IMMUNOMODULATION INHIBITS THE DEVELOPMENT
OF ENDOMETRIOSIS IN RATS
INTRODUCTION
Endometriosis is an oestrogen-dependent disease in which endometrium glands and stroma proliferate outside the uterine cavity. It is associated with chronic inflammation, leading to pelvic pain and reduced fertility. Endometriosis affects 10% of women of reproductive age; 70% to 80% of affected women are diagnosed with chronic pelvic pain, and 30% are diagnosed with infertility (1, 2). Although there are various treatment approaches for endometriosis (surgical and pharmacological), their primary goal is in symptom alleviation. More often than not, these treatments yield undesirable outcomes such as disease recurrence and lasting infertility (2, 3). More knowledge of the molecular pathophysiology of endometriosis will lead to targeted therapies with better outcomes and perhaps even prevent the development of the disorder (4).
Numerous in vivo and in vitro studies have shown that hormones, oxidative stress, and proinflammatory cytokines regulate cell proliferation and apoptosis in endometriosis (5-9). Some hypotheses suggest that increased cell proliferation and defective apoptotic mechanisms are responsible for the pathologic changes that shift endometrial tissue from eutopic to ectopic. Therefore, inhibition of cell proliferation and induction of apoptosis could represent promising treatment strategies (10-12).
In humans and mouse models, several proteins regulate the balance between cell proliferation and death. For instance, Bcl2 and Bax inhibit and activate apoptosis, respectively (13, 14). The combined action of these factors is critical for determining cell survival during the menstrual cycle. An excellent marker for estimating the proliferative activity of cells is the nuclear protein MKI67, which is present during all active phases of the cell cycle (14, 16). MKI67 also has a high predictive value for cancer, especially lung, prostate, and breast cancers (15, 16). Additionally, the enzyme telomerase is highly active in proliferating cells (17). Telomerase could potentially show increased activity in eutopic endometrium cells of patients with endometriosis and could represent an effective marker of the disease (18).
The most common hypothesis conceives that the source of the ectopic endometrium is eutopic endometrium, which enters the peritoneal cavity by retrograde reflux of menstrual blood (1). Recent research revealed a large number of immune abnormalities in endometriosis patients (10, 19-21). Moreover, patients with endometriosis have autoantibodies against endothelial cells and cellular components such as nuclei, histones, and phospholipids (10, 20-22). Thus, there may be a mechanism related to an aberrant immune cell population, causing a reduction in the rate of apoptosis in both the eutopic and ectopic endometrium. Therefore, immunological therapy could represent a promising treatment approach for endometriosis.
Endometriosis immunotherapy aims to restore the role of activated natural killer cells and increase the response of T-cells against the ectopic endometrium invasion. To date, two methods of immunomodulation have been used in research; RESAN vaccines and V-ENDO. Both trigger a specific T-cell immune response against endometriosis, which has been demonstrated by clinical research (23).
Understanding the pathogenesis of endometriosis and the mechanisms responsible for the various stages of its development has been the subject of many studies in recent years (1, 22-23). In accordance with the leading theory, we sought to assess whether immunomodulation by RESAN could prevent the early invasion of endometriotic implants. We used a rat model of endometriosis to analyse changes in the expression of genes involved in the regulation of cellular proliferation and apoptosis following immunotherapy by RESAN.
MATERIAL AND METHODS
Ethical statement
The Institutional Animal Research Committee approved this study (approval no. 42/2017), in accordance with the EU Directive 2010/63/EU for animal experiments.
RESAN vaccine characterisation
The RESAN vaccine is a complex of molecules derived from Gallus domesticus xenogeneic tissues. It contains glycoproteins (fraction α2β), peptides, and carbohydrate fragments of more than 40 different common tumour antigens. The glycoproteins imitate 6 – 50 fragments (a length of 7 – 30 amino acids) of each antigen (24).
Study design
The study included a total of 56 Wistar rats. The animals were divided into three groups: prophylaxis (vaccinated 3 months before eutopic endometrium implantation, n = 23), therapeutic (vaccinated 3 months after eutopic endometrium implantation - at the expected time of endometriosis foci excision, n = 23), and non-treatment control (n = 10). In the prophylaxis and therapeutic groups, 0.5 mL of the RESAN vaccine suspension (200 mg/mL of the antigens) was delivered intramuscularly and subcutaneously into the upper part of the buttock, according to the manufacturer's instructions (21).
