SERUM GALECTIN 3, GALECTIN 9 AND GALECTIN 3-BINDING PROTEINS IN PATIENTS WITH ACTIVE AND INACTIVE INFLAMMATORY BOWEL DISEASE
2Department of Integrated Dentistry, Jagiellonian University Medical College, Cracow, Poland
INTRODUCTION
Galectins are lectins that bind β-galactosides due to the presence of a carbohydrate recognition domain (1). To date, 12 galectin-related proteins have been identified in humans (1, 2). They are involved in physiological processes such as cell proliferation, apoptosis, migration, immune responses, inflammation, signalling, angiogenesis, and pathological phenomena such as tumour progression and metastasis (3-6, 7). The activities of galectins differ depending on the specific tissue and subcellular location (1). They are found both intracellularly and extracellularly (4, 6). Galectins-1, -2, -3, -4, -7, -8, -9, and -12 induce T-cell apoptosis and modulate inflammation (1). They are expressed by many immune cells such as macrophages, dendritic cells, B and T lymphocytes, as well as endothelial cells, epithelial cells, stromal cells of different tissues, and sensory neurons (2, 7-9).
Galectin-3 (Gal-3) is the only chimera galectin. It can be both anti-apoptotic and pro-apoptotic, and has anti-inflammatory and pro-inflammatory effects (1, 3). Gal-3 is highly expressed in the epithelium of the gastrointestinal tract, lungs, and urinary bladder, but is also found in most other tissues (10-12). Galectin-3 (Gal-9) is a tandem repeat galectin with two carbohydrate recognition domains (1). In mouse models, it is expressed in the liver, small intestine, thymus, kidney, spleen, lung, cardiac and skeletal muscles, and brain (13). Its alternate splicing isoform is only expressed in the small intestine (13). Patients with food allergies show increased Gal-9 expression in intestinal epithelial cells (1).
There is some evidence that galectins play important roles in the pathogenesis of several chronic inflammatory disorders including rheumatoid arthritis, osteoarthritis, juvenile idiopathic arthritis, asthma, exacerbated chronic obstructive pulmonary disease, inflammatory bowel disease (IBD), and Helicobacter pylori infection (6, 11, 14-19). Gal-3 expression has also been reported in many types of fibrosis including the liver, pancreas and lungs (11). It is also related to thrombogenesis and increased serum levels of Gal-3 and Gal-3BP have been found in both mice and humans with venous thrombosis (12).
Galectin-3-binding protein (Gal-3BP), also known as MAC-2-binding protein or 90K, is important for the modification of galectin-mediated biological effects (12, 20, 21). It promotes cell adhesion and binds to type V and VI collagens, integrins, and fibronectin (22, 23). It can also be involved in cancer growth and progression (21). In most adult tissues, it is detectable at low levels, while its high expression has been found in several malignancies such as lymphoma, melanoma, and different types of cancers including head, neck, oesophagus, breast, lung, prostate, and ovarian cancers (21).
Gal-3BP is a serum marker of inflammation in patients with autoimmune hepatitis, rheumatoid arthritis, juvenile idiopathic arthritis, asthma. It can also be a marker of liver fibrosis in viral hepatitis, non-alcoholic fatty liver disease, autoimmune hepatitis, primary biliary cholangitis, idiopathic pulmonary fibrosis, and chronic pancreatitis (22-26). In addition, patients with viral infections express Gal-3BP in greater amounts (27).
IBD including ulcerative colitis (UC) and Crohn’s disease (CD) are disorders with an etiopathogenesis that is not fully understood (28-31). Interactions among genetic predispositions, changes in microbiota, several environmental factors, and pathological immune responses lead to chronic intestinal inflammation, and endothelial dysfunction and overproduction of reactive oxygen species also play important roles (29, 30, 32).
Plasma biomarkers are of increasing interest in IBD and can provide prognostic value and insight into the pathophysiology of the disease. To date, the expression of galectins in patients with IBD has mainly been assessed within intestinal epithelial cells (6, 33-35). To the best of our knowledge, only one study has reported the association between serum levels of Gal-3 in IBD patients in connection with the clinical activity of the disease (36). Therefore, we measured the serum levels of Gal-3, Gal-9, and Gal-3BP and evaluated their possible correlations with clinical characteristics and levels of inflammatory markers in patients with IBD.
MATERIALS AND METHODS
Study population
Consecutive adult patients (18 – 75 years of age) with diagnosed IBD (48 UC patients and 77 CD patients) were involved in this study. The diagnoses of IBD were based on standard clinical, radiological, endoscopic, and histological criteria (37). The patients were recruited from the Department of Gastroenterology and Hepatology, Jagiellonian University Hospital, Cracow, Poland. The controls included 30 healthy volunteers. The exclusion criteria were pregnancy, concomitant acute or chronic inflammatory disorders, and severe diseases, including heart failure, arrythmias, renal insufficiency, respiratory insufficiency, liver injury, diabetes, and malignancies.
