EXPOSURE TO AIR POLLUTION AND OXIDATIVE STRESS MARKERS IN PATIENTS WITH POTENTIALLY MALIGNANT ORAL DISORDERS
INTRODUCTION
One of the most common toxic gases polluting atmosphere is carbon monoxide (CO). This odorless, tasteless, colorless gas is third most common cause of intoxication. Its’ most frequently described exogenous sources are: cigarette smoke, traffic exhaust, wooden and gas ovens, incomplete combustion of fossil fuels, mining industry and power stations. Most important variables that determine the nosogenic potential of CO are: its level in exhaled air, length of exposure on its increased concentration, coexistence of other toxic gases, general health condition of patient and its age. CO’s harmful effects are not caused only by acute intoxication with high concentrations, but also long-term exposure to low dosage affects nervous, cardiovascular (1) and respiratory systems (2, 3). General air pollution is also known as a risk factor for nasopharyngeal and oral cavity diseases (4, 5). Other mechanisms of CO toxicity include ion channels dysregulation, formation of reactive oxygen species, cytochrome C oxidase alteration (6) and release of endothelial nitric oxide or the cellular respiration (7).
Production of oxidants seems to be the major biological effect responsible for tissue injury and inflammatory response to air pollution or the response of host immune system to it. Oxidative stress plays important role in the pathogenesis of various vascular, neurologic and skin diseases, it may also lead to neoplasia (8-11). The oral cavity is chronically exposed to air pollution, thus the oxidative damage and inflammation may lead to different disorders like oral lichen planus (OLP) and leukoplakia, which are classified as potentially malignant oral disorders (PMOD) (12).
However, the etiopathogenesis of OLP is not fully understood, there are hypotheses considering specific and non-specific immunological mechanisms, as the disease is related to T-lymphocytic inflammatory response to the antigen of keratinocytes located in the basal layer of the epithelium (13, 14). Recent results suggest that oxidative stress may be involved in the etiology of OLP, as it is dependent on T lymphocytes (15). Thus the role of oxidative stress is intensively investigated in the pathomechanism of OLP and leukoplakia (16-18).
The leukoplakia is most commonly encountered type of PMOD. The disease is promoted by poor dental hygiene, mechanical damage to the mucous membrane and smoking. Latest study reveals that occurrence of PMOD is also correlated with concentration of air pollution (19). Untreated leukoplakia in 4 – 6% of patients, within about 5 years transforms into squamous cell carcinoma (20-22).
Our previous work showed that exhaled CO may be useful in evaluation of exposure to pollution (23). Further studies are needed to find out if changes of exhaled CO measurement are correlated with oxidation damage and if correction coefficient can be established to enable comparison of subjects from low and high polluted areas.
The aim of this study was to compare the oxidative stress markers (8-OHdG and MDA) and antioxidant potential (TAC, GSH) in saliva of PMOD subjects and healthy controls in two different time points (periods of high and low air pollution).
MATERIAL AND METHODS
Study design
This study was conducted in a Polish city of Cracow reported to be the 11th of the most polluted cities in the European Union (24, 25).
The level of exhaled carbon monoxide (eCO) was measured and saliva was collected from PMOD patients after treatment of oral cavity lesions and healthy controls at two consecutive visits in June when air pollution is low and November when it is increased. Four oxidative stress parameters: 8-hydroxy-2'-deoxyguanosine (8-OHdG), malondialdehyde (MDA), reduced glutathione (GSH) total antioxidant capacity (TAC) were measured in saliva.