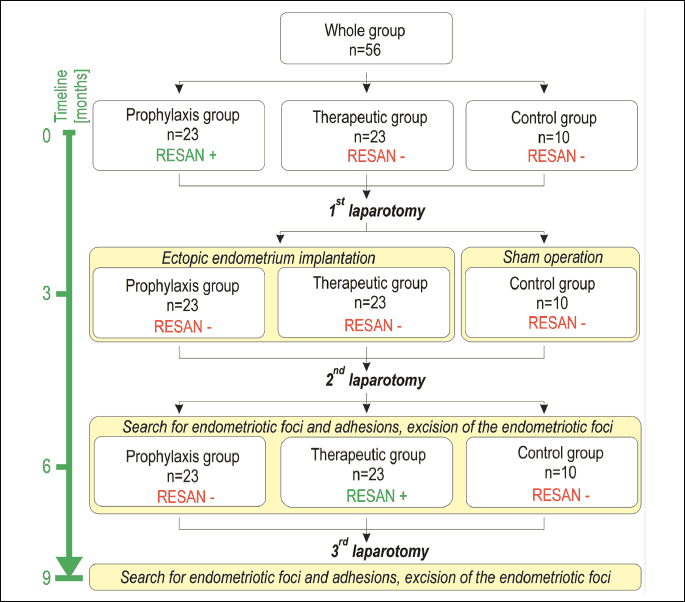
Three months after vaccination in the prophylaxis group, the 1st laparotomy was performed in all three groups (autotransplantation and sham operation in the control group). The 2nd laparotomy was performed three months later, again in all three groups. Endometriotic foci in the study groups and the nylon sutures of the control group were removed after photographic documentation and description. Subsequently, RESAN injections were given to the therapeutic group, only. Three months later, the 3rd laparotomy was performed in all animals. Description and photographic documentation were carried out, and suspected foci were excised. Fragments of the eutopic endometrium were collected from all animals during the 1st and 3rd laparotomies. A schematic of the experiment is shown in Fig. 1. Surgical procedures (autotransplantation of uterine squares to the peritoneal cavity) were carried out as previously described (24).
Histological analysis
All suspected endometriosis foci were surgically removed during the 2nd and 3rd laparotomies. The tissue specimens were fixed in 4% formaldehyde, cut on a microtome, and stained with haematoxylin and eosin. The tissue sections were assessed for endometriosis by the same technician in a blind analysis. Histological assessment was performed by two independent histologists who did not know the study groups.
Tert, Mki67, Bax and Bcl2 expression in eutopic endometrium
For gene expression analyses, the obtained tissue specimens were immediately submerged in RNA protective medium (RNA Stabilizer, Novazym, Poznan, Poland) and stored in –80ºC until use. Total RNA was isolated and processed as previously described (26) with some modifications; RNA precipitation was carried out at –80ºC instead of room temperature. The cDNA synthesis was performed using 1 µg total RNA and the QuantiTect® Reverse Transcription kit (Qiagen, Mainz, Germany) following the manufacturer’s protocol. The procedure was performed with the integrated removal of genomic DNA contamination for use in real-time two-step PCR. The cDNA was synthesised in a reaction volume of 20 µL and carried out in the MJ Mini™ Personal Thermal Cycler (Bio-Rad, Hercules, CA, USA) with the following program: 30 minutes (min) at 42ºC followed by enzyme inactivation at 95ºC for 3 min. The cDNA was used for the quantitative polymerase chain reaction (qPCR) analysis.
qPCR analysis
qPCR analysis was used to establish the expression level of Tert, Mki67, Bax, and Bcl2 mRNAs (GenBank IDs: NM_053423.1, NM_001271366.1, NM_017059.2 and NM_016993.1, respectively). We used the DyNAmo HS SYBR Green qPCR Kit (Finnzymes, Finland) and a set of gene-specific primers (details in Tables 1-3).

Oligonucleotides were purchased in TIB MolBiol, Poznan, Poland.