This study was approved by the Jagiellonian University Medical College Bioethics Committee, and the study was performed in accordance with the ethical principles of the Helsinki Declaration of 2008.
Clinical assessment
Demographics and clinical information including cigarette-smoking habits, the presence of comorbidities, and medications were obtained during patient interviews. Then body mass index (BMI) was calculated. In IBD patients, the following parameters were evaluated: disease duration, disease location and extent, disease activity, complications, and past surgical procedures. Complications were defined as abscesses, fistulae, and stenoses resulting in post-obstructive symptoms or presence of extraintestinal symptoms. To determine the location of lesions in UC and CD patients, the Montreal classification was used (38). To assess CD and UC activities, Crohn’s Disease Activity Index (CDAI) (39) and disease activity index (DAI) were used (40).
Data collection
Fasting blood samples were collected from the antecubital vein in the morning. The complete blood count, C-reactive protein (CRP), fibrinogen, albumin, glucose, and creatinine were determined using routine laboratory techniques. Human Gal-3 and Gal-9 were determined using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Human Galectin-3 Quantikine ELISA and Human Galectin-9 Quantikine ELISA; R&D Systems, Minneapolis, MN, USA). Optical density was measured on a plate reader using 450 nm, and data were collected using Gen 5 (BioTek, Winooski, VT, USA) software. A log/log curve fit was used to generate the standard curve. Results for both parameters were expressed as ng/ml. Human Gal-3BP was determined using an ELISA according to the manufacturer’s instructions (Mac-2BP Human ELISA Kit; Abcam, Cambridge, UK). The optical density was measured on a plate reader using 450 nm, and data were collected using Gen 5 software (BioTek). A four parametric curve fit was used to generate the standard curve. The results for both parameters were expressed in ng/ml.
Statistical analysis
Comparisons of quantitative variables in two groups were conducted using the Student’s t-test (in case of normal distributions in both groups) or Mann-Whitney U test (otherwise). Comparisons of quantitative variables in more than two groups were conducted using analysis of variance (in case of a normal distribution in each group) or the Kruskal-Wallis test (otherwise). Fisher’s LSD test (in case of normal distributions in each group) or the Dunn test (otherwise) were used as post hoc procedures. Correlations among quantitative variables were assessed with Pearson’s (in case of normal distribution of both variables) or Spearman’s (otherwise) correlation coefficients. The strength of association was judged using the following schema: |r| ≥ 0.9, very strong; 0.7 ≤ |r| < 0.9, strong; 0.5 ≤ |r| < 0.7, moderate; 0.3 ≤ |r| < 0.5, weak; and |r| < 0.3, very weak. Normality was assessed using the Shapiro-Wilk test. Analyses were conducted at a 0.05 level of significance using R software, version 3.5.1 (R Foundation, Vienna, Austria).