Subjects
Forty participants were enrolled in the study: 20 PMOD patients and 20 healthy volunteers. The diagnosis of PMOD was given before enrollment to the study based on symptoms and histopathological examination in accordance with the World Health Organization (WHO) guidelines. The PMOD patients were at least 3 months after surgical or cryotherapy treatment. None of the participants took any antibiotics, non-steroid anti-inflammatory drugs, corticosteroids or multivitamin supplements within the last 3 months. They had to be non-smokers for minimum 5 years, free from oral cavity disorders such as: caries, periodontal disease, epithelial dysplasia and inflammatory lesions of the oral mucosa. History of rheumatic disorders (Sjogren disorder), asthma and sinusitis were also excluding from participation in the study as they can influence quality and quantity of saliva.
The subjects gave informed consent to participate in the study and official approval for the study protocol from the Jagiellonian University Ethics Committee was obtained (1072.6120.163.2018).
Saliva preparation for the oxidant-antioxidant tests
Unstimulated saliva samples (4 ml) were collected in sterile plastic tubes using the Salivette® Cotton Swab system (Sarstedt, Nuembrecht, Germany). The biological material was collected in the morning, between 9:00 and 11:00 AM. The subjects rinsed their mouths with tap water for 30 s and expectorated it before the saliva was collected. The study subjects did not eat, drink, brush their teeth or chew gum for a minimum of 2 hours prior to the sampling. The samples were placed in ice and transported to the laboratory for further processing within a period of no more than 1 hour. The saliva was centrifuged at 900 g for 10 minutes at a temperature of 4ºC. Then the entire filtrate was transferred to sterile 1.5 ml micro test-tubes (Eppendorf type) and frozen at –80ºC until analysis.
Measurement of exhaled carbon monoxide
The level of CO in exhaled air (eCO) was measured with the use of PiCO+ Smokerlyzertools (Bedfont Scientific Ltd, England); all devices were calibrated in the same licensed company Synecpol S.C. The examination proceeded according to the following scheme: after a period of normal breathing, subjects were asked to hold breath during a 15-s countdown and, after hearing the machine beep, to blow continuously and slowly into the smokerlyzer mouthpiece. The level of eCO was expressed in ppm.
The data concerning CO levels in ambient air were obtained from local air quality monitoring stations run by the Regional Inspectorate of Environmental Protection in Cracow (http://monitoring.krakow.pios.gov.pl/), as all of the study participants lived in Cracow. The mean, minimum and maximum values of CO levels, reported in the months of the testing, were taken into account in the final analysis.
Measurement of oxidative stress parameters
1. Reduced glutathione
Concentration of reduced glutathione was determined by the method described by Beutler et al. (26). The method is based on the formation of a persistent yellow color after the addition of 5,5'-dithiobis (2-nitrobenzoic acid) to the sulfhydryl groups in the test material. Measurement of the resulting color was made at wavelength λ = 412 nm. Concentration of reduced glutathione was performed in µmol/l unit, using the Biotek ELX808 microplate reader. All reagents used in the assay are from Sigma Aldrich (St. Louis, MO USA). Intra-assay and inter-assay precision were 8.0 and 12.0%, respectively.
2. Total antioxidant capacity
Total antioxidant capacity was determined using the methodology provided by Benzie et al. (27). Based on the assessment of the ability to reduce Fe3+ ions present in complex form with tripyridyl triazine (Fe3+ –TTPTZ) by low molecular weight antioxidants contained in the test biological material. The resulting Fe2+ –TPTZ complex is characterized by intense blue color and has a maximum absorption at wavelength λ = 593 nm. The sample's antioxidative capacity is determined by comparing the changes in absorbance ΔA. With the value of ΔA of the standard solution Fe2+. The final result was expressed in the form of mmol/l, using the Biotek ELX808 microplate reader. All reagents used in the assay are from Sigma Aldrich (St. Louis, MO USA). Intra-assay and inter-assay precision were 3.2 and 7.0%, respectively.
3. Malonylodialdehyde
Malonylodialdehyde (MDA) was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using commercial kits MyBioSource (San Diego, CA, USA). The result was expressed in mmol/l. Intra-assay and inter-assay precision were 5.3 and 6.4%, respectively.
