VISCOELASTIC PROPERTIES OF PLASMA FIBRIN CLOTS ARE SIMILAR
IN PATIENTS ON RIVAROXABAN AND VITAMIN K ANTAGONISTS
INTRODUCTION
Fibrin is formed from fibrinogen in the presence of thrombin, and is the main protein component of blood clots and intravascular thrombi in all locations (1). Fibrin together with platelets form a plug to stop bleeding over a wound site or thrombi at pathological conditions. Fibrin fibers are characterized by extremely large extensibility. The most endurable fibers can extend their length over six times before rupturing and cross-linked fibers resist with no permanent damage up to 2.8-fold extension (2).
Fibrin also affects cell adhesion, proliferation, migration, differentiation, wound healing, and inflammation (3, 4). It has been reported that developing fibrin clots may be modified by many environmental and genetic factors (5-7). Previous studies on fibrin clot structure demonstrated that there is a diagnostic and prognostic potential of some clot structure characteristics assessed in vitro and improvement of its architecture might be used to prevent thrombosis (8). We have demonstrated that fibrin clots generated from plasma of patients with venous thromboembolism (VTE) and their relatives were less compact, permeable, and had prolonged clot lysis time compared to the control subjects (9). Moreover, we have shown that recurrences of deep vein thrombosis (DVT) and pulmonary embolism (PE) were associated with a prothrombotic fibrin clot phenotype reflected by faster formation of denser clots (10, 11). Fibrin clot properties have been shown to be improved on vitamin K antagonists (VKAs) and rivaroxaban, including increased clot permeability, a measure of clot density and clot lysis time (5, 12).
Little is known about the impact of oral anticoagulants on plasma clots viscoelastic parameters described by storage modulus (G'), representing elastic properties characterizing the stiffness of the polymer, and loss modulus (G'') representing viscous properties of the fibrin clot (13). Another viscoelastic parameter, fractal dimension (Dƒ), has been used to characterize nonlinear growth of branching network structures (14, 15). It has been demonstrated to be lower in clots formed from a whole blood after heparinization compared with non-heparinized samples and there was a correlation between fibrinogen levels and Dƒ (14). Reduced Dƒ was also observed following dual antiplatelet therapy compared with aspirin alone (14).
To our knowledge, there have been no studies on viscoelastic properties of fibrin clots in VTE patients treated with VKAs or newer oral anticoagulants in association with their blood concentrations. Thus, we sought to investigate whether rivaroxaban and VKAs, namely warfarin or acenocoumarol, alter to a similar extent the stiffness and viscosity of fibrin clots generated from plasma of anticoagulated VTE patients.
MATERIALS AND METHODS
Patients
We studied four age- and sex-matched groups as follows:
(1) 15 VTE patients taking rivaroxaban 20 mg/day in whom blood was drawn after at least 2 hours up to 24 hours since the last dose intake, in whom the drug’s concentration was above 30 ng/ml;
(2) 15 VTE patients taking VKAs with a history of DVT and/or PE treated with warfarin or acenocoumarol, in whom INR was in the therapeutic range (between 2 and 3);
(3) 15 VTE patients without any oral anticoagulant therapy (OAT) who stopped anticoagulant and antiplatelet therapy at least 1 month before the enrolment;
(4) the control group of 25 healthy volunteers.
All patients (groups 1 – 3) fulfilled the diagnostic criteria for VTE at least 6 months prior to enrolment.
The exclusion criteria for VTE subjects were:
• acute thromboembolic events within preceding 6 months,
• use of anticoagulants other than non-vitamin K anticoagulants (NOACs) or VKAs,
• known malignancy,
• signs of acute infection,
• pregnancy or postpartum period,
• end-stage kidney disease.
The exclusion criteria for healthy volunteers were:
• personal history of arterial or venous thrombosis,
• any acute illness,
• hepatic and renal dysfunction,
• use of any medication within previous 14 days,
• use of hormone replacement therapy and/or oral contraceptives.