RESULTS
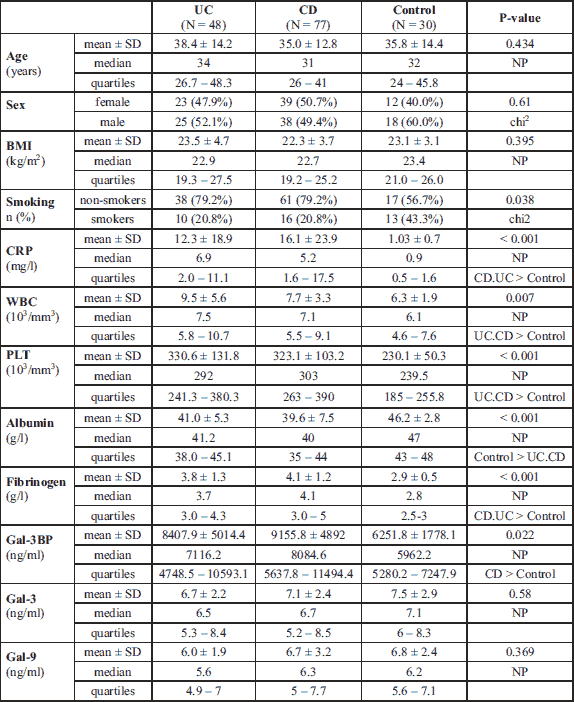
Patient characteristics
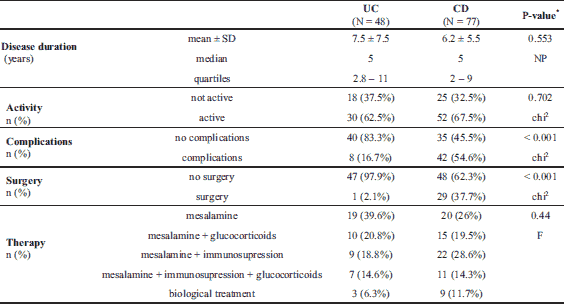
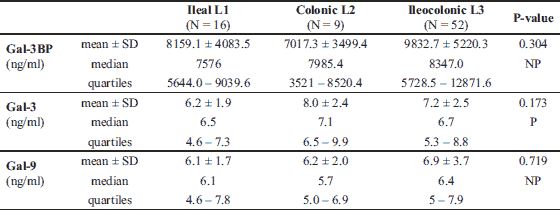
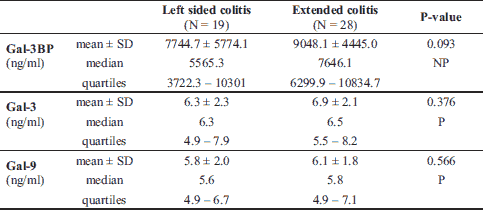
The characteristics of IBD patients and controls are listed in Table 1. UC and CD groups as well as controls did not significantly differ in terms of sex, median age, and median BMI. There were fewer smokers in the IBD groups than in the control group (approximately 20% versus > 40%, P = 0.04). Median levels of inflammatory markers such as white blood cells, CRP, platelets, and fibrinogen were significantly higher in IBD groups than in the healthy control group, whereas the median levels of albumin were lower (Table 1). Data for the UC and CD groups regarding IBD characteristics are listed in Table 2. CD patients presented with more intestinal complications than UC patients, and more frequently underwent abdominal surgery (P < 0.001). In the CD group, there were 16 patients (20.8%) with L1, 9 (11.7%) with L2, and 52 (67.5%) with L3 disease locations. In the UC group, there was only 1 patient (2.1%) with an E1 location, 19 (39.6%) with an E2, and 28 (58.3%) with an E3 disease location.
Galectins and Gal-3BP
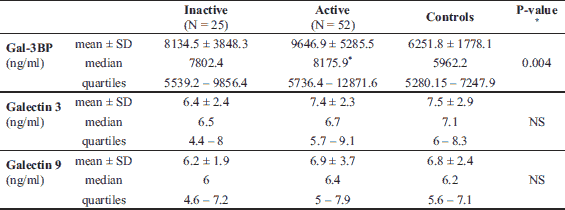
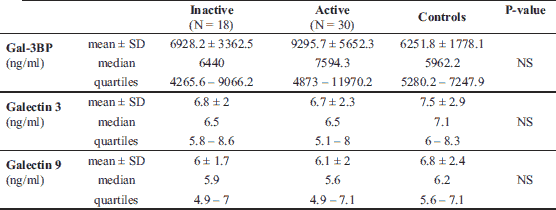
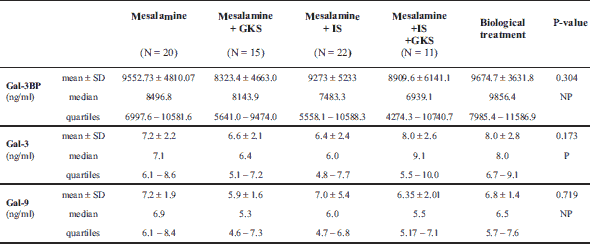
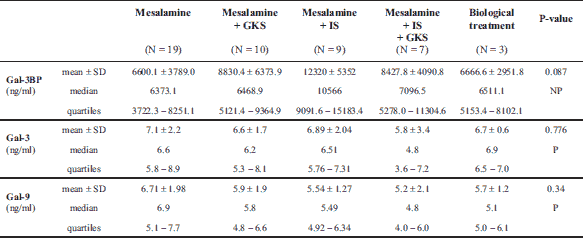
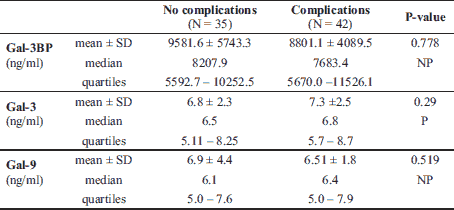
The median Gal-3 and Gal-9 levels did not differ between patients and controls (Table 1). Only the Gal-3BP median level was significantly higher in CD patients than in healthy controls (8084.6 (5637.8 – 1494.4) ng/ml versus 5962.2 (5280.15 – 247.92) ng/ml, P = 0.02). No significant differences in Gal-3, Gal-9, or Gal-3BP between active and inactive CD and UC subgroups were found (Tables 3 and 4, respectively). However, the median Gal-3BP level was higher in the subgroup with active CD than in the controls (8175.9 (5736.4 – 2871.62) ng/ml versus 5962.2 (5280.15 – 7247.92) ng/ml, P = 0.004). Galectins levels did not correlate with disease duration (Table 5). No differences in the galectins or Gal-3BP level were also found in dependence of disease location (Tables 6 and 7), type of implemented therapy (Tables 8 and 9), or the presence of complications (Table 10). No sex-related difference was found in Gal-3 and Gal-3BP serum concentrations. Only the mean Gal-9 level was elevated in UC female patients compared to male patients (6.66 ± 2.16 ng/ml versus 5.46 ± 1.33 ng/ml, P = 0.02). The mean Gal-3 level was higher in smoking patients than in non-smoking UC patients (7.12 ± 2.22 ng/ml versus 5.23 ± 1.13 ng/ml, P = 0.01) (Fig. 1), whereas in CD patients, the median Gal-3BP was higher in smoking patients (10830.2 (7161.9 – 19103.23) ng/ml than in non-smoking patients 7782.8 (5539.2 – 0150.7) ng/ml, P = 0.03), UC patients, and controls (Fig. 2).
