NONSTEROIDAL ANTI-INFLAMMATORY DRUGS-INDUCED FAILURE
OF LOWER ESOPHAGEAL AND PYLORIC SPHINCTER AND COUNTERACTION
OF SPHINCTERS FAILURE WITH STABLE GASTRIC
PENTADECAPEPTIDE BPC 157 IN RATS
INTRODUCTION
We focused on NSAIDs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincter failure with stable gastric pentadecapeptide BPC 157 (1-7).
Common GI-toxicity of NSAIDs (8-14) and the role on prostaglandins (15) are generally known. However, the effect of NSAIDs on lower esophageal sphincter and pyloric sphicter pressure is far less studied, and is inconsistent and not fully described (16-23). Thereby, we focused on the lower esophageal and pyloric sphincters failure that may be induced by different NSAIDs, both COX-1 (aspirin, diclofenac, ibuprofen, paracetamol) and COX-2 (celecoxib) inhibitors (24).
To show that the sphincters failure is a part of the NSAIDs-toxicity, COX-1 and COX-2 inhibitors were used in dose regimens shown to induce gastric and small intestine ulcers, hepatotoxicity and encephalopathy (8-14). These effects were counteracted by stable gastric pentadecapeptide BPC 157.
Furthermore, the damaging effect of NSAIDs in the stomach was essential for the concept of Robert's cytoprotection (25), although the influence of NSAIDs on near sphincters was not considered. Contrary to this, the stable gastric pentadecapeptide BPC 157 has a special effect on both lower esophageal and pyloric sphincter (26-33) as a novel mediator of Robert's cytoprotection and adaptive cytoprotection (1-7). Also, it may have a particular effect on NSAIDs-lesions (3, 8-14). Namely, stable and not degraded in human gastric juice (more than 24 hours), as a particular anti-ulcer peptide (GEPPPGKPADDAGLV, M.W. 1419, PL-10, PLD-116, PL 14736), in trials for inflammatory bowel disease (LD1 not achieved), wound and fistulas treatment (1-7, 27, 29, 33-37) (i.e., stimulation of expression of the early growth response 1 (egr-1) gene (38), and other molecular pathways (38-42), effective alone without carrier (1-7) it has a particular effect on endothelium protection along with prominent angiogenesis (1, 43-47) and an effect on NO-system (1-7). Thereby, cumulatively, the beneficial effect on NSAIDs may be extensive (3, 8-14). Besides counteraction of bleeding aspirin-gastric ulcers (14), BPC 157 also counteracts other NSAIDs-toxicity, using the same dose range (8-14), namely, gastric and small intestine ulcers (i.e., induced by indomethacin, diclofenac, ibuprofen, celecoxib (8-14)), hepatotoxicity (paracetamol, diclofenac, ibuprofen, celecoxib) (8-14) and encephalopathy (paracetamol, diclofenac, ibuprofen, celecoxib) (8-14). Recently, it was found that BPC 157 also counteracts aspirin-prolonged bleeding and thrombocytopenia (along with counteraction of heparin and warfarin prolonged bleeding and throbocytopenias) (13).
Thus, because of the wide range of use of NSAIDs and the counteraction of their toxicity with pentadecapeptide BPC 157, this suggests that BPC 157 may be useful in antagonization of NSAIDs side effects (3, 8-14) and particularly, possible NSAIDs-sphincter failure. Similar to the BPC 157 effect on sphincters function, BPC 157 exhibited a particular anti-reflux effect in healthy (i.e., increase of pressure within lower esophageal sphincter, decrease of pressure within pyloric sphincter), while also rescuing sphincters failure (i.e., decreased pressure) in esophagitis rats (increase toward the normal values) (31, 32). In relation to the improved sphincter function and smooth muscle function (26-33), BPC 157 may additionally rescue the function of other failed sphincters as well (48, 49).
MATERIAL AND METHODS
Animals
Wistar Albino male rats (200 g b.w.) were randomly assigned to the experiments (10 animals, at least, per each experimental group), all of which were approved by the Local Ethics Committee. Furthermore, all experiments were carried out under blind protocol, and the effect was assessed by examiners who were completely unaware of the given protocol.