Data on demographics, medical history and current treatment were collected using a standardized questionnaire. The diagnosis of DVT was established by a positive finding of color duplex sonography (visualization of an intraluminal thrombus in calf, popliteal, femoral or iliac veins). The diagnosis of PE was based on the presence of typical symptoms and positive results of high-resolution spiral computed tomography. Unprovoked (idiopathic) VTE episode was defined as having no history of cancer, surgery requiring general anesthesia, major trauma, plaster cast, immobilization or hospitalization in the past month, oral contraceptive use or hormone replacement therapy and pregnancy or delivery in the past 3 months. Antiphospholipid syndrome (APS) was diagnosed according to the revised classification criteria by Miyakis et al. (16).
The Jagiellonian University Medical College Ethical Committee approved the study and participants provided informed consent in accordance with the Declaration of Helsinki.
Laboratory analysis
After an overnight fast venous blood drawn from the antecubital vein into citrated tubes (9:1 of 0.106 M sodium citrate) was centrifuged at 2500 g at 20ºC for 20 minutes, and stored in small aliquots at –80ºC until analysis. Blood drawn into serum tubes was centrifuged at 1600g at 4ºC for 10 minutes and stored at –80ºC. To determine glucose, lipid profile, international normalized ratio (INR), and activated partial thromboplastin time (APTT), routine laboratory assays were used. Fibrinogen was measured by the von Clauss method (Instrumentation Laboratory, Bedford, MA, USA). Immunoturbidimetric assay was used to determine plasma D-dimer (Siemens, Marburg, Germany). Rivaroxaban concentrations were measured by the anti-factor Xa chromogenic assay, Biophen DiXaI (Hyphen Biomed, Neuilly-sur-Oise, France) according to the manufacturer’s instruction (17-19). High-sensitivity C-reactive protein (hsCRP) was determined using immunoturbidimetry (Roche Diagnostics, Mannheim, Germany). Factor V Leiden (FVL) and prothrombin G20210A polymorphisms were determined by the polymerase chain reaction followed by restriction fragment length polymorphism analysis as described (9).
Rheology of a fibrin clot
Rheological measurements were performed using the rheometer DHR-1 (TA Instruments, New Castle, DE, USA) with 20 mm parallel plates geometry and 200 µm gap between plates. Plasma samples were activated with tissue factor (TF, Innovin, Siemens, Marburg, Germany) at a final concentration of 5 pM, 20 mM calcium chloride and 10.5 µM phospholipids (Rossix, Molndal, Sweden) in Tris-buffered saline, pH 7.5. The reaction components were thoroughly mixed and transferred immediately (< 30 seconds) to parallel plate geometry. Time sweep tests were performed for 30 minutes under an oscillation procedure of 2% strain at an angular frequency of 5 radians/seconds. All measurements were performed at 37ºC. G' and G'' moduli, which correspond to the elastic and viscous properties of the clot, respectively, were recorded in duplicate for each sample. G' and G'' were calculated on the basis of average values obtained at the plateau phase within the time interval from 1250 to 1800 seconds. G' and G'' values obtained for control clots were considered as normal values, while clots characterized by higher G’/G” were recognized as more rigid and viscous. The coefficients of intra- and inter-assay variations were 10% and 12%, respectively.
Scanning electron microscopy
Fixation was performed after the permeability measurement using 2.5% glutaraldehyde (in 0.1 M phosphate-buffered saline solution, pH 7.4) for 2 hours. Fixed clots were gently removed from tubes, washed with distilled water, and then dehydrated in graded water-ethanol solutions, dried by the critical point procedure, and sputter coated with gold. Finally, the treated clots were scanned in nine different areas (microscope JEOL JCM-6000; JEOL Ltd., Tokyo, Japan). For each clot 4 micrographs were performed at machine magnification of 20,000 × to evaluate fibrin diameter based on 40 – 50 fibers per each clot using ImageJ (US National Institutes of Health, Bethesda, MD, USA) (20).
