by using RQ software (Applied Biosystems).
Collection of volatile organic compounds from C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi panels
VOCs of C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi were collected by trapping gas with a SHIBATA minipump (MP-Σ300, SHIBATA, Saitama, Japan). The composition of the obtained VOCs was analyzed by gas chromatography-mass spectrometry (GC-MS) using a Trace 1310/ISQ-LT (Thermo Scientific, USA). A TR-5MS capillary column (30 cm × 0.25 mm × 0.25 µm; Thermo Scientific, USA) was used for entrapping the gas, and He (1 mL/min, 25 psi) was used as the carrier gas. The entrapped gas was analyzed by using thermal desorption (TD) GC-MS performed with GC model Agilent 7890 and MS model Agilent 5975 (Agilent, Santa Clara, USA). Components were detached from the TA tube at 270°C and concentrated in a –20°C cold trap. Concentrated components were then analyzed via GC-MS. The compounds analyzed in the VOCs were matched with the total ion chromatogram and the W9N08 library.
Statistical analysis
Data are presented as mean ± standard deviation of the mean values and were analyzed by one-way analysis of variance followed by Tukey’s multiple comparison test. Statistical analyses were performed by using GraphPad Prism software (version 4.0; GraphPad Software Inc., La Jolla, CA, USA). A value of P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Cytological examination of bronchoalveolar lavage fluid
To confirm the infiltration and distribution of inflammatory cells in lung tissue, bronchoalveolar lavage (BAL) fluid was stained with Diff-Quik stain. The number of immune cells in the OVA-treated group was significantly increased (by 2.5-fold), whereas the number of immune cells in the other groups were not significantly decreased from that in the OVA-treated group (Fig. 1). The cell composition in the BAL fluids was altered; granulocytes such as mast cells and basophils were observed in the OVA-treated group. The positive control, C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi groups showed similar cell compositions to that in the vehicle group. Thus, the VOCs given off by the four wood types did not significantly change the number of immune cells in the BAL fluids, but the cell compositions did change with VOC treatment.
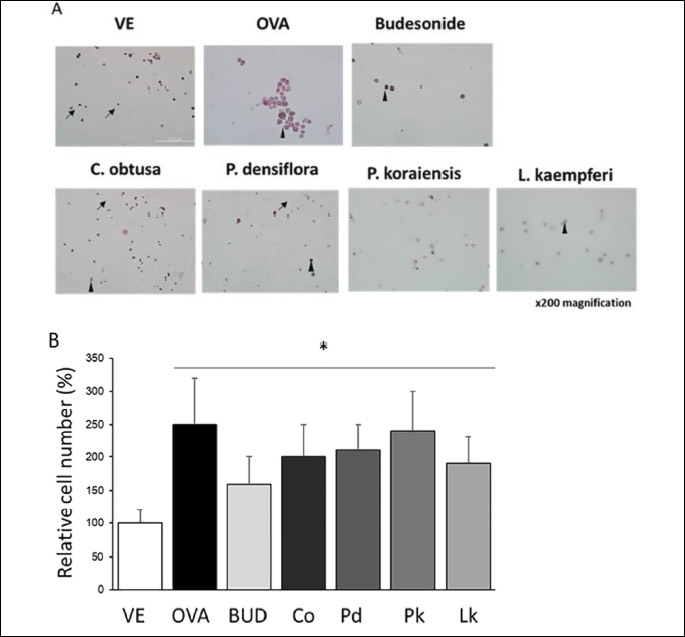
Histological examination of lung and trachea tissues
To assess the OVA-induced pathophysiological changes in bronchial wall thickness, lung and trachea tissues were sectioned and H&E stained. The thickening of the bronchial wall gradually increased from thinnest in the vehicle group, ascending in order through the budesonide, P. koraiensis, L. kaempferi, C. obtusa, and P. densiflora groups, to thickest in the OVA group. In the OVA-treated group, the bronchial wall changed from a simple cuboidal epithelium to a stratified cuboidal epithelium that included metaplastic changes. The positive control group had a much thinner wall thickness than those in the VOC-treated groups, due to the group’s anti-inflammatory response to budesonide, a steroid agent. In the P. densiflora, C. obtusa, P. koraiensis, and L. kaempferi groups, the bronchus walls were thicker than those in the positive control group but thinner than that in the OVA-treated group.