The gene-specific primers were designed using the Primer3 Plus algorithm (27). In silico analysis of the properties of the primers was carried out using the OligoAnalyzer 1.2 (https://eu.idtdna.com/calc/analyzer; an online tool). The specificity of the primers was confirmed using the Basic Local Alignment Search Tool program (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi). The quality of the qPCR product was confirmed by melting temperature analysis and by electrophoretic separation. Rattus norvegicus glyceraldehyde-3-phosphate dehydrogenase (Gapdh, NM_017008.4) and actin, beta (Actb, NM_031144.3) were used as reference genes. The expression levels of both genes were compared between the analysed groups. Spearman’s rank R = 0.967 (P < 0.0001) and thus randomisation and bootstrapping for designated Ct values for reference and analysed genes was used. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in qPCR was used (28).
Reaction efficiencies for the analysed genes and references were calculated as previously described (26). The quality of the standard curves was evaluated based on Pearson’s determination coefficient r2 (ranged from 0.996 to 0.999). Each reaction set included the negative template control (pooled RNA from different samples and isolations in RT reaction lack of reverse transcriptase) and positive control. All reactions were made in triplicates. The reaction compounds, as well as reaction conditions, are shown in Tables 2 and 3.

Statistical analysis
The Wilcoxon rank-sum and Kruskal-Wallis tests with Dunn's post-test were used to compare gene expression levels. The R-coefficient for the Spearman test was calculated (Statistica ver. 13.3 software, TIBCO Software Inc., 2017). A P-value of ≤ 0.05 was considered statistically significant.
RESULTS
Outcomes of autotransplantation to model endometriosis
To study the effects of immunomodulation in endometriosis, we performed a surgical induction of peritoneal endometriosis through autotransplantation. The rats were divided into three groups: the prophylaxis group, the therapeutic group and the control group. The prophylaxis group were vaccinated using RESAN, and three months later, the first laparotomy was performed in all groups (a sham operation was carried out in the control group). Three months later, a second laparotomy was performed under the same conditions. Subsequently, RESAN injections were given to the therapeutic group, only. Three months later, the 3rd laparotomy was performed in all animals.
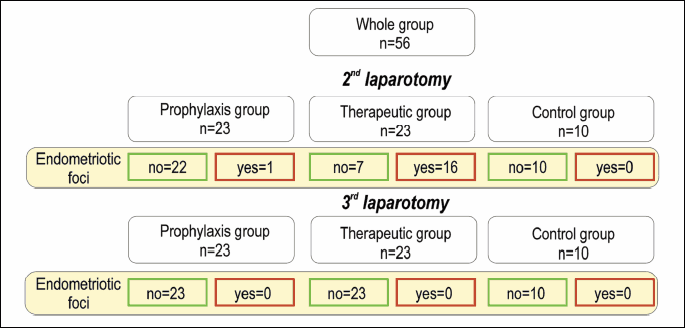
This procedure was successful in 17 rats. After the 2nd laparotomy, 22 animals in the prophylaxis group and 7 animals in the therapeutic group had not developed endometriosis (Fig. 2). Following the 3rd laparotomy, no macroscopic and histological evidence of endometriosis was found in any of the studied groups.
Histological analysis of tissue specimens
The presence or absence of endometriosis was determined using the histological examination. This analysis confirmed peritoneal endometriosis in 17 rats. The consultant histologist performed a second assessment and confirmed or excluded endometriosis. Their findings were entirely concordant with the initial assessments, demonstrating this was a reliable and reproducible method for detecting endometriosis.
Comparison of the expression of Bax, Tert, Mki67, Bcl2 and Bax/Bcl2 after the first laparotomy
To assess the impact of immunomodulation on the cells forming the eutopic endometrium, we assessed the expression of Bax, Tert, Mki67, and Bcl2 genes, as well as the ratio of Bax/Bcl2 expression. The prophylaxis, therapeutic, and control groups differed only in Bax gene expression (Fig. 3), with the prophylaxis group showing significantly higher expression than the control group (P = 0.026). The expression of Bcl2 did not change between the study groups. However, we did detect a difference when comparing the Bax/Bcl2 gene expression ratio between the groups. The Bax/Bcl2 expression ratio in the prophylaxis (P = 0.003), and therapeutic groups (P = 0.002) were significantly higher than the control group (Fig. 3).