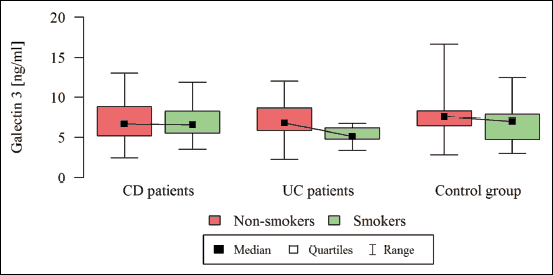 |
Fig. 1. Galectin 3 in dependence of smoking status. |
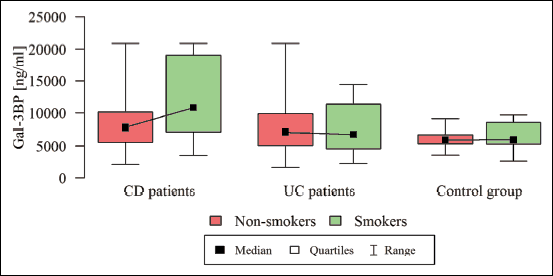 |
Fig. 2. Galectin 3 binding protein in dependence of smoking status. |

Correlations between selected markers
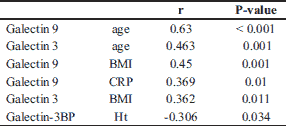
In both the UC and CD groups, Gal-3 levels moderately correlated with Gal-9 levels (r = 0.54, P < 0.001). In the CD group, the Gal-3 and Gal-9 levels very weakly correlated with age (r = 0.25, P = 0.03; r = 0.27, P = 0.02, respectively). Gal-9 also correlated very weakly with the BMI (r = 0.27, P = 0.02). Correlations between Gal-3, Gal-9, Gal-3BP, and biochemical parameters, age, and BMI in UC patients are shown in Table 11. In the CD group, the Gal-3 level very weakly correlated with the level of CDAI (r = 0.26, P = 0.02). There was no correlation among Gal-9, Gal-3BP, and disease activity in both CD patients, and no correlation among galectins, Gal-3BP, and the DAI scale was found in the UC group.
DISCUSSION
To the best of our knowledge, this is the first study to show that in patients with active CD, the serum level of Gal-3BP was higher than in healthy controls. We also showed that in UC and CD patients, regardless of the disease activity, there was no increase in the serum levels of Gal-3 and Gal-9.
Johannes et al. suggested that the presence of galectins in serum may result from leakage from tissue, thereby making it a possible disease biomarker (3). Our findings contradicted the results of Frol'ova et al., who showed an elevation of plasma Gal-3 activity in both active and inactive UC and CD patients compared to levels in controls (36). This discrepancy might have resulted from the occurrence of some differences in factors that affected the levels of Gal-3 such as age, renal function, and concomitant diseases as well as various sources of commercial ELISA kits used for Gal-3 measurement (BMS 279; Bender Medsystems GmbH, Vienna, Austria); also, the control group in Frol’ova’s study was twice as big as ours. They also reported the upregulation of Gal-3 in intestinal tissue samples of IBD patients, with strong expression of Gal-3 on CD14-positive cells (monocytes/macrophages). Expression of CD14 was increased in the tissues of patients with active IBD relative to people in remission, while in healthy subjects Gal-3 was not present on CD14-positive cells but on enterocytes (36). On murine model the researches confirmed that fluorescein isothiocyanate-labeled dextran sodium sulfate failed to stain CD14-positive cells in tissue samples from healthy mice, whereas reacted with leukocytes in colon samples from IBD (36). The authors suggest that Gal-3 expressed by CD14-positive cells in inflamed mucosa might contribute to further development of inflammatory process (36).