Statistical analysis
Categorical variables were presented as numbers and percentages and were analyzed by Pearson’s χ2 or Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM) or median with interquartile range. Normality of the data was assessed using Shapiro-Wilk test. Differences between 2 groups were compared using the Student's test for normally distributed continuous variables and for non-normally distributed continuous variables the Mann-Whitney U test was used. Analysis of covariance (ANCOVA) was used to compare continuous variables across > 2 groups with adjustment for fibrinogen. Multiple comparisons were performed with Tukey-Kramer adjustment. Associations between nonparametric and parametric variables were assessed by Spearman’s and Pearson’s tests, respectively. P-values of < 0.05 were considered statistically significant. All statistical analyses were performed using JMP® Version 13.1.0 and SAS 9.4 (SAS Institute Inc.).
RESULTS
VTE patients and healthy volunteers did not differ with regard to most variables, except for lower BMI observed in controls compared with VTE patients (P = 0.006; Table 1). The VTE patients on rivaroxaban and VKA had higher fibrinogen and hsCRP levels compared to controls (all P < 0.05; Table 1). No intergroup differences were found in other laboratory parameters (Table 1). Patients on VKAs had time in therapeutic range above 60% in the 3 preceding months. None of the VTE patients experienced recurrent VTE prior to enrolment while on OAT. The highest prevalence of FV Leiden mutation was observed in patients taking rivaroxaban compared with the 3 other groups, while the prevalence of prothrombin G20210A mutation was observed with similar frequency in all groups (Table 1).
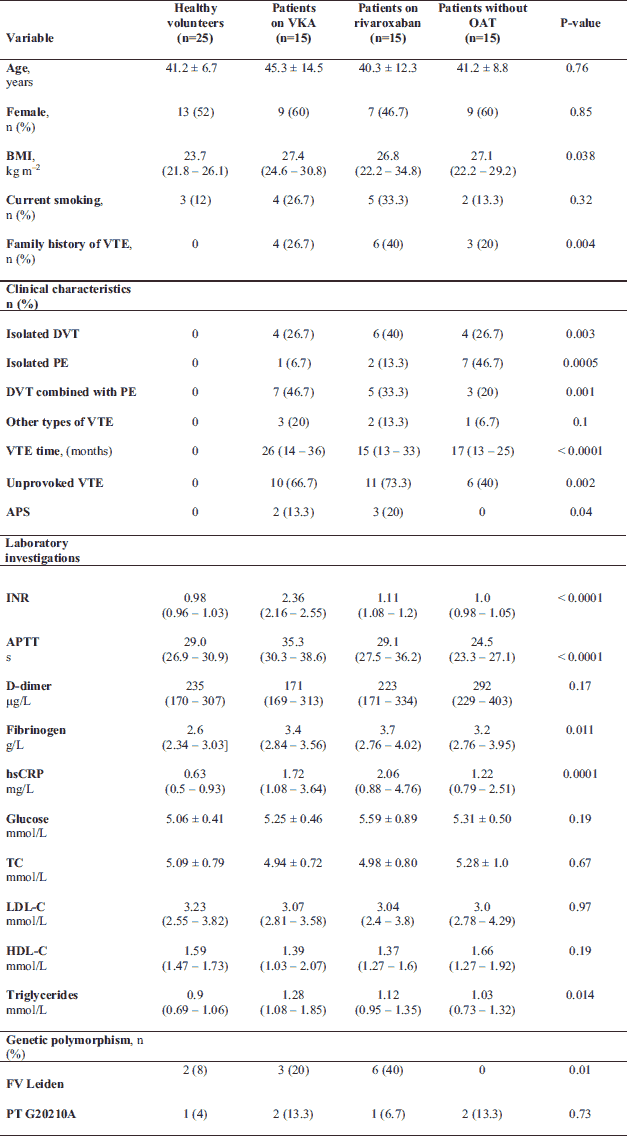
APS, antiphospholipid syndrome; APTT, activated partial thromboplastin time; BMI, body mass index; DVT, deep vein thrombosis; HDL-C, high density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; INR, international normalised ratio; LDL-C, low density lipoprotein cholesterol; OAT, oral anticoagulant therapy; PE, pulmonary embolism; PT G20210A, prothrombin G20210A mutation; TC, total cholesterol; VKA, vitamin K antagonists; VTE, venous thromboembolism; VTE time, months from the diagnosis of the last VTE incident;