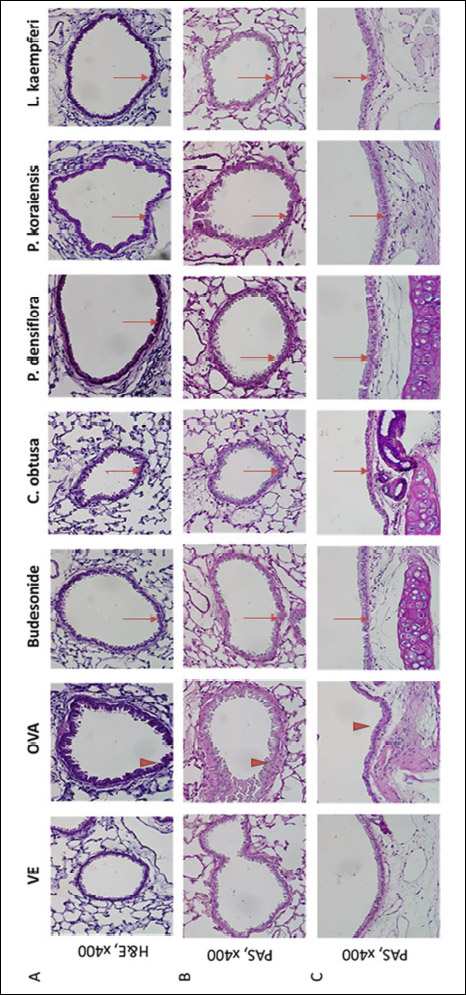 |
Fig. 2. Histopathological analysis of airway damage from OVA-induced asthma model mice. Lung and trachea tissue samples were fixed by 4% paraformalin. Bronchioles in lung tissue and trachea were assessed damage by applying H&E and Periodic acid-Schiff (PAS) stains. H&E staining with bronchiole in lung (A), PAS staining with bronchiole (B), and tracheal transverse staining with PAS (C). Harris hematoxylin solution 3 min, eosin 2 min. Thick arrow indicates OVA-induced hyperplasia, arrow indicates alleviated hyperplasia of epithelial cells. Each image was taken at × 400 magnification. |
Tumor necrosis factor-α and interleukin-4 concentrations in serum
ELISA was used to determine the serum levels of TNF-α and IL-4. Fig. 3A shows the results for TNF-α and reveals that the OVA, P. densiflora, P. koraiensis, and L. kaempferi groups had significantly higher concentrations of serum TNF-α than those in the vehicle- and budesonide-treated groups. In the budesonide and C. obtusa groups, the serum TNF-α levels were similar to that in the vehicle group. Fig. 3B shows the IL-4 concentrations in serum. The IL-4 level was higher in the OVA-treated group than in the vehicle group, whereas it was lower than that in the OVA-treated group in the budesonide, C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi groups. The IL-4 level in the L. kaempferi group was the lowest among the tested groups, indicating that L. kaempferi VOCs had the greatest effect on alleviating the OVA-induced asthmatic responses and lowering the IL-4 concentration in serum.