4. 8-hydroxy-2'-deoxyguanosine
8-hydroxy-2'-deoxyguanosine (8-OHdG) was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using commercial kit Japan Institute for the Control of Aging (Fukuroi, Shizuoka Pref., Japan). The result was expressed in ng/ml. Intra-assay and inter-assay precision were 2.0 and 4.4%, respectively.
Statistical analysis
Categorical data were summarized as absolute and relative values, while continuous data as median and 25th – 75th percentile, mean and standard deviation. Normality was checked using the Shapiro-Wilk W test. Statistical significance of differences between groups was analyzed using Wilcoxon signed-rank test and Mann-Whitney U-test for pairwise comparisons. Fisher exact test was used to assess the differences between categorical data in different groups. Significance level was established as P value < 0.05. All other statistical analyses were performed with the use of Dell Statistica (v.13) and Origin Pro (v.9.1).
RESULTS
Clinical characteristics of study groups
PMOD patients (mean age 56.35 ± 9.02) were predominantly female (90%). Two types of PMOD were diagnosed and successfully treated: leukoplakia in 55% (Van der Waal classification I degree - 3 patients; II degree - 8 patients. All lesions located on cheeks mucosal membrane) and lichen planus in 45% (all with reticular forms, on the mucosal membrane of cheeks - 6 patients; tongue - 1 patient and gums - 2 patients) (28).
Mean time from operative treatment of the lesion was 5 months (3 – 7 months). The healthy control group was sex and age matched.
Ambient air pollution
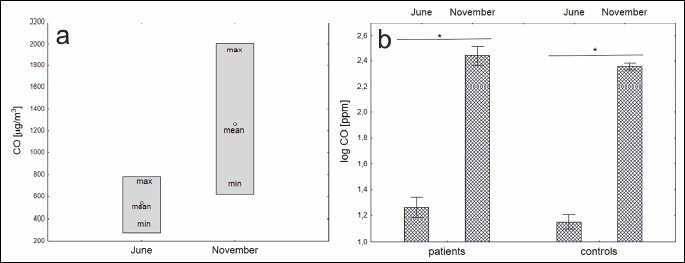
The ambient CO levels were as follows, in June: mean 559 µg/m³; minimum 277 µg/m³; maximum 781 µg/m³ and in November: mean 1152 µg/m³; minimum 621 µg/m³; maximum 2006 µg/m³ (Fig. 1).
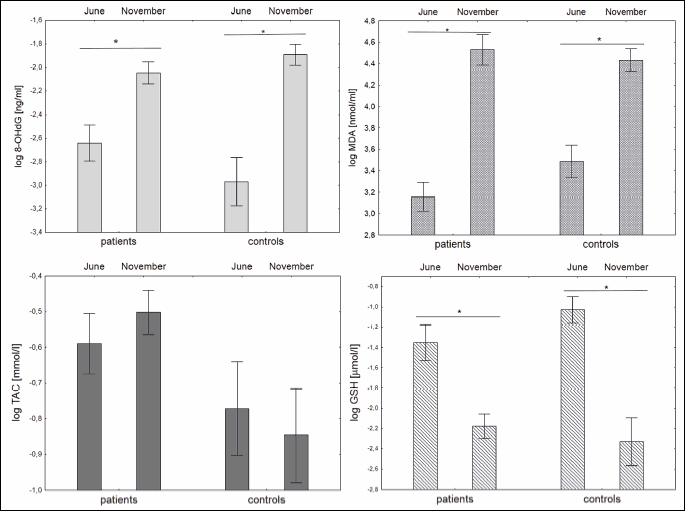
Salivary oxidative stress metabolites
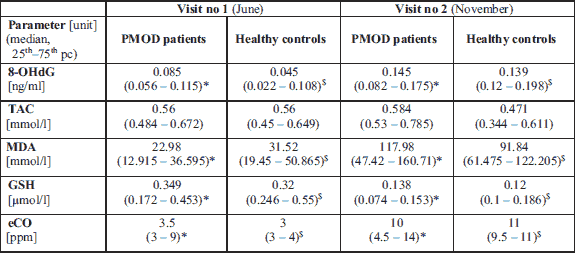
In periods of high air pollution (November) significantly higher concentrations of 8-OHdG (P < 0.001 for PMOD patients and P = 0.001 for healthy controls), MDA (P = 0.002 and P = 0.012, respectively) and eCO (P < 0.001 and P < 0.001, respectively) were observed in both groups. The concentration of salivary GSH (P < 0.001 and P < 0.001 for both groups) decreased when compared between consecutive visits. The concentration of TAC did not change between visits as described in Fig. 2. Concentrations of selected oxidative stress markersmeasured in saliva in both study groups measured at two consecutive visits in June and November are shown in Table 1. There were no statistically significant differences in oxidative status between PMOD and healthy controls neither at visit in June or November.