Drugs
Pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419), (Diagen, Ljubljana, Slovenia) dissolved in saline, was used in all experiments. The peptide BPC 157 is part of the sequence of human gastric juice protein BPC, and is freely soluble in water at pH 7.0 and saline. It was prepared as described previously (8-14) with 99% high pressure liquid chromatography (HPLC) purity, expressing 1-des-Gly peptide as an impurity (18-20).
Aspirin (400 mg/kg i.p. or i.g.), diclofenac (12.5 mg/kg or 40 mg/kg i.p.), paracetamol (5 g/kg i.p.), ibuprofen (400 mg/kg/day i.p. for 4 weeks), and celecoxib (0.5 g/kg or 1 g/kg i.p.) (Sigma, USA) were used as before (8-14). The assessment was made after NSAIDs application as follows: after 15 min, 30 min, 60 min, 3 h, 24 h, 48 h, and 72 for diclofenac; after 25 min, 3 h, and 24 h for paracetamol; after 3 h for aspirin; after 15 min, 30 min, 24 h, 48 h, 72 h, and 7 days for celecoxib; and with chronic use of ibuprofen for 4 weeks, 30 min after last application.
BPC 157 (10 µg/kg, 10 ng/kg) was given immediately after NSAIDs administration, intraperitoneally (aspirin, diclofenac, paracetamol, ibuprofen, celecoxib) or intragastrically (aspirin, paracetamol) while controls received an equivolume (5 ml/kg) of saline. Also, BPC 157 was given perorally in drinking water for 4 weeks (12 ml/rat/day, 0.16 µg/ml, 0.16 ng/ml) in rats which underwent ibuprofen application for 4 weeks while controls were drinking water only.
Likewise, BPC 157 (10 µg/kg, 10 ng/kg) was given in healthy rats. This should demonstrate the effect of BPC 157 alone in order to better understand its effect on NSAIDs-induced disturbances. Due to the different application protocols of NSAIDs, intraperitoneal (aspirin, diclofenac, paracetamol, celecoxib), intragastric (aspirin) application or prolonged applications (ibuprofen), BPC 157 (10 µg/kg, 10 ng/kg) was given as intraperitoneal and intragastric administration, or prolonged administration in drinking water (12 ml/rat/day; 0.16 µg/ml, 0.16 ng/ml) and its effect was assessed at the corresponding intervals.
Lower esophageal sphincter pressure assessment and pyloric sphincter pressure assessment. As described before (16, 17) all rats received manometrical evaluation (cmH2O) with a water manometer connected to the drainage port of the Foley catheter as described (the values of 68 – 76 cmH2O for lower esophageal sphincter and 68 – 4 cmH2O for pyloric sphincter were considered to be normal as determined before). The proximal side of the esophageal or distal side of the duodenal incision was ligated to prevent regurgitation.
Statistical analysis
Statistical analysis was performed using parametric two-way mixed model ANOVA (one factor is repeated-measures) and Student Newman-Keuls test to compare the difference between groups. A P value of 0.05 or less was considered statistically significant.
RESULTS
All of the NSAIDs induced both lower esophageal and pyloric sphincters pressure fall, and thereby the induced sphincter failure may be a common phenomenon of NSAIDs, with some particularities for the used non-specific (diclofenac, ibuprofen, aspirin, paracetamol) and specific (celecoxib) NSAIDs (Figs. 1-5).
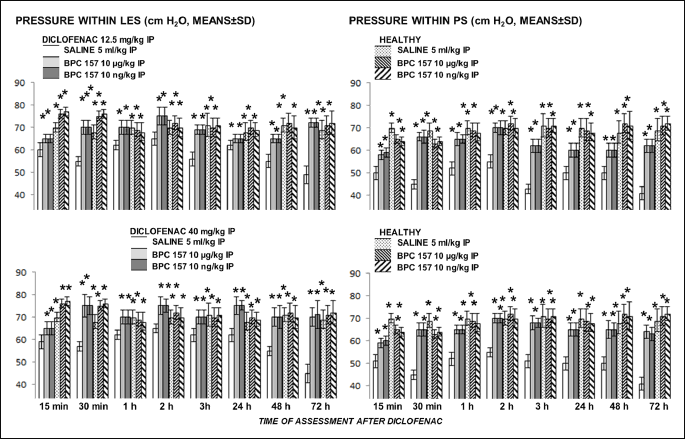
Diclofenac
We noted an immediate and then sustained effect on pressure in lower esophageal sphincter and pyloric sphincter after either 12.5 mg/kg or 40 mg/kg intraperitoneal challenge. Pyloric sphincter seems to be more affected. BPC 157 reversed disturbance of both sphincters (Fig. 1).