VTE patients treated with rivaroxaban in whom the blood was drawn minimum 2 hours after drug admission had a median drug’s concentration of 145 ng/ml (interquartile range 67 – 217 ng/ml). Higher G' but not G'' was observed for clots generated from plasma of VTE patients taking rivaroxaban (+34%; post hoc, P = 0.029) compared to controls (Table 2). As reflected by lower G' and G'', patients taking rivaroxaban (–19% and –30%; post hoc, P = 0.0013 and P < 0.0001, respectively) formed less stiff and viscous clots compared to VTE patients after OAT withdrawal (Fig. 1A and 1B), also after adjustment for FVL mutation. All differences remained significant after adjustment for fibrinogen. G' and G'' correlated with fibrinogen concentrations (r = 0.62, P = 0.0009 and r = 0.58, P = 0.002 for the control group and r = 0.51, P = 0.049, and r = 0.59, P = 0.02 for rivaroxaban, respectively). G' and G'' correlated also with plasma rivaroxaban concentrations (r = –0.67, P = 0.005 and r = –0.59, P = 0.021, respectively), and the time from the last dose of rivaroxaban intake (median, 4.5 (3 – 19) h) (r = 0.59, P = 0.02 and r = 0.58, P = 0.022, respectively).
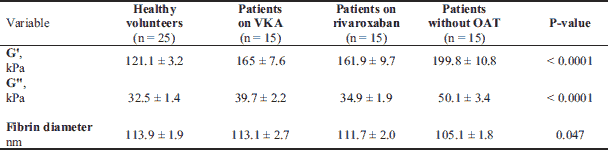
G', storage modulus; G'', loss modulus, OAT, oral anticoagulant therapy; VKA, vitamin K antagonists.
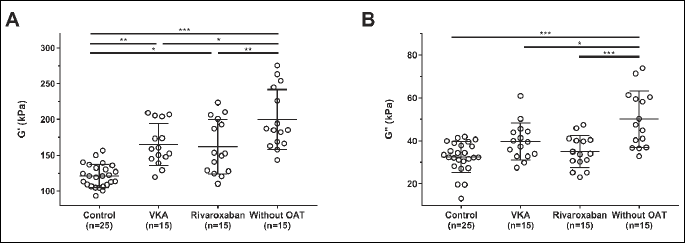
Panel (A) storage modulus – G', Panel (B) loss modulus – G''. VTE, venous thromboembolism; VKA, vitamin K antagonists; OAT, oral anticoagulant therapy.
Multiple comparisons were performed with Tukey-Kramer adjustment. Significance is shown as *P < 0.05; **P < 0.001; ***P < 0.0001.
Similarly like in patients taking rivaroxaban, subjects anticoagulated with VKA had higher G' but not G'' (+36%; post hoc, P = 0.0013) compared to controls (Fig. 1A and 1B). As expected, VTE patients treated with VKA (–17% and –21%; post hoc, P = 0.015 and P = 0.017, respectively) formed less rigid and viscous clots compared to VTE patients without OAT (Table 2). All differences remained significant after adjustment for fibrinogen and FVL mutation (all P < 0.05). Moreover, we showed no differences in G' and G'' in VTE patients treated with acenocoumarol (n = 8) or warfarin (n = 7) (P = 0.71, P = 0.38, respectively). Of note, VTE patients treated with rivaroxaban and VKA had similar viscoelastic properties of the fibrin clots (post hoc, P = 0.85 for G' and P = 0.29 for G'') (Table 2) (Fig. 1A and 1B).
Lower G' and G'' were observed in healthy volunteers (–39% and –35%; post hoc, both P < 0.0001) as compared to VTE patients after anticoagulation cessation (Table 2) (Fig. 1A and 1B).