DISCUSSION
In our study we have chosen saliva as material for redox status monitoring. The advantages of this sample type include availability for collection and further diagnosis and ability to become a tool for screening diseases of oral cavity (29, 30) as well as general conditions. Correlation between salivary markers, clinical condition of a patient and the severity of the disease has been observed and documented (31). Additionally, the oxidation status in saliva is characterized by antioxidant potential (TAC, GSH) (29) and oxidative damage (MDA, 8OHdG) (32, 33), thus results are more informative due to use of biomarkers panel instead of a single parameter, which enables a better understanding of the underlying pathomechanisms.
Our study revealed the correlation between eCO and oxidative stress markers in saliva. We used the exhaled CO measurements to monitor exposure to air pollution as previously described. This method proved to be efficient to distinguish subjects from low and high polluted areas, and also was able to monitor smoking status (23, 34). Results monitoring correlation between exposure to air pollution and oxidative status measured in saliva, (35) plasma and urine (36) are present in literature. In first paper oxidative status of traffic and no traffic police were compared, group exposed to exhausted fumes has higher concentration of oxidation products in saliva. However work published by Ambroz et al. (36) compared mothers and newborns from low and high polluted areas, the oxidation markers were measured in blood and urine. Results showed higher oxidative damage in newborns from polluted region, but not in mothers. In both studies the exposure to pollution were defined by occupation specificity or area of living, thus the exposure to high pollution was not confirmed by any parameters measured in study subjects.
Surprisingly the TAC parameter did not change between visits as it could be expected - the increase of oxidative damage should lead to decrease od antioxidant potential as observed for GSH. The review paper by Tothova et al. (30) described the same effect in many studies of subjects with and without carries, the increase of 8-OHdG and MDA was followed by decrease of GSH and even increase of TAC in group with carries. The explanation of this phenomenon include adoptive response to prolonged oxidative stress.
Our results also showed no difference between the studied groups suggesting that effective treatment leads to decrease of the oxidation damage and restores antioxidant potential. Previous study published by Darczuk et al. (29) proved that patients with active OLP are characterized by higher concentration of oxidation products and lower antioxidant potential. This observation seems to be effect of active disease. It should be noticed that oxidative status in oral cavity after treatment normalizes in time. This assumption is based on article showing that 2 weeks after treatment of OLP the concentration of oxidative markers remained higher in group with OLP compared to healthy controls (37).