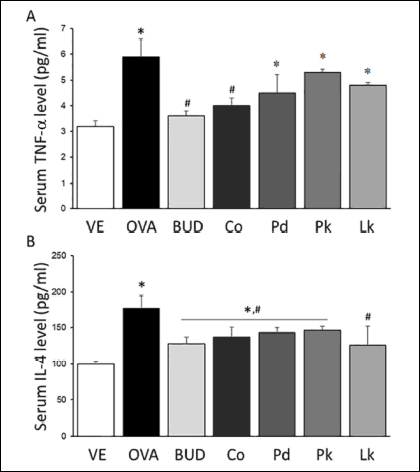 |
Fig. 3. Serum concentration of inflammatory cytokines TNF-α and IL-4. Serum concentrations of TNF-α (A) and IL-4 (B) were measured by ELISA. VE, vehicle; OVA, ovoalbumin - negative control; BUD, budesonide - positive control; Co, C. obtusa; Pd, P. densiflora; Pk, P. koraiensis; Lk, L. kaempferi. Values are expressed as means ± standard deviation. *P < 0.05 versus VE, #P < 0.05 versus OVA. |
Interleukin expressions in lung tissue
Real-time PCR was used to measure the IL-4, IL-5, IL-9, and IL-13 concentrations in lung tissue. The expression levels of IL-4, IL-5, IL-9, and IL-13 were higher in the OVA-treated group than in the vehicle-treated group (Fig. 4). The steroidal anti-inflammatory agent budesonide successfully recovered the OVA-induced increases in IL-4, IL-5, IL-9, and IL-13 expressions in lung tissue. The VOCs from C. obtusa recovered the OVA-induced IL-4, IL-9, and IL-13 expression levels, while the VOCs from P. densiflora, P. koraiensis, and L. kaempferi recovered the OVA-induced IL-4 and IL-13 expressions. The inhibitory effects on IL-4, IL-9, and IL-13 expression levels were more apparent in the C. obtusa group than in the other VOC-treated groups.
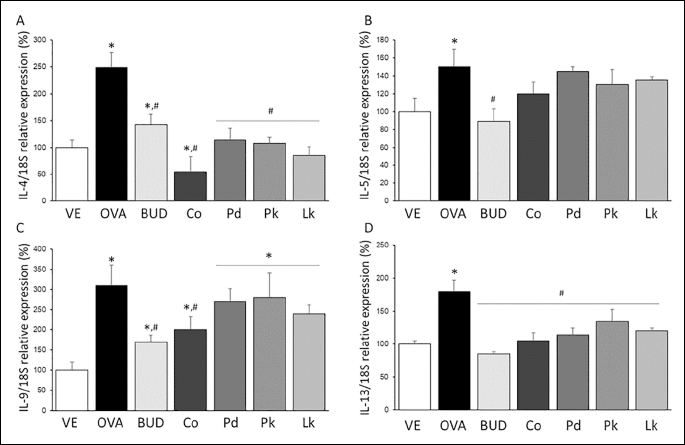
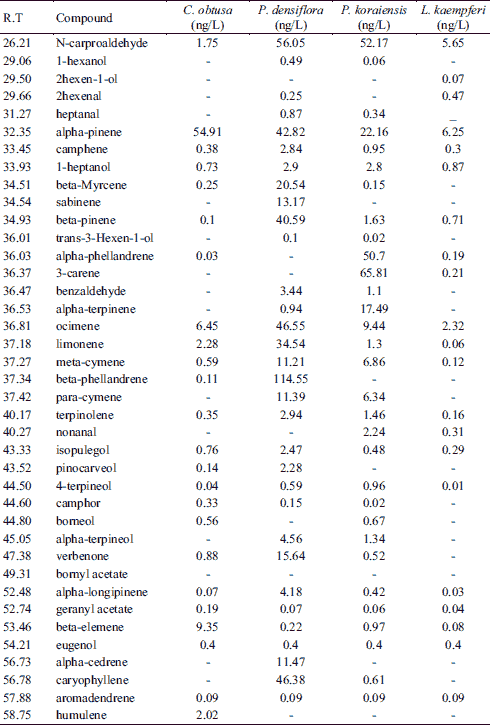
Volatile organic compound contents of C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi
Over a period of four weeks, the VOCs emitted from the four wood types were entrapped in a closed system and the VOC components and their concentrations were analyzed every other day. Results of a quantitative analysis of the components of the VOCs from each type of wood are shown in Table 1. Both the concentration levels and numbers of components that diffused from L. kaempferi were higher than those from P. densiflora. In both species, the VOCs included alpha-pinene, camphene, 1-heptanol, beta-pinene, ocimene, limonene, meta-cymene, terpinolene, isopulegol, 4-terpineol, alpha-longipinene, geranyl acetate, beta-elemene, eugenol, aromadendrene, and n-carproaldehyde; however, there were differences in the concentrations of those substances between the two species. C. obtusa wood emitted alpha-pinene and elemene; P. densiflora wood emitted alpha-pinene, beta-pinene, beta-phellandrene, and caryophyllene; P. koraiensis wood emitted alpha-phellandrene, 3-carene, and alpha-terpinene; and L. kaempferi wood emitted alpha-pinene and ocimene. The overall concentrations of VOCs also differed by wood type; the C. obtusa VOC concentration was 82.76 ng/L, the P. densiflora VOC concentration was 494.68 ng/L, the P. koraiensis VOC concentration was 249.56 ng/L, and the L. kaempferi VOC concentration was 18.63 ng/L.