In VTE patients after OAT withdrawal, positive associations between G' and G'' and the time since the end of anticoagulation (median, 6 (3 – 12) months) were observed (r = 0.85, P < 0.0001, and r = 0.71, P = 0.003, respectively). There were no associations between G'/G'' and demographic or clinical factors, INR, D-dimer, hsCRP, glucose, and lipid profile.
Representative scanning electron microscopy images demonstrated that clots generated from plasma of the VTE subjects without OAT were composed of thinner fibrin fibers than clots of healthy controls or anticoagulated VTE patients with rivaroxaban and VKA (Fig. 2). This observation was confirmed by the measurement of fibrin fiber thickness (post hoc, P = 0.017, P = 0.022 and P = 0.024, respectively; Table 2) and remained significant after adjustment for fibrinogen. No associations were found between G' or G'' and fibrin diameter in either group.
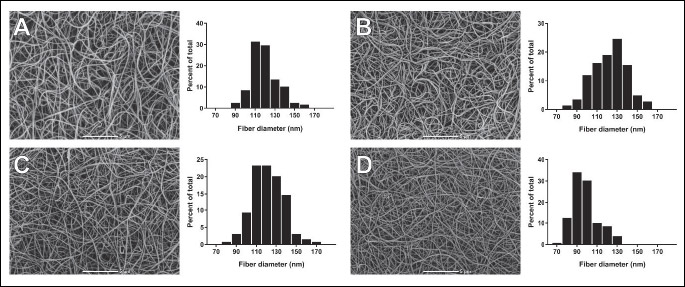
VTE, venous thromboembolism, VKA, vitamin K antagonists; OAT, oral anticoagulant therapy. Machine magnification × 5000; scale bar 5 µm.
DISCUSSION
This study is the first to investigate whether rivaroxaban and VKAs alter the two major plasma clot rheological properties such as G' and G'' in VTE patients. Rheology is a well-established method for studying the viscoelastic properties of liquid materials in response to applied force and has been used to evaluate biological samples, however data generated in plasma fibrin clots are limited (13-15). Using the hybrid rheometer and protocol developed in our lab, we demonstrated that rivaroxaban and VKAs are associated with improved viscoelastic properties of fibrin clots prepared from citrated plasma of VTE patients. Importantly, there were strong relationships between plasma rivaroxaban concentrations and the G' and G'' values, suggesting a potent impact of FXa inhibition on fibrin properties, which is in contrast with the VKA group where INR values were not associated with either G' or G'' values. Interestingly, studies on mechanical properties of whole blood clots using a biomarker of clot microstructure - Df in patients on warfarin did not demonstrate associations with INR. Moreover, procoagulant state in these VTE subjects was not detected by INR, thromboelastography or thrombin generation (26). We found associations between the storage and loss moduli with time from the last dose of rivaroxaban intake. This suggests a direct beneficial impact of rivaroxaban and possibly other FXa inhibitors such as apixaban and edoxaban on viscoelastic properties measured in plasma clots using rheometry. The present study adds new data on physical plasma clot characteristics by showing, to our knowledge for the first time, similarities in viscoelastic properties of plasma fibrin clots in anticoagulated VTE patients on two different agents.
It has been demonstrated that VTE and coronary artery disease are associated with the prothrombotic fibrin clot phenotype, including its impaired susceptibility to lysis and lower permeability (9, 21-23). Fibrin as a viscoelastic polymer demonstrates both elastic and viscous properties, therefore clots which structure is looser and more permeable are less stiff and elastic. It is known that higher concentrations of fibrinogen cause higher clot stiffness (24), which is in line with our present findings. Our observations indicate that G' or G'' reflect specific clot physical features, beyond key functional plasma clot characteristics which highlights benefits from comprehensive plasma clot analysis in VTE patients and possibly in other prothrombotic disease states. In our opinion rheometry allow to investigate other aspects of fibrin clot properties, different from clot porosity, or fiber thickness on clot imaging, and offers an additional value by making this analysis more refined. It might be speculated that G' and G'' might be of clinical importance through its potential for identifying patients at risk of recurrent VTE. This hypothesis is worth investigating on larger patient groups.