It should be emphasized that although saliva is convenient and easy to obtain material for oxidation damage assessment, the interpretation of oxidation status based only on the measured parameters may be influenced by several factors. On the one hand concentration of oxidation products may rise in presence of local lesions like OLP (29) as well as in systemic disorders as Crohn disease (31) and on the other hand may be decreased by chemopreventive agents present in the various food components (38). Additionally air pollution may also influence the results of oxidation stress markers measurements. There is another seasonal factor influencing the oxidative status, that has to be taken under the consideration - the presence of allergic process (39). Multiple studies showed that in patients with ongoing allergic diseases, such as allergic rhinitis (40), asthma (41) or atopy (42), the biomarkers of oxidative stress are elevated, however it still hasn’t been proved if this is the cause or result or allergy. However allergy is an important factor in oxidative status assessment, it is mostly correlated with season of spring and early summer. Our results show that the oxidative status biomarkers are elevated in the November, when the exacerbation of allergic diseases is less frequent. This let as assume that in this particular case the allergy-based factors did not biased the results in significant way. The next step in assessment of cause-effect relationship between air pollution and salivary oxidative status should be comparison in the same period of the year two groups with different exposure to air pollution confirmed by i.e. eCO. Results of oxidative status panel in such groups would give additional knowledge on influence of air pollution on oxidative stress markers.
We conclude that the exhaled carbon monoxide, reflecting exposure to air pollution, correlates with the oxidative stress markers in saliva of patients with PMOD and healthy controls.
Abbreviations: 8-OHdG, hydroxy-2'-deoxyguanosine; eCO, exhaled carbon monoxide; GSH, reduced glutathione; MDA, malondialdehyde; OLP, oral lichen planus; PMOD, potentially malignant oral disorders; TAC, total antioxidant capacity.
Conflict of interests: None declared.
REFERENCES
- Levy RJ. Carbon monoxide pollution and neurodevelopment: a public health concern. Neurotoxicol Teratol 2015; 49: 31-40.
- Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res 2014; 129: 11-19.
- Pope D, Diaz E, Smith-Sivertsen T, et al. Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environ Health Perspect 2015; 123: 285-292.
- Ceretti E, Feretti D, Viola GCV, et al. DNA damage in buccal mucosa cells of pre-school children exposed to high levels of urban air pollutants. PLoS One 2014; 9: e96524. doi: 10.1371/journal.pone.0096524
- Josyula S, Lin J, Xue X, et al. Household air pollution and cancers other than lung: a meta-analysis. Environ Health 2015; 14: 24. doi: 10.1186/s12940-015-0001-3
- Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal 2009; 13: 157-192.
- Roderique JD, Josef CS, Feldman MJ, Spiess BD. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 2015; 334: 45-58.
- Buczko P, Zalewska A, Szarmach I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J Physiol Pharmacol 2015; 66: 3-9.
- Lopez-Jornet P, Martinez-Canovas A, Pons-Fuster A. Salivary biomarkers of oxidative stress and quality of life in patients with oral lichen planus. Geriatr Gerontol Int 2014; 14: 654-659.
- Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res 2017; 113: 389-398.
- Iseme RA, McEvoy M, Kelly B, et al. A role for autoantibodies in atherogenesis. Cardiovasc Res 2017; 113: 1102-1112.
- Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol 2018; 125: 612-627.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc 2002; 68: 494-499.
- Barbosa JF, de Figueiredo SM, Monteiro FM, et al. New approaches on Leishmaniasis treatment and prevention: a review of recent patents. Recent Pat Endocr Metab Immune Drug Discov 2015; 9: 90-102.
- Shirzad A, Pouramir M, Seyedmajidi M, Jenabian N, Bijani A, Motallebnejad M. Salivary total antioxidant capacity and lipid peroxidation in patients with erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects 2014; 8: 35-39.
- Upadhyay RB, Carnelio S, Shenoy RP, Gyawali P, Mukherjee M. Oxidative stress and antioxidant defense in oral lichen planus and oral lichenoid reaction. Scand J Clin Lab Invest 2010; 70: 225-228.
- Senghore T, Li Y-F, Sung F-C, et al. Biomarkers of oxidative stress associated with the risk of potentially malignant oral disorders. Anticancer Res 2018; 38: 5211-5216.
- Srivastava KC, Austin RD, Shrivastava D, Pranavadhyani G. Oxidant-antioxidant status in tissue samples of oral leukoplakia. Dent Res J 2014; 11: 180-186.