DISCUSSION
Plants are of high importance to humans, and essential oils extracted from trees are reported to be beneficial for animal health (17, 18). Previous studies have revealed that essential oils from wood have an inhibitory effect on inflammatory cells (17, 18). However, in the residential environment, more research is needed to determine whether vapors emitted by trees are beneficial to health because we are more likely to be exposed to tree vapors than to tree essential oils. Thus, to determine whether inflammatory responses are suppressed by tree vapors, we exposed mice to wood from four tree species. In order to mimic a wood-based residential environment, mice and a wood panel were placed inside a polyacrylamide chamber. Experiment groups consisted of a vehicle group in which the mice were only administered 0.9% saline, a negative control group in which the mice were administered OVA to induce allergic asthma, a positive control group that received OVA and budesonide to suppress the inflammatory response, and four VOC-treated groups that were exposed to C. obtusa, P. densiflora, P. koraiensis, or L. kaempferi wood panels.
In each VOC-treated group, two wood panels of the selected tree species were placed in the residential chamber so that the mice were living in the presence of wood VOCs. On the 14th day of the experiment, albumin with alum was administered to the abdominal cavity to induce a systemic primary immune response. Subsequently, on the 22nd day of the experiment, albumin was administered through the nasal cavity for five consecutive days to induce local secondary immune responses in the airways and lungs. At the end of the experimental period, real-time PCR, tissue staining, and ELISA were used to examine the changes in IL-4 and TNF-α concentrations in serum and expressions of IL-4, -5, -9, and -13 in lung tissue. In addition, BAL fluids were stained to assess morphological and numerical changes in inflammatory cells.
Analysis of the numbers and morphology of immune cells in BAL fluid indicated that there was no significant decrease in the numbers of immune cells in the P. densiflora and C. obtusa groups from that in the negative control group. But the abundances of granulocytes in the budesonide-, P. densiflora-, and C. obtusa-treated groups showed that those treatments could recover the effect of the OVA treatment. After the migrated granulocytes contact with an allergen, rapid degranulation and release of various mediators occurs, which leads to tissue remodeling (21). Asthma is well-characterized as being correlated with eosinophilic and neutrophilic inflammation (22, 23). When an inflammatory response occurs in the lung, various histological changes can occur; bronchial epithelial cells become metaplastic, changing from simple cuboidal to stratified cuboidal epithelial cells, and inflammatory cells infiltrate the submucosal layer. Also, the basement membrane and smooth muscle cells thicken. H&E staining of the lung tissue sections of the mice in this study showed that the thickness of the bronchiolar wall increased in ascending order from the positive control group, to the vehicle, C. obtusa, P. densiflora, P. koraiensi, L. kaempferi, and OVA treatment groups. In the OVA-treated group, the bronchiolar wall appeared to be metaplastic, exhibiting a change from a simple cuboidal epithelium to a stratified cuboidal epithelium. In the positive control, C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi groups there was alleviation of the OVA-induced histopathological changes to the bronchiolar wall. The anti-inflammatory effects of C. obtusa, P. densiflora, and P. koraiensis were identified in previous studies (18, 24, 25). The present results suggest that the VOCs from the four species of wood treated to the asthma model mice had alleviatory effects on the OVA-induced inflammatory histological changes. Morphological study of the bronchial epithelium has revealed the presence of transitional cells indicating that epidermoid metaplasia may result from the conversion of airway epithelial cells (26, 27).