Data on viscoelastic properties of fibrin clots in VTE patients are limited. Previous studies have demonstrated that patients with acute PE compared to DVT had shorter lag time and increased rate of fibrin formation (25). Recent studies on mechanical properties of fibrin clots prepared from a whole blood of VTE and non-VTE patients taking warfarin (mean INR, 2.7 for both groups) have shown that VTE subjects are still characterized by 'abnormal' clot microstructure and probably require more efficient anticoagulant therapy (26). We extended this observation by showing that OAT either with rivaroxaban or VKA improves clot microstructure almost to the extent observed in controls, while a few months after OAT cessation the clot phenotype is prothrombotic regardless of the VTE type (provoked/unprovoked), which possibly reflects a prolonged prothrombotic state. It remains to be established whether G'/G'' tend to improve with time since the index event at least following provoked episodes. It is also unknown whether assessment of clot mechanical properties, reflected by G' and G'', could be a useful tool for evaluating the anticoagulation effectiveness or safety. Current evidence indicates that both the approach using fractal dimension based on a measurement of the gel point of whole blood clot as well as typical markers describing clot mechanical properties, such as G' and G'', are able to detect changes in the clot microstructure related to anticoagulant therapy and deserve to be considered as global markers of hemostasis (14, 15, 26).
This study has several limitations. The group size was limited and type II errors cannot be avoided. However, this study is preliminary and hypothesis generating, suggesting potent effects of OAT on viscoelastic features assessed in VTE patients, which is a novel finding. Second, the current findings cannot be easily extrapolated on subjects with acute DVT and PE or other oral anticoagulants (16, 26). Third, the application limitations of rheological methods are linked with two phenomena; (1) difference between time of rheological measurement and rate of fibrin polymerization and (2) measurement conditions: the value of strain amplitude should be optimized for both initial (start of fibrin polymerization) and final clot (gel state) conditions. We did not evaluate viscoelastic properties of whole blood clots. The contribution of erythrocytes or platelets to clot rheological features is however significant as described (27). Therefore, the results of our study should be interpreted with caution. Finally, serial analysis of G' and G'' before and during anticoagulation could provide more insights into the phenomena studied, however the current study was cross-sectional by design.
Our study provides evidence that rheology of plasma clots is needed to comprehensively analyse fibrin clot properties in VTE patients and their modulation by anticoagulant medication. Analysis of plasma clot microstructure reflected by G' and G'' can expand the available routine coagulation workup and might help optimize OAT in particular with rivaroxaban with close association with the drug concentration. Since it is known that some unfavourably altered clot characteristics can predict recurrent VTE events (11), the pathophysiological role of increased clot stiffness and its on-treatment values might have potential clinical implications. Therefore, the presented data indicate that VTE patients taking rivaroxaban or VKA have similarly improved clot viscoelastic properties compared to VTE patients after OAT cessation. Further studies on real-life patients on other anticoagulants are needed to better describe fibrin clot properties in various prothrombotic diseases.
Acknowledgments: We thank to Joanna Wzorek for technical support.
The study was supported by the Polish National Science Center (DEC-2018/02/X/NZ5/00004, to TG).
Conflict of interest: None declared.
REFERENCES
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005; 3: 1894-1904.
- Liu W, Jawerth LM, Sparks EA, et al. Fibrin fibres have extraordinary extensibility and elasticity. Science 2006; 313: 634. doi: 10.1126/science.1127317
- Rybarczyk BJ, Lawrence SO, Simpson-Haidaris PJ. Matrix-fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood 2003; 102: 4035-4043.
- Laurens N, Koolwijk P, de Maat MPM. Fibrin structure and wound healing. J Thromb Haemost 2006; 4: 932-939.