Ibuprofen
Given for 4 weeks, ibuprofen severely disturbed both lower esophageal sphincter and pyloric sphincter. Pyloric sphincter appears to be even more affected. BPC 157 reversed disturbance of both sphincters, given either immediately after ibuprofen or continuously in drinking water (Fig. 2).
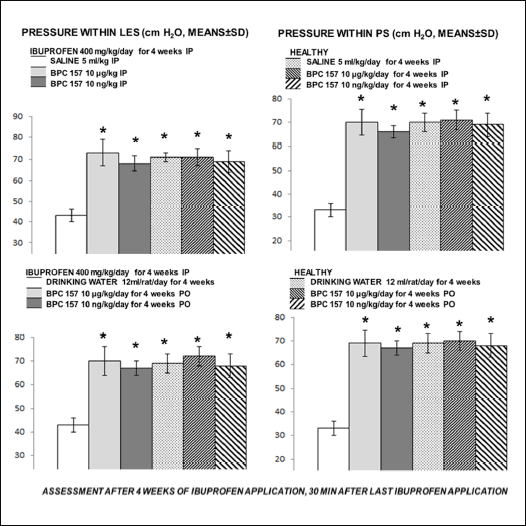 |
Fig. 2. Given for 4 weeks, ibuprofen severely disturbed both lower esophageal sphincter and pyloric sphincter (white bars). BPC 157 reversed disturbance of both sphincters, given either immediately after ibuprofen or continuously in drinking water (gray bars). Innate effect of BPC 157, given alone, was assessed at the corresponding intervals (healthy rats, dashed bars). Always, the noted effects were within the values for lower esophageal sphincter and for pyloric sphincter which were considered to be normal as determined before. Pressure within LES and PS (cm H2O, means ± S.D.), assessment after NSAID application. Medication immediately after NSAID or in drinking water. *P ≤ 0.05, at least, versus control. |
Aspirin
Given as an intraperitoneal or as an intragastric challenge, aspirin disturbed both lower esophageal sphincter and pyloric sphincter, but the effect appears to be relatively mild. Pyloric sphincter appears to be even more affected. BPC 157 reversed disturbance of both sphincters, given either intraperitoneally or intragastrically (Fig. 3).
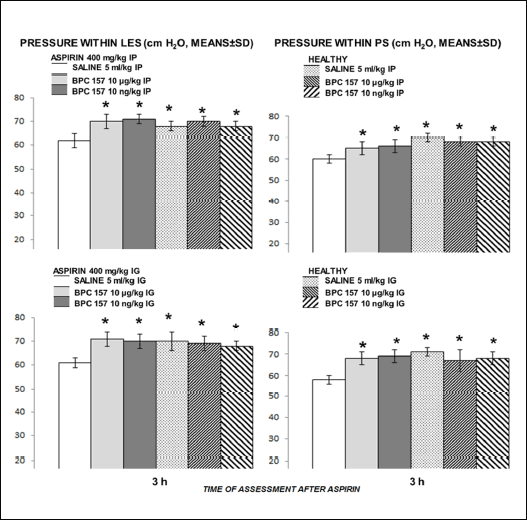 |
Fig. 3. Given as an intraperitoneal or as an intragastric challenge, aspirin disturbed both lower esophageal sphincter and pyloric sphincter, but the effect appears to be relatively mild (white bars). BPC 157 reversed disturbance of both sphincters given either intraperitoneally or intragastrically (gray bars). Innate effect of BPC 157 given alone, was assessed at the corresponding intervals (healthy rats, dashed bars). Always, the noted effects were within the values for lower esophageal sphincter and for pyloric sphincter which were considered to be normal as determined before. Pressure within LES and PS (cm H2O, means ± S.D.), assessment at 3 h after NSAID application. Medication immediately after NSAID. *P ≤ 0.05, at least, versus control. |