Several studies of IBD mouse models have reported the importance of galectins in the inflammatory process (33); however, the results were not conclusive. Papa Gobbi et al., in biopsies taken from the inflamed mucosa of IBD patients, found deregulated expression levels of Gal-1, Gal-3, Gal-4, and Gal-9 mRNA, with only an increase in Gal-1 mRNA expression, whereas the expression of Gal-3, Gal-4, and Gal-9 was reduced. The authors did not find any difference in Gal-3 expression between non-inflamed mucosa in IBD patients and the control group, or between CD and UC patients (33). Decreased expression of Gal-3 in inflamed mucosa of patients with active CD was reported by Jansen-Jarolim et al., as well as in active IBD patients by Muller et al. (35, 41). It should be emphasized that the study performed using Jansen-Jarolim included only a small group of patients. However, the authors explained this phenomenon based on in vitro studies, reporting that the incubation of intestinal biopsies with tumour necrosis factor-alpha (TNF-α) resulted in downregulation of Gal-3 mRNA, which may be of importance, particularly in CD patients with TNF-alpha as the main inflammatory mediator (35, 41). There are also reports that TNF-α mRNA levels are upregulated in inflamed colonic tissue of CD patients as well as in both inflamed and non-inflamed colonic mucosa of UC patients, compared to the controls (8, 42).
Gal-3 is also an activator of colonic lamina propria fibroblasts (6). Lippert et al. found that in patients with CD in fistulae and stenoses, the expression of Gal-3 mRNA was almost undetectable, while no statistically significant difference was observed between inflamed mucosa and normal mucosa. The authors also reported that in CD patients, there was a lower expression of Gal-3 mRNA in the terminal ileum compared to the colon of controls (6). However, they did not report a difference in Gal-3 mRNA expression in colonic epithelial cells between UC patients and healthy controls (6). The authors suspected that increased levels of TNF-α contributed to the lower expression of Gal-3 (6). Chronic inflammatory processes also result in injury to epithelial cells, which might release Gal-3, and increased Gal-3 levels will increase migration of fibroblasts, leading to formation of fistulae and stenoses.
Gal-3 is a chemoattractant and adhesion factor, which activates monocytes/macrophages, promotes adhesion of neutrophils, and takes part in eosinophil recruitment (17). Xue et al. reported that expression of CD98+ eosinophils in intestine biopsies was upregulated in IBD patients (43). After activation, dendritic cells express Gal-3, which is a ligand for CD98 (43, 44). Gal-3 binds CD98, resulting in activation of eosinophils, which release inflammatory mediators and induce inflammation in the intestine (43).
The objective of current treatment in IBD patients is to achieve deep remission, including mucosal healing, which improves the long-term outcome, and reduces the risk of disease relapse and the occurrence of complications (45, 46). Shallow wounds heal quickly with the involvement of few biological processes, whereas deep or large wounds require supplementary processes (47). Puthenedam et al. showed in vitro that cleavage of Gal-3 by matrilysin-1 leads to inhibition of wound healing in IBD patients, which may be one of the explanations of prolonged ulcer healing in IBD patients (48).
There is minimal data on the relationship between Gal-9 with IBD. Intestinal epithelial cells express Gal-2, Gal-3, Gal-4, and Gal-9 (13, 49). In our study, there were no differences in the serum levels of Gal-9 between IBD patients and healthy controls. Papa Gobbi et al. reported the downregulated expression of Gal-9 mRNA in biopsies taken from the inflamed mucosa of IBD patients (33). Kivit et al. found that intestinal epithelium-derived Gal-9 is involved in the immunomodulating effects of nondigestible oligosaccharides (50). Gal-9 has an immunomodulatory effect on many cell types including vascular endothelial cells and leukocytes (15). In studies by O’Brien et al. it was observed that Gal-9 increases monocyte migration in vitro, while it increases angiogenesis in vivo (15). It also induces apoptosis of Th1 cells independently or through interaction with T-cell immunoglobulins and the mucin domain containing-3 (51).
Except for the increased concentration of Gal-9 in females with UC, we did not find any difference between groups, which was consistent with some recent reports (52), but which contradicted other studies that reported higher Gal-3 levels in females (53, 54).
Our study showed a moderate positive correlation between age and Gal-9 and a weak positive correlation between age and Gal-3 in the UC group, but a very weak correlation between age and galectins in the CD group. These observations were consistent with some previous studies (53, 55, 56), as Meeusen et al. did not show dependence on age, although only children participated in the study (57). In our study, Gal-3BP levels did not differ between groups in terms of sex and age, which was consistent with other findings (58).
We also found a positive correlation between BMI and galectins in UC patients and between BMI and Gal-9 in CD patients. Similar observations regarding Gal-3 have been reported by others in children (59) and in adults (53).
Smoking affects IBD, which is a risk factor for CD and a protective factor for UC (60). It was notable that in our study, the concentration of Gal-3 was higher in UC smokers than in non-smokers. However, in CD smokers, the Gal-3BP level was lower than in non-smokers, UC patients, and controls. There is a possibility that these differences might play a role in modifying the impact of smoking on CD and UC patients. It has recently been proposed that smoking may affect the development of IBD through nicotine acetylcholine receptors of the bowel epithelial cells or T cells. The alternative mechanisms include the involvement of factors other than nicotine chemical substances, which may alter cytokine levels (61).