- Janion-Sadowska A, Natorska J, Siudut J, Zabczyk M, Stanisz A, Undas A. Plasma fibrin clot properties in the G20210A prothrombin mutation carriers following venous thromboembolism: the effect of rivaroxaban. J Thromb Haemost 2017; 117: 1739-1749.
- Krzek M, Ciesla Dul M, Zabczyk M, Undas A. Fibrin clot properties in women heterozygous for factor V Leiden mutation: effects of oral contraceptives. Thromb Res 2012; 30: e216-e221.
- Brzezińska-Kolarz B, Kolarz M, Walach A, Undas A. Weight reduction is associated with increased plasma fibrin clot lysis. Clin Appl Thromb Hemost 2014; 20: 832-837.
- Ariens RA. Denser matters. Blood 2009; 114: 3978-3979.
- Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 2009; 114: 4272-4278.
- Cieslik J, Mrozinska S, Broniatowska E, Undas A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: a cohort study. Blood 2018; 131: 797-807.
- Zabczyk M, Plens K, Wojtowicz W, Undas A. Prothrombotic fibrin clot phenotype is associated with recurrent pulmonary embolism after discontinuation of anticoagulant therapy. Arterioscler Thromb Vasc Biol 2017; 37: 365-373.
- Zabczyk M, Majewski J, Karkowski G, Malinowski KP, Undas A. Vitamin K antagonists favourably modulate fibrin clot properties in patients with atrial fibrillation as early as after 3 days of treatment: Relation to coagulation factors and thrombin generation. Thromb Res 2015; 136: 832-838.
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem 2004; 112: 267-276.
- Knowles RB, Lawrence MJ, Ferreira PM, et al. Platelet reactivity influences clot structure as assessed by fractal analysis of viscoelastic properties. Platelets 2018; 29: 162-170.
- Evans PA, Hawkins K, Morris RHK, et al. Gel point and fractal microstructure of incipient blood clots are significant new markers of hemostasis for healthy and anticoagulated blood. Blood 2010; 116: 3341-3346.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295-306.
- Zalewski J, Rychlak R, Goralczyk T, Undas A. Rivaroxaban concentration in patients with deep vein thrombosis who reported thrombus progression or minor hemorrhagic complications: first Polish experience. Pol Arch Med Wewn 2014; 124: 553-555.
- Weitz JI, Jaffer IH. Optimizing the safety of treatment for venous thromboembolism in the era of direct oral anticoagulants. Pol Arch Med Wewn 2016; 126: 688-696.
- Raschi E, Bianchin M, Ageno W, De Ponti R, De Ponti F. Adverse events associated with the use of direct-acting oral anticoagulants in clinical practice: beyond bleeding complications. Pol Arch Med Wewn 2016; 126: 552-561.
- Hotaling NA, Bharti K, Kriel H, Simon CG. A validated open source nanofiber diameter measurement tool. Biomaterials 2015; 61: 327-338.
- Siudut J, Swiat M, Undas A. Altered fibrin clot properties in patients with cerebral venous sinus thrombosis: association with the risk of recurrence. Stroke 2015; 46: 2665-2668.
- Zabczyk M, Undas A. Plasma fibrin clot structure and thromboembolism: clinical implications. Pol Arch Intern Med 2017; 127: 873-881.
- Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol 2006; 26: 2567-2573.
- Ryan EA, Mockros LF, Weisel JW, Lorand L. Structure origins of fibrin clot rheology. Biophys J 1999; 77: 2813-2826.
- Martinez MR, Cuker A, Mills AM, et al. Enhanced lysis and accelerated establishment of viscoelastic properties of fibrin clots are associated with pulmonary embolism. Am J Physiol Lung Cell Mol Physiol 2014; 306: 397-404.
- Lawrence MJ, Sabra A, Mills G, et al. A new biomarker quantifies differences in clot microstructure in patients with venous thromboembolism. Br J Haematol 2015; 168: 571-575.
- Tutwiler V, Litvinov RI, Lozhkin AP, et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood 2016; 127: 149-159.
A c c e p t e d : February 28, 2019