Paracetamol
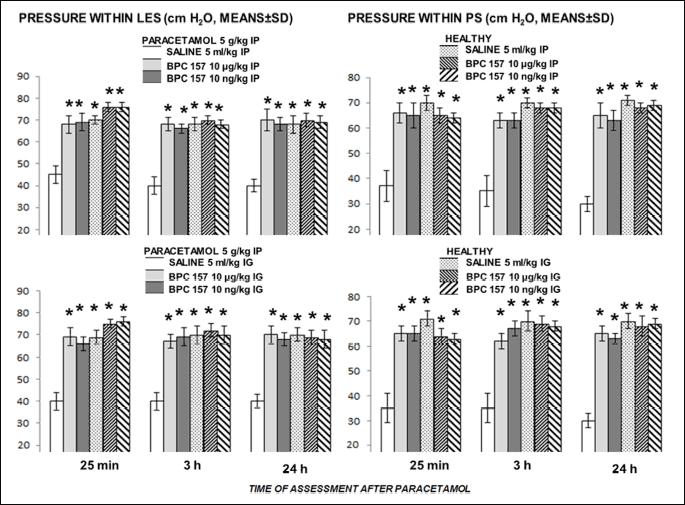
Given as an intraperitoneal challenge, paracetamol instantly and severely disturbed both lower esophageal sphincter and pyloric sphincter (note, in pyloric sphincter, pressure values are even below 50% of the normal values). BPC 157 reversed disturbance of both sphincters, given either intraperitoneally or intragastrically (Fig. 4).
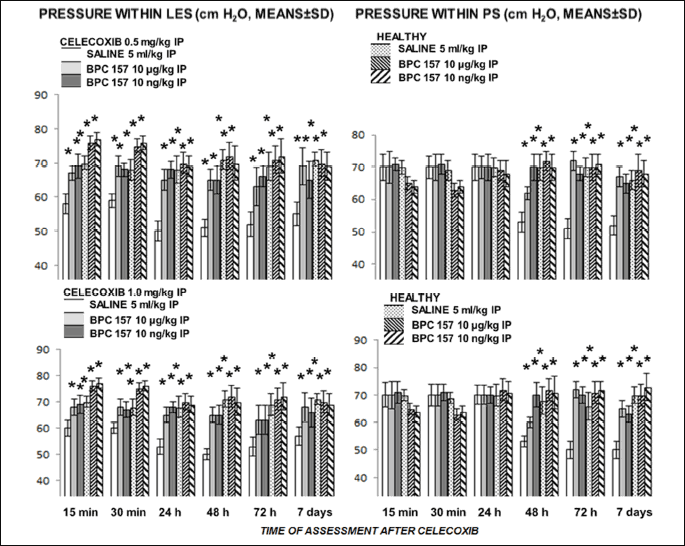
Celecoxib
We noted an immediate and then sustained effect on pressure in lower esophageal sphincter, and a postponed effect on pyloric sphincter after either 0.5 mg/kg or 1.0 mg/kg intraperitoneal challenge. Thereby, pyloric sphincter seems to be less affected. BPC 157 reversed disturbance of both sphincters (Fig. 5).
BPC 157 alone
To make needed distinction between the effect of BPC 157 itself and the effect of BPC 157 with administration of NSAIDs, BPC 157 was given as intraperitoneal and intragastric administration, or prolonged administration in drinking water in healthy rats to adequately correspond to different application protocols of NSAIDs, intraperitoneal (aspirin, diclofenac, paracetamol, celecoxib), intragastric (aspirin) application or prolonged applications (ibuprofen). Innate BPC 157 effect was assessed at the corresponding intervals. Always, the noted effects were within the values for lower esophageal sphincter and for pyloric sphincter which were considered to be normal as determined before (Figs. 1-5).
In summary, non-specific NSAIDs (i.e., diclofenac, aspirin, ibuprofen, aspirin, and paracetamol) immediately bring down pressure within lower esophageal and pyloric sphincters. Once established, the pressure fall has a debilitating effect on both sphincters that persists and seems to be not dose-dependent. A specific NSAID, celecoxib, affected firstly the lower esophageal sphincter, and then, after a day, the pyloric sphincter as well. BPC 157 given in either regimen (µg or ng; parenterally or perorally) consistently antagonizes the NSAIDs effect on lower esophageal sphincter and pyloric sphincter, thus counteracting both the early effects and the late effects induced by non-specific and specific NSAIDs.