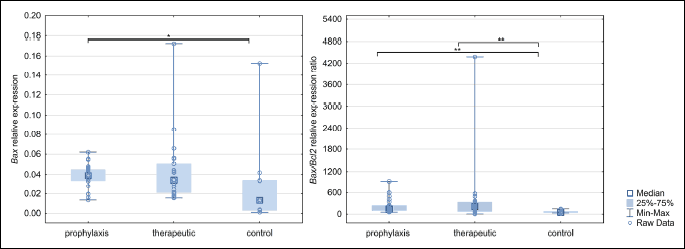
*P < 0.05, **P < 0.01.
Comparison of the expression of Bax, Tert, Mki67, Bcl2 and Bax/Bcl2 after the third laparotomy
Endometrial expression of all studied genes was different between groups after the 3rd laparotomy (Fig. 4). Analysis of the Bax gene expression indicates a statistically significant increase in its activity in the rat endometrium of the therapeutic group relative to prophylaxis (P = 0.003) and control (P = 0.003) groups. Similarly, Tert expression in the therapeutic group was statistically significantly higher compared to the prophylaxis (post-test P < 0.001) and control groups (P < 0.001). Mki67 showed higher expression in the therapeutic group relative to the control group (P < 0.001). Bcl2 showed higher expression in the prophylaxis group compared the control group only (P < 0.001). Analysis of the Bax/Bcl2 expression ratio indicated higher expression of Bax relative to Bcl2 in the therapeutic group compared to the prophylaxis group (P < 0.001) and control group (P = 0.042).
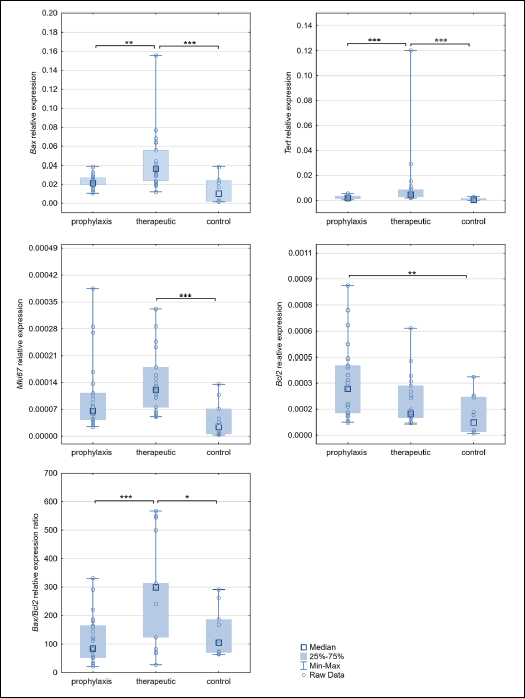 |
Fig. 4. Bax, Tert, Mki67, Bcl2 expression changes, and Bax/Bcl2 ratio differences in studied groups after third laparotomy. *P < 0.05, **P < 0.01. |
Analysis of gene expression changes throughout the experiment
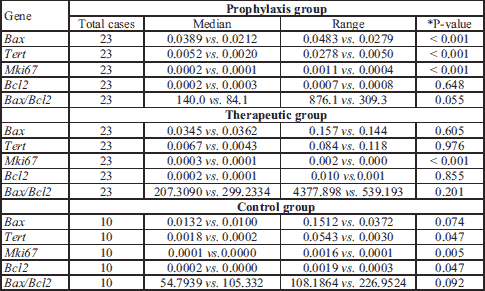
The relative changes in expression of candidate genes were assessed in all three groups from the 1st laparotomy to the end of the experiment (Table 4). The prophylaxis group showed decreasing expression of Bax, Tert, and Mki67 over time. The therapeutic group only showed a decrease in Mki67 expression. The control group showed decreasing expression of Tert, Mki67, and Bcl2.