TNF-α is a pro-inflammatory cytokine that has been implicated in the airway pathology of asthma (19). IL-4 mediates pro-inflammatory responses in asthma including the induction of IgE isotypes, expression of vascular cell adhesion molecule-1 (VCAM-1), mucus secretion, and differentiation of T helper type 2 lymphocytes (20). The changes in serum TNF-α concentration were statistically significant in all experimental groups. The concentration in the OVA-treated group was higher than that of the vehicle group, while the TNF-α concentration of the budesonide-treated group was the lowest among the tested groups. The results also showed that the OVA-induced increased concentrations of two inflammatory cytokines, TNF-α and IL-4, were lowered by C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi treatments. The TNF-α and IL-4 results show that, among the four wood types, C. obtusa was most effective in inhibiting expression of TNF-α (a type 1 cytokine) while L. kaempferi was most effective in inhibiting IL-4 expression (a type 2 cytokine). IL-4 is secreted by activated helper T cell and acts on Th2 and B cells, thymocytes, mast cells, and eosinophils to promote differentiation and proliferation (6, 13); moreover, it is critical for IgE synthesis and eosinophil chemotaxis (6). Also, IL-4 induces class switching of IgE through a synergistic effect with IL-5. In addition, IL-4 is associated with airway eosinophilia, airway remodeling, mucus metaplasia, and profibrotic effects (13). IL-5 gives rise to the recruitment of inflammatory cells such as eosinophils and neutrophils and their exit through small holes on the surface of blood vessels via amoebic movement (6, 13, 16). IL-5 is essential for eosinophil production, maturation, and activation (17). It also contributes to airway epithelial fibrosis and mucus metaplasia (13). IL-9 increases IL-4-mediated production of IgE in human and murine B cells, and it induces eosinophils to mature through a synergistic effect with IL-5 (15). In addition, IL-9 promotes transcription of mucin, which is produced in respiratory endothelial cells (15, 16). IL-13 has biological activities similar to those of IL-4 (13). Similar to the actions of IL-4, IL-13 induces IgE class switching, chemotaxis, activates fibroblasts, and stimulates mucus production (7, 11). It is also needed for the production of IgE (7).
The IL-4, IL-5, IL-9 and IL-13 mRNA expressions in lung tissues showed that recovery of OVA-induced asthmatic inflammation could be accomplished by application of the steroidal agent budesonide and by the presence of VOCs from C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi. The alleviating effect of VOCs on OVA-induced lung inflammation was more apparent in the C. obtusa group than in the other wood treatment groups. Such tendencies suggest that differences among the wood types tested may be associated with differences in the composition and amount of VOCs in each type of wood. The VOCs from C. obtusa included 24 compounds, while P. densiflora and P. koraiensis contained 32 compounds each, and L. kaempferi contained 20 compounds. Each wood’s VOCs included monoterpenes and sesquiterpenes; limonene and alpha-pinene were particularly abundant. Among the contents detected in the VOCs emitted from the tested wood types, alpha-pinene, beta-pinene, 3-carene, alpha-terpinene, limonene, alpha-terpineol, and verbenone have been previously reported as having anti-inflammatory properties (28, 29). However, in this study, the individual contents of the VOCs were not matched with the alleviatory effect of the total VOCs from the wood panels. Therefore, future studies are needed to determine the effects of individual extracted compounds from among the VOCs of the four wood types in this study.
In conclusion, the results suggest that VOCs from C. obtusa, P. densiflora, P. koraiensis, and L. kaempferi produce their alleviatory effects on asthma through the inhibition of Th2 cytokines (IL-4, -9, and -13) that cause granulocyte migration.
Conflict of interests: None declared.
REFERENCES
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454: 445-454.
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med 2002; 53: 477-498.
- Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol 2015; 5 (Suppl 1): S2-S6.