In our study, we demonstrated an increase in Gal-3BP levels in active CD patients. To the best of our knowledge, this is the first study to assess serum Gal-3BP levels in IBD patients. Gal-3BP promotes cell adhesion, cytokine secretion (natural killers, interleukin 1, and interleukin 6), modifies the inflammatory response, and contributes to wound healing (62-64). It is a human homologue of murine cyclophilin C-associated protein (CyC-AP), with 69% identity to this protein (64). Kong et al. showed in vivo and in vitro that CyC-AP functions as a mediator for fibronectin fragment-induced matrix metalloproteinase 13 expression, and concluded that it takes part in host defences during inflammatory disease (64). In another study, the authors reported that CyC-AP is upregulated during skin wound healing (65).
Gal-3BP should also be considered a biomarker of both the inflammatory process and fibrosis. A glycosylation isomer of Gal-3BP has been found to be a biomarker for detecting moderate and severe liver fibrosis (66). Migita et al. confirmed that serum levels of Gal-3BP glycosylation isomer were increased in patients with untreated autoimmunological hepatitis, and that steroid therapy led to reduction of these isomers, so they concluded that it reflects both inflammation and fibrosis (24).
Recent studies show that oligosaccharides may play an important role in the development of inflammation in IBD patients. Alterations in the cell surface oligosaccharides structure have an impact on immune function (67). In serum of IBD patients, an increase in fucosylated agalactosyl IgG oligosaccharides is observed what is correlated with the severity of the disease. Changes in oligosaccharides structure in serum and tissues may be used as disease biomarkers and “glyco-immunology” may contribute to the explanation of IBD pathogenesis and development of new treatment strategy (67).
Our study had several limitations. First, the sample size was small. Second, we did not evaluate the endoscopic activity of IBD patients as well as the stage of potential fibrosis. We also did not assess the epithelial expression of galectins. Finally, the study was also limited by its observational design, so we could only identify associations, and no cause-and-effect effect was implied.
In conclusion, our results showed that serum levels of Gal-3 and Gal-9 should not be considered biomarkers of IBD. Despite not being a specific marker for CD, serum Gal-3BP might be used as an adjuvant biomarker for disease activity. However, further studies using a larger cohort are required to confirm its clinical usefulness.
Abbreviations: BMI, body mass index; CD, Crohn's disease; CDAI, Crohn's disease activity index; CRP, C-reactive protein; CyC-AP, cyclophilin C-associated protein; DAI, disease activity index; Gal-3, galectin-3; Gal-3BP, galectin 3 binding protein; Gal-9, galectin 9; IBD, inflammatory bowel disease; TNF, tumor necrosis factor; UC, ulcerative colitis
Acknowledgements: This study was supported by the grant from the Jagiellonian University Medical College in Cracow, Poland.
Conflict of interest: None declared.
REFERENCES
- Brinchmann MF, Patel DM, Iversen MH. The role of galectins as modulators of metabolism and inflammation. Mediators Inflamm 2018; 2018: 9186940. doi: 10.1155/2018/9186940
- Golden-Mason L, Rosen HR. Galectin-9: diverse roles in hepatic immune homeostasis and inflammation. Hepatology 2017; 66: 271-279.
- Johannes L, Jacob R, Leffler H. Galectins at a glance. J Cell Sci 2018; 131: jcs208884. doi: 10.1242/jcs.208884
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta 2006; 1760: 616-635.
- Dings RPM, Miller MC, Griffin RJ, Mayo KH. Galectins as molecular targets for therapeutic intervention. Int J Mol Sci 2018; 19: E905. doi: 10.3390/ijms19030905
- Lippert E, Gunckel M, Brenmoehl J, et al. Regulation of galectin-3 function in mucosal fibroblasts: potential role in mucosal inflammation. Clin Exp Immunol 2008; 152: 285-297.
- Rabinovich GA, Toscano MA. Turning 'sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 2009; 9: 338-352.
- Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol 2013; 19: 8831-8849.
- van Stijn CM, van den Broek M, van de Weerd R, et al. Regulation of expression and secretion of galectin-3 in human monocyte-derived dendritic cells. Mol Immunol 2009; 46: 3292-3299.
- Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419. doi: 10.1126/science.1260419
- Sciacchitano S, Lavra L, Morgante A, et al. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int J Mol Sci 2018; 19: E379. doi: 10.3390/ijms19020379
- DeRoo EP, Wrobleski SK, Shea EM, et al. The role of galectin-3 and galectin-3-binding protein in venous thrombosis. Blood 2015; 125: 1813-1821.
- Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem 1997; 272: 6078-6086.