Correlation of gene expression in the eutopic endometrium after the first laparotomy
We next studied the correlation in the expression of the candidate genes after the 1st laparotomy. In the prophylaxis group, a positive correlation was found between Bax and Tert (R = 0.52, P = 0.012), Bax and Bcl2 (R = 0.46, P = 0.028), Bcl2 and Tert (R = 0.84, P < 0.001) and a negative correlation was observed between Tert and Bax/Bcl2 (R = –0.69, P = 0.001). In the therapeutic group, we found positive correlations between Bax and Tert (R = 0.71, P < 0.001), Bax and Bcl2 (R = 0.57, P = 0.004), Bcl2 and Tert (R = 0.75, P < 0.001), and a negative correlation between Tert and the Bax/Bcl2 (R = –0.86, P = 0.009). In the control group, positive correlations were found between the expression of Bax and Mki67 (R = 0.93, P < 0.001), Bax and Bcl2 (R = 0.88, P < 0.001), Bax and Tert (R = 0.96, P < 0.001), Tert and Bcl2 (R = 0.81, P = 0.005) and Mki67 and Bcl2 (R = 0.85, P = 0.002).
Correlation of gene expression in the eutopic endometrium after the third laparotomy
We also analysed the correlation in expression between all tested genes following the 3rd laparotomy. In the prophylaxis group, positive correlations were found between Tert and Bcl2 (R = 0.92, P < 0.001) and negative correlations between Tert and the Bax/Bcl2 (R = –0.81, P < 0.001). In the therapeutic group, positive correlations were found between Bax and Tert (R = 0.72, P < 0.001), Bax, and Mki67 (R = 0.56, P = 0.005), and Mki67 and Tert (R = 0.43, P = 0.038). In the control group, positive correlations were found between Bax and Tert (R = 0.76, P = 0.011), Bax and Mki67 (R = 0.73, P = 0.016), Bax and Bcl2 (R = 0.71, P = 0.022), Tert and Mki67 (R = 0.83, P = 0.003), Tert and Bcl2 (R = 0.90, P < 0.001) and Mki67 and Bcl2 (R = 0.88, P < 0.001).
DISCUSSION
The limited treatment options for those who struggle with endometriosis have provoked many to investigate new strategies. One line of enquiry is in using immunotherapy to control the inflammatory response to ectopic endometrial tissue. Examples of immunomodulating agents include pentoxifylline, leflunomide, rosiglitazone, pioglitazone, metformin, and natural compounds such as resveratrol and epigallocatechin gallate (EGCg) (29). The immunomodulator we used in this study is a complex of molecules extracted from xenogeneic tissues (G. domesticus), known as RESAN (24).
The effectiveness of RESAN in eliminating endometriosis was demonstrated in our previous study (24). After vaccination, we observed a reduction in endometriosis foci at both macroscopic and microscopic levels in Wistar rats. These promising findings inspired us to investigate the immunomodulatory effects of RESAN at the molecular level. Therefore, we measured the expression levels of four candidate genes in the eutopic endometrium in RESAN-treated versus control rats. The genes were selected based on their known roles in cellular proliferation and apoptosis, and we predicted they would display significant differences in expression patterns between, treated and control subjects.
Our prediction was confirmed for the pro-apoptotic Bax gene, which showed much higher expression in therapeutic versus control groups. Additionally, the ratio of Bax to its anti-apoptotic counterpart, Bcl-2 (Bax/Blc2), was also higher in endometriosis compared to control groups. These observations are consistent with the results of a previous study in humans, which demonstrated that in the granular cells of individuals with endometriosis, Bax expression and Bax/Bcl2 expression ratios were increased compared to healthy individuals (30, 31). The observation that Bax is dysregulated in endometriosis, as well as evidence in our study indicating that Bax expression levels can be controlled through immunomodulation, highlights the potential therapeutic value of RESAN. Indeed, within the nine months of the study, it was shown that Bax expression increased in RESAN treated groups compared to controls.
A recent in vitro study assessed another pro-apoptotic protein - BTG1 - and found that its downregulation is significantly associated with reduced caspase-3 expression and overall increased migration potential of human endometrial stromal cells (HESCs) (32). It would be interesting to use the procedures employed in this study to determine whether Btg1 expression would, like Bax, increase upon RESAN treatment. Moreover, a recent review reported that proteins involved in inflammation and apoptosis, such as the caspases, were highly expressed in endometriosis-associated ovarian carcinoma, and this correlated with poor survival (33). An intriguing and vital investigation would be to uncover whether immunomodulation through RESAN will downregulate inflammatory proteins to avoid the associated carcinomas and increase overall survival.