- Leomicronn B. T cells in allergic asthma: key players beyond the Th2 pathway. Curr Allergy Asthma Rep 2017; 17: 43. doi: 10.1007/s11882-017-0714-1
- Hill VL, Wood PR. Asthma epidemiology, pathophysiology, and initial evaluation. Pediatr Rev 2009; 30: 331-335; quiz 5-6.
- Killeen K, Skora E. Pathophysiology, diagnosis, and clinical assessment of asthma in the adult. Nurs Clin North Am 2013; 48: 11-23.
- Wieczorek M, Abualrous ET, Sticht J, et al. Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front Immunol 2017; 8: 292. doi: 10.3389/fimmu.2017.00292
- Deckers J, Branco Madeira F, Hammad H. Innate immune cells in asthma. Trends Immunol 2013; 34: 540-547.
- Kaufman G. Asthma: pathophysiology, diagnosis and management. Nurs Stand 2011; 26: 48-56, quiz 8.
- Zhao C, Wu AY, Yu X, et al. Microdomain elements of airway smooth muscle in calcium regulation and cell proliferation. J Physiol Pharmacol 2018; 69: 151-163.
- Kips JC. Cytokines in asthma. Eur Respir J Suppl 2001; 34: 24s-33s.
- Rosenwasser LJ, Klemm DJ, Dresback JK, et al. Promoter polymorphisms in the chromosome 5 gene cluster in asthma and atopy. Clin Exp Allergy 1995; 25 (Suppl 2): 74-78, discussion 95-96.
- Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999; 104: 1001-1006.
- Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18: 673-683.
- Horka H, Staudt V, Klein M, et al. The tick salivary protein sialostatin L inhibits the Th9-derived production of the asthma-promoting cytokine IL-9 and is effective in the prevention of experimental asthma. J Immunol 2012; 188: 2669-2676.
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol 2007; 19: 676-680.
- Yang H, Jung EM, Ahn C, et al. Elemol from Chamaecyparis obtusa ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int J Mol Med 2015; 36: 463-472.
- Yang H, Ahn C, Choi IG, et al. Estimation of the environmental effect of natural volatile organic compounds from Chamaecyparis obtusa and their effect on atopic dermatitis-like skin lesions in mice. Mol Med Rep 2015; 12: 345-350.
- Berry M, Brightling C, Pavord I, Wardlaw A. TNF-αlpha in asthma. Curr Opin Pharmacol 2007; 7: 279-282.
- Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res 2001; 2: 66-70.
- Mokry J, Urbanova A, Medvedova I, et al. Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. J Physiol Pharmacol 2017; 68: 721-730.
- Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 2009; 6: 256-259.
- Bazan-Socha S, Zuk J, Plutecka H, Marcinkiewicz C, Zareba L, Musial J. Collagen receptors alpha(1)beta(1) and alpha(2)beta(1) integrins are involved in transmigration of peripheral blood eosinophils, but not mononuclear cells through human microvascular endothelial cells monolayer. J Physiol Pharmacol 2012; 63: 373-379.
- Ahn C, Lee JH, Kim JW, Park MJ, Lee SS, Jeung EB. Alleviation effects of natural volatile organic compounds from Pinus densiflora and Chamaecyparis obtusa on systemic and pulmonary inflammation. Biomed Rep 2018; 9: 405-414.
- Kang SA, Kim DH, Hong SH, et al. Anti-inflammatory activity of Pinus koraiensis cone bark extracts prepared by micro-wave assisted extraction. Prev Nutr Food Sci 2016; 21: 236-244.
- Reader JR, Tepper JS, Schelegle ES, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol 2003; 162: 2069-2078.
- Trevisani L, Sartori S, Bovolenta MR, et al. Structural characterization of the bronchial epithelium of subjects with chronic bronchitis and in asymptomatic smokers. Respiration 1992; 59: 136-144.
- Almeida JR, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Scientific World Journal 2013; 2013: 808460. doi: 10.1155/2013/808460
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules 2010; 15: 9252-9287.
A c c e p t e d : December 30, 2018