- Salamanna F, Veronesi F, Frizziero A, Fini M. Role and translational implication of galectins in arthritis pathophysiology and treatment: a systematic literature review. J Cell Physiol 2019; 234: 1588-1605.
- O'Brien MJ, Shu Q, Stinson WA, et al. A unique role for galectin-9 in angiogenesis and inflammatory arthritis. Arthritis Res Ther 2018; 20: 31. doi: 10.1186/s13075-018-1519-x
- Ezzat MH, El-Gammasy TM, Shaheen KY, Osman AO. Elevated production of galectin-3 is correlated with juvenile idiopathic arthritis disease activity, severity, and progression. Int J Rheum Dis 2011; 14: 345-352.
- Gao P, Simpson JL, Zhang J, Gibson PG. Galectin-3: its role in asthma and potential as an anti-inflammatory target. Respir Res 2013; 14: 136. doi: 10.1186/1465-9921-14-136
- Feng W, Wu X, Li S, et al. Association of serum galectin-3 with the acute exacerbation of chronic obstructive pulmonary disease. Med Sci Monit 2017; 23: 4612-4618.
- Park AM, Hagiwara S, Hsu DK, Liu FT, Yoshie O. Galectin-3 plays an important role in innate immunity to gastric infection by Helicobacter pylori. Infect Immun 2016; 84: 1184-1193.
- Inohara H, Akahani S, Koths K, Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res 1996; 56: 4530-4534.
- Giansanti F, Capone E, Ponziani S, et al. Secreted Gal-3BP is a novel promising target for non-internalizing antibody-drug conjugates. J Control Release 2018; 294: 176-184.
- Bellan M, Castello LM, Pirisi M. Candidate biomarkers of liver fibrosis: a concise, pathophysiology-oriented review. J Clin Transl Hepatol 2018; 6: 317-325.
- Ohshima S, Kuchen S, Seemayer CA, et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum 2003; 48: 2788-2795.
- Migita K, Horai Y, Kozuru H, et al. Serum cytokine profiles and Mac-2 binding protein glycosylation isomer (M2BPGi) level in patients with autoimmune hepatitis. Medicine (Baltimore) 2018; 97: e13450. doi: 10.1097/ MD.0000000000013450
- Kalayci O, Birben E, Tinari N, Oguma T, Iacobelli S, Lilly CM. Role of 90K protein in asthma and TH2-type cytokine expression. Ann Allergy Asthma Immunol 2004; 93: 485-492.
- Cecamore C, Marsili M, Salvatore R, et al. 90K immunostimulatory glycoprotein in children with juvenile idiopathic arthritis. Mod Rheumatol 2018; 28: 637-641.
- Loimaranta V, Hepojoki J, Laaksoaho O, Pulliainen AT. Galectin-3-binding protein: a multitask glycoprotein with innate immunity functions in viral and bacterial infections. J Leukoc Biol 2018; 104: 777-786.
- Szczeklik K, Krzysciak W, Domagala-Rodacka R, et al. Alterations in glutathione peroxidase and superoxide dismutase activities in plasma and saliva in relation to disease activity in patients with Crohn's disease. J Physiol Pharmacol 2016; 67: 709-715.
- Cibor D, Domagala-Rodacka R, Rodacki T, et al. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol 2016; 22: 1067-1077.
- Szczeklik K, Krzysciak W, Cibor D, et al. Evaluation of plasma concentrations of selected antioxidant parameters in patients with active Crohn's disease. Folia Med Cracov 2018; 58: 119-130.
- Krakowska-Stasiak M, Cibor D, Domagała-Rodacka R, et al. Insulin like growth factor system in remission and flare of inflammatory bowel diseases. Pol Arch Intern Med 2017; 127: 832-839.
- Szczeklik K, Krzysciak W, Cibor D, et al. Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active Crohn disease. Pol Arch Intern Med 2018; 128: 362-370.
- Papa Gobbi R, De Francesco N, Bondar C, et al. A galectin-specific signature in the gut delineates Crohn's disease and ulcerative colitis from other human inflammatory intestinal disorders. Biofactors 2016; 42: 93-105.
- Muglia CI, Gobbi RP, Smaldini P, et al. Inflammation controls sensitivity of human and mouse intestinal epithelial cells to galectin-1. J Cell Physiol 2016; 231: 1575-1585.
- Muller S, Schaffer T, Flogerzi B, et al. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm Bowel Dis 2006; 12: 588-597.
- Frol'ova L, Smetana K, Borovska D, et al. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res 2009; 58: 503-512.
- Stenson WF. Inflammatory bowel diseases. In: Textbook of Gastroenterology, Yamada T, Alpers DH et al. (eds). Philadelphia, Lippincott Wiliams and Wilkins, 1995, pp. 1761-1772.
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55 (Suppl. 6): 749-753.
- Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s activity disease index. National Cooperative Crohn’s disease study. Gastroenterology 1976; 70: 439-444.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 1987; 317: 1625-1629.