Later stages of the experiment (3rd laparotomy) showed that the cells forming the eutopic endometrium of all groups differed in expression of almost all studied genes. An increase in the activity of the Bax, Bax/Bcl2, Tert, and Mki67 was observed in the therapeutic group. In this group, all endometriosis foci were removed during the 2nd laparotomy, and then the animals were immunised. Interestingly, Bcl2 expression remained unchanged in the therapeutic group. These results can be explained as an effect of the influence of inflammation from necrotic cells (not necessarily from apoptotic cells), which may stimulate the proliferation of the neighbouring cells, and therefore, the increase in Mki67 and Tert gene expression. It should be noted that increased telomerase expression in the eutopic endometrium of women with endometriosis has already been described (18).
To analyse whether the obtained contradictory results of gene expression are the effect of immunomodulation as well as ageing of the endometrium, we assessed the gene expression changes after nine months. In the prophylaxis group, we found that, in the six-month duration of the experiment, the expression of the Bax, Tert, and Mki67 genes decreased. In the therapeutic group, a similar decrease was observed with the Mki67 gene. A significant decrease in the expression of Tert, Mki67 and Bcl2 genes was found in the control group. The reduction in expression of the studied genes can be explained by the suppression of endometrial senescence by immunomodulation. However, the results indicate that the gene expression profile of the endometrium changes significantly with the age of animals.
Importantly, we also analysed the effects of immunisation timing on gene expression. In the group immunised before endometrium implantation (prophylaxis group), we observed that, after the 1st laparotomy, the network of relationships did not express Mki67, and after the 3rd laparotomy, Bax gene expression was also switched off. In the therapeutic group, in which immunisation was carried out after the 2nd laparotomy, Mki67 expression was switched off after the 1st laparotomy, and Bcl2 after the 3rd laparotomy. In the control group, the network of connections between expressions is a strictly complex network except for the significance of the Bax to Bcl2 ratio. Analysis of the gene expression relationship indicates that, in the prophylaxis group, Mki67 and Bax did not have a significant association. Similar results were obtained for the therapeutic group in the expression of Mki67 and Bcl2. In the control group, the network of connections between expressions is strictly complexed.
Although our results seem promising, we acknowledge some limitations of the study. Firstly, animal models are almost always less complicated than clinical cases. Only some animal observations may be transferred to humans. Secondly, we examined the expression of genes, but not the final protein products that carry out the activities of the genes. However, our results did show reliable statistical power, with relatively high n-values for in vivo rat model work. Despite this, the lack of functional studies, as well as protein-based investigations into the gene cascade, encourage us to draw careful conclusions. In future studies, the observation of such changes in ectopic sites would be valuable. The challenge of this is obtaining enough biopsy material to collect samples for histology and protein expression analysis. Thirdly, surgeons knew which group each animal belonged to during surgery.
Our data support the exploration of immunotherapy in the treatment and prevention of endometriosis. The mechanisms responsible for the control of apoptosis and proliferation should be considered as an immunotherapy target to prevent endometriotic changes. These factors could also be targeted during the removal of ectopic lesions, to prevent recurrence of the disease.
Acknowledgements: The authors acknowledge the help of Andrey Yantchenko from The Scientific Research Enterprise RESAN, Vitebsk, Belarus for kindly sending us the RESAN vaccine.
Source of funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interests: None declared.
REFERENCES
- Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction 2016; 152: R63-R78.
- Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 2017; 6: 34-41.
- Zhu G, Jiang C, Yan X, Zhao S, Xu D, Cao Y. Shaofu Zhuyu decoction regresses endometriotic lesions in a rat model. Evid Based Complement Alternat Med 2018; 2018: 3927096. doi:10.1155/2018/3927096
- Zito G, Luppi S, Giolo E, et al. Medical treatments for endometriosis-associated pelvic pain. Biomed Res Int 2014; 2014: 1-12. doi:10.1155/2014/191967
- Dziekonski M, Zmijewska A, Czelejewska W, Wojtacha P, Okrasa S. The effect of selective agonists of opioid receptors on in vitro secretion of steroid hormones by porcine endometrium during the estrous cycle and early pregnancy. J Physiol Pharmacol 2018; 69: 727-735.