- Gregorczyk-Maga I, Wachsmann A, Olszewska M, Partyka L. Exhaled carbon monoxide levels correlate with incidence of oral mucosal lesions independent of smoking status. Int J Environ Health Res 2019; 29: 290-300.
- Kaur J, Jacobs R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J Korean Assoc Oral Maxillofac Surg 2015; 41: 171-175.
- Carrard VC, van der Waal I. A clinical diagnosis of oral leukoplakia; a guide for dentists. Med Oral Patol Oral Cir Bucal 2018; 23: e59-e64.
- Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007; 36: 575-580.
- Maga M, Janik MK, Wachsmann A, et al. Influence of air pollution on exhaled carbon monoxide levels in smokers and non-smokers. A prospective cross-sectional study. Environ Res 2017; 152: 496-502.
- WHO/Oral health surveys: basic methods, 5th edition [Internet, cited 2019 Feb 4]. Available from: http://www.who.int/ oral_health/publications/9789241548649/en/
- Air Quality in Europe - 2017 Report, European Environment Agency. [Internet, cited 2019 Feb 4]. Available from: https://www.eea.europa.eu/publications/air-quality-in-europe-2017
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61: 882-888.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 1996; 239: 70-76.
- van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol 2009; 45: 317-323.
- Darczuk D, Krzysciak W, Vyhouskaya P, et al. Salivary oxidative status in patients with oral lichen planus. J Physiol Pharmacol 2016; 67: 885-894.
- Tothova L, Kamodyova N, Cervenka T, Celec P. Salivary markers of oxidative stress in oral diseases. Front Cell Infect Microbiol 2015; 5: 73. doi: 10.3389/fcimb.2015.00073
- Szczeklik K, Krzysciak W, Cibor D, et al. Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active Crohn disease. Pol Arch Intern Med 2018; 128: 362-370.
- Babiuch K, Bednarczyk A, Gawlik K, et al. Evaluation of enzymatic and non-enzymatic antioxidant status and biomarkers of oxidative stress in saliva of patients with oral squamous cell carcinoma and oral leukoplakia: a pilot study. Acta Odontol Scand 2019; Mar 11: 1-11. doi: 10.1080/00016357.2019.1578409
- Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci 2010; 52: 251-256.
- Gregorczyk-Maga I, Maga M, Wachsmann A, et al. Air pollution may affect the assessment of smoking habits by exhaled carbon monoxide measurements. Environ Res 2019; 172: 258-265.
- Baliga MS, Shivashankara AR, Rao S, et al. Saliva as an important body fluid in the detection of oxidative stress in community based studies: Preliminary study with police personnel’s exposed to automobile exhaust. Int J Appl Res 2017; 3: 372-376.
- Ambroz A, Vlkova V, Rossner P, et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health 2016; 219: 545-556.
- Hashemy SI, Gharaei S, Vasigh S, et al. Oxidative stress factors and C-reactive protein in patients with oral lichen planus before and 2 weeks after treatment. J Oral Pathol Med 2016; 45: 35-40.
- Scrobota I, Bolfa P, Filip AG, et al. Natural chemopreventive alternatives in oral cancer chemoprevention. J Physiol Pharmacol 2016; 67: 161-172.
- Twardoch MA, Lodwich M, Mazur B. Allergies and oxidative stress [in Polish] Ann Acad Medicae Silesiensis 2016; 70: 15-23.
- Emin O, Hasan A, Aysegul D, Rusen D. Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. J Investig Allergol Clin Immunol 2012; 22: 188-192.
- Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Severe Asthma Research Program. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol 2009; 123: 146-152.
- Nazaryan R, Kryvenko L. Salivary oxidative analysis and periodontal status in children with atopy. Interv Med Appl Sci 2017; 9: 199-203.
A c c e p t e d : February 28, 2019