DISCUSSION
We investigated the effects that BPC 157 administration has on decreased lower esophageal and pyloric sphincter pressure, due to non-specific and specific NSAIDs administration. Aspirin, diclofenac, ibuprofen, paracetamol and celecoxib were given in dose regimens shown to induce gastric and small intestine ulcers, hepatotoxicity and encephalopathy (8-14), and their effects were timely investigated. The results revealed that BPC 157 may consistently antagonize the effect of all NSAIDs on lower esophageal sphincter and pyloric sphincter.
All of the NSAIDs induced both lower esophageal and pyloric sphincters pressure fall and, thereby, the induced sphincter failure may be a common phenomenon for NSAIDs, with some particularities for the NSAIDs used, namely, diclofenac, ibuprofen, aspirin, paracetamol and celecoxib. Generally, the NSAIDs-induced sphincter failure may be quite prominent, at least in the range of that induced mechanically by prolonged stretch, after tube insertion into the lower esophageal and/or pyloric sphincter and sphincter failure in esophagitis rats (30-32), surgically by anastomosis and fistulas creation (26, 27, 29), or chemically, by huge potassium-overdose application (28). Providing that it may be induced rapidly, and then persisting for hour and day periods, this damaging effect is obviously part and parcel of general NSAIDs-toxicity, including gastric and small intestine ulcers, hepatotoxicity and encephalopathy (3, 8-14).
Moreover, sphincter failure soon after NSAIDs administration seems to be a common occurrence and may be responsible for later NSAIDs toxicity development. Possibly, this toxicity, in addition to lower esophageal sphincter and pyloric sphincter failure, involves sole NSAIDs-gastric ulcers, presenting with aspirin (and thereby, aspirin was used intragastrically) (14) and ibuprofen (10), as well as with those agents combining gastric and small intestine ulcers (i.e., diclofenac (9, 11)). Also, the sphincter failure results from other agents with either apparently less gastrointestinal toxicity, i.e., celecoxib (12, 50), or without gastrointestinal lesions (i.e., paracetamol) (3, 8). Furthermore, it may be interesting to present sphincters failure together with hepatotoxicity as common points for NSAIDs (paracetamol, diclofenac, ibuprofen), as well as encephalopathy due to NSAIDs (paracetamol, diclofenac, ibuprofen) (3, 8-14).
Considering non-specific NSAIDs (24) (diclofenac, aspirin, ibuprofen, aspirin, paracetamol) (an immediate effect of both sphincters) versus specific NSAID, celecoxib, we evidenced that celecoxib affected firstly lower esophageal sphincter, and then, after a day, the pyloric sphincter as well. BPC 157 may accordingly antagonize both COX 1- and COX 2-NSAIDs effect on lower esophageal sphincter and pyloric sphincter. This may be along with general COX 1 and COX 2 specificity (24), with the pyloric sphincter less sensitive to COX 2 and the lower esophageal sphincter more sensitive, but ultimately both affected by inhibition of both COX 1 and COX 2. Previously, pyloric sphincter function was found to be responsible for the biofeedback loop which is critical for tissue integrity maintenance. Pyloric sphincter dysfunction exhibits prolonged esophagitis, with a constantly lowered pressure not only in the pyloric but also in the lower esophageal sphincter, and a failure of both sphincters, which are counteracted by BPC 157 (30-32).
Thus, stable gastric pentadecapeptide BPC 157 has beneficial effects to counteract all NSAIDs side-effects on both sphincters (3, 8-14). Interestingly, BPC 157 also prevents and reverses adjuvant arthritis (14). Therefore, it is likely that pentadecapeptide BPC 157 provides counteracting potential in NSAIDs-sphincter failure, along with its proven ability to reduce NSAIDs toxicity (3, 8-14). In support of this is beneficial effect that BPC 157 has on both lower esophageal sphincter and pyloric sphincter. Note, the results were obtained with parenteral and per-oral application (i.e., BPC 157 is stable in gastric juice more than 24 h, unlike standard growth factors) (1-7). For instance, in rat esophagitis and failed function of both lower esophageal sphincter and pyloric sphincter, BPC 157 increased pressure in both sphincters to normal and also reduced esophagitis. However, while always being within the range of normal values, in healthy rats, it may decrease (pyloric sphincter) or increase (lower esophageal sphincter) the pressure in sphincters and, thereby, it may participate in the anti-reflux mechanism (30-32). Thus, in NSAIDs-rats the noted counteracting effect of BPC 157 (routinely, the initial fall in pressure was minimized and pressure values restored to normal values) seems to be convincingly demonstrated.