- Schink M, Konturek PC, Herbert SL, et al. Different nutrient intake and prevalence of gastrointestinal comorbidities in women with endometriosis. J Physiol Pharmacol 2019; 70: 255-268.
- Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril 2012; 98: 520-528.
- Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 17βE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFκB-dependent pathway. Mol Cell Endocrinol 2010; 317: 31-43.
- Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol 2009; 175: 225-234.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001; 75: 1-10.
- Wolun-Cholewa M. Expression profile of selected genes in eutopic and ectopic endometrium in women with endometriosis [in Polish: Profil ekspresji wybranych genow w eutopowym i ektopowym endometrium u kobiet z endometrioza]. Poznan, Polska Akademia Nauk, 2011.
- Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet 2002; 76: 117-126.
- Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril 2000; 74: 760-766.
- Wang X, Yan Y, Yang L, Li M, Zhong X. Effect of quercetin on the expression of Bcl-2/Bax apoptotic proteins in endometrial cells of lipopolysaccharide-induced-abortion. J Tradit Chinese Med [Chung i tsa chih ying wen pan] 2016; 36: 737-742.
- Niklaus AL, Aubuchon M, Zapantis G, et al. Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum Reprod 2007; 22: 1778-1788.
- Sobecki M, Mrouj K, Colinge J, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res 2017; 77: 2722-2734.
- Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 2013; 4: 2185. doi:10.1038/ncomms3185
- Hapangama DK, Turner MA, Drury JA, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod 2008; 23: 1511-1519.
- Dmowski PW, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 2004; 18: 245-263.
- Barrier BF. Immunology of Endometriosis. Clin Obstet Gynecol 2010; 53: 397-402.
- Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol 2011; 7: 611-626.
- Polina ER, Araujo RRCV, Sbruzzi RC, et al. Relationship of polymorphisms in the tissue inhibitor of metalloproteinase (TIMP)-1 and -2 genes with chronic heart failure. Sci Rep 2018; 8: 9446. doi:10.1038/s41598-018-27857-5
- Vililininovich YV, Vililininovich YA, Yanchenko LK. Immitators of the tumor antigens. Belarus Patent Database. Pantent no 5942. http://bypatents.com/11-5942-imitator-opuholevyh-antigenov.html
- Szymanowski K, Chmaj-Wierzchowska K, Yantczenko A, et al. Endometriosis prophylaxis and treatment with the newly developed xenogenic immunomodulator RESAN in an animal model. Eur J Obstet Gynecol Reprod Biol 2009; 142: 145-148.
- Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 2002; 123: 217-226.
- Andrusiewicz M, Slowikowski B, Skibinska I, Wolun-Cholewa M, Dera-Szymanowska A. Selection of reliable reference genes in eutopic and ectopic endometrium for quantitative expression studies. Biomed Pharmacother 2016; 78: 66-73.
- Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 2007; 35 (Web Server issue): W71-W74. doi: 10.1093/nar/gkm306.
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30: e36. doi: 10.1093/ nar/30.9.e36
- Jimenez-Lopez J, Munoz-Hernando L, Marqueta- Marques L, et al. Endometriosis: alternative methods of medical treatment. Int J Womens Health 2015; 7: 595. doi: 10.2147/ IJWH.S78829
- Wiweko B, Muna N, Gunawarti DP, Nasution RU, Zesario A. High bax-bcl-2 ratio expression on granulosa cells from endometriosis patients. Adv Sci Lett 2017; 23: 6720-6722.
- Marquardt RM, Kim TH, Shin J-H, Jeong J-W. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int J Mol Sci 2019; 20: 3822. doi: 10.3390/ijms20153822
- Kim JS, Choi YS, Park JH, et al. Role of B-cell translocation gene 1 in the pathogenesis of endometriosis. Int J Mol Sci 2019; 20: 3372. doi: 10.3390/ijms20133372
- Sapalidis K, Machairiotis N, Zarogoulidis P, et al. Genes’ interactions: a major contributor to the malignant transformation of endometriosis. Int J Mol Sci 2019; 20: E1842. doi:10.3390/ijms20081842
A c c e p t e d : February 28, 2020