- Jensen-Jarolim E, Gscheidlinger R, Oberhuber G, et al. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn's disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur J Gastroenterol Hepatol 2002; 14: 145-152.
- Dionne S, Hiscott J, D'Agata I, Duhaime A, Seidman EG. Quantitative PCR analysis of TNF-αand IL-1β mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci 1997; 42: 1557-1566.
- Xue FM, Zhang HP, Hao HJ, et al. CD98 positive eosinophils contribute to T helper 1 pattern inflammation. PLoS One 2012; 7: e51830. doi: 10.1371/ journal.pone.0051830
- Matsuda R, Koide T, Tokoro C, et al. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naive ulcerative colitis during active and quiescent disease. Inflamm Bowel Dis 2009; 15: 328-334.
- Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: What does it mean? World J Gastroenterol 2013; 19: 7552-7560.
- Budzynska A, Gawron-Kiszka M, Nowakowska-Dulawa E, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) correlates with clinical and endoscopic activity in ulcerative colitis but fails to predict activity in Crohn's disease. J Physiol Pharmacol 2017; 68: 859-865.
- Basson MD. Hierarchies in healing in gut mucosal injury. J Physiol Pharmacol 2017; 68: 789-795.
- Puthenedam M, Wu F, Shetye A, Michaels A, Rhee KJ, Kwon JH. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm Bowel Dis 2011; 17: 260-267.
- Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J Histochem Cytochem 2009; 57: 41-50.
- de Kivit S, Kraneveld AD, Knippels LM, et al. Intestinal epithelium-derived galectin-9 is involved in the immunomodulating effects of nondigestible oligosaccharides. J Innate Immun 2013; 5: 625-638.
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12: 492-499.
- Agnello L, Bellia C, Sasso BL, et al. Establishing the upper reference limit of galectin-3 in healthy blood donors. Biochem Med (Zagreb) 2017; 27: 030709. doi: 10.11613/BM.2017.030709
- Arora P, Agarwal Z, Venkatraman A, et al. Galectin-3 and risk of ischaemic stroke: reasons for geographic and racial differences in stroke cohort. Eur J Neurol 2017; 24: 1464-1470.
- Yan J, Xu Y, Zhang L, et al. Increased expressions of plasma galectin-3 in patients with amyotrophic lateral sclerosis. Chin Med J (Engl) 2016; 129: 2797-2803.
- de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 2012; 272: 55-64.
- Weigert J, Neumeier M, Wanninger J, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab 2010; 95: 1404-1411.
- Meeusen JW, Johnson JN, Gray A, et al. Soluble ST2 and galectin-3 in pediatric patients without heart failure. Clin Biochem 2015; 48: 1337-1340.
- Nigjeh EN, Chen R, Allen-Tamura Y, Brand RE, Brentnall TA, Pan S. Spectral library-based glycopeptide analysis-detection of circulating galectin-3 binding protein in pancreatic cancer. Proteomics Clin Appl 2017; 11: doi: 10.1002/prca.201700064
- Dencker M, Arvidsson D, Karlsson MK, Wollmer P, Andersen LB, Thorsson O. Galectin-3 levels relate in children to total body fat, abdominal fat, body fat distribution, and cardiac size. Eur J Pediatr 2018; 177: 461-467.
- Ye Y, Pang Z, Chen W, Ju S, Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med 2015; 8: 22529-22542.
- Frolkis A, Dieleman LA, Barkema HW, et al. Environment and the inflammatory bowel diseases. Can J Gastroenterol 2013; 27: e18-e24.
- Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA. Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem 2008; 283: 18573-18581.
- Trahey M, Weissman IL. Cyclophilin C-associated protein: a normal secreted glycoprotein that down-modulates endotoxin and proinflammatory responses in vivo. Proc Natl Acad Sci USA 1999; 96: 3006-3011.
- Kong W, Longaker MT, Lorenz HP. Cyclophilin C-associated protein is a mediator for fibronectin fragment-induced matrix metalloproteinase-13 expression. J Biol Chem 2004; 279: 55334-55340.
- Kong W, Li S, Longaker MT, Lorenz HP. Cyclophilin C-associated protein is up-regulated during wound healing. J Cell Physiol 2007; 210: 153-160.
- Moon HW, Park M, Hur M, Kim H, Choe WH, Yun YM. Usefulness of enhanced liver fibrosis, glycosylation isomer of Mac-2 binding protein, galectin-3, and soluble suppression of tumorigenicity 2 for assessing liver fibrosis in chronic liver diseases. Ann Lab Med 2018; 38: 331-337.
- Shinzaki S, Iijima H, Fujii H, et al. A novel pathogenesis of inflammatory bowel disease from the perspective of glyco-mmunology. J Biochem 2017; 161: 409-415.
A c c e p t e d : February 28, 2019