In addition, considering the important concept, cytoprotection (initially based on the essential consistent ulcerogenic role of NSAIDs agents (25)) can now provide the consistently demonstrated NSAIDs-sphincter failure as an additional clue to further accommodate all of the NSAIDs effects. At the same time, this supports the likely role of BPC 157, as a novel mediator of cytoprotection (1-7), in providing a beneficial effect in help rescuing failed sphincter function. Also, pentadecapeptide BPC 157, given peripherally, could affect sphincter function through serotonin (51, 52) or dopamine (in the same dose-range that BPC 157 prevented/reversed catalepsy or stereotypy and all concomitant gastrointestinal lesions) (53-56), likely providing a particular balance between peripheral and central effects (57).
Finally, from a general point of view, the consistent effect of NSAIDs (24, 25), and thereby, the consistent NSAIDs effects on sphincters failure in rat, and vice versa, the consistent sphincter failure related to NSAIDs, thus, a commonality for both NSAIDs and sphincter failure, could be hardly combined with the quite inconsistent effects of NSAIDs on sphincter pressure in different species so far reported (17-23). For instance, aspirin decreased pyloric sphincter pressure (23), while indomethacin was variously shown to have increased lower esophageal sphincter (18, 20, 22), decreased (19, 21) or had no effect (23) on lower esophageal sphincter pressure. Interestingly, indomethacin may also have a dual effect, namely, increased then abolished lower esophageal sphincter tone (19). On the other hand, as claimed in this study, a common damaging effect of NSAIDs on sphincters may be much more likely, all NSAIDs share the same characteristics.
In conclusion, we evidenced failed sphincter function after application of different NSAIDs, and the beneficial effect of BPC 157 on lower esophageal sphincter and pyloric sphincter and its counteraction against NSAIDs-toxicity. Now, in regards to the failed sphincter function after different NSAIDs, and later, to the improved sphincter function and smooth muscle function when BPC 157 was given, BPC 157, as a particular anti-ulcer pentadecapeptide, LD1 could be not achieved, effective alone without carrier (1-7) may be a suitable peptide candidate to additionally rescue such extended NSAIDs toxicity (1-14).
Acknowledgements: This research was supported by a grant from Ministry of Science, Education and Sports, Republic of Croatia (grant number 108-1083570-3635).
Conflict of interests: None declared.
REFERENCES
- Seiwerth S, Brcic L, Vuletic LB, et al. BPC 157 and blood vessels. Curr Pharm Des 2014; 20: 1121-1125.
- Sikiric P, Seiwerth S, Rucman R, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des 2014; 20: 1126-1135.
- Sikiric P, Seiwerth S, Rucman R, et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des 2013; 19: 76-83.
- Sikiric P, Seiwerth S, Rucman R, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem 2012; 19: 126-132.
- Sikiric P, Seiwerth S, Rucman R, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des 2011; 17: 1612-1632.
- Sikiric P, Seiwerth S, Brcic L, et al. Revised Robert's cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des 2010; 16: 1224-1234.
- Sikiric P, Seiwerth S, Rucman R, et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des 2017; doi: 10.2174/1381612823666170220163219. (epub ahead of print).
- Ilic S, Drmic D, Zarkovic K, et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol 2010; 61: 241-250.
- Ilic S, Drmic D, Franjic S, et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci 2011; 88: 535-542.
- Ilic S, Drmic D, Zarkovic K, et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol 2011; 667: 322-329.
- Lojo N, Rasic Z, Zenko Sever A, et al. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 hour-short-bowel rats. PLoS One 2016; 11: e0162590. doi: 10.1371/journal.pone.0162590
- Drmic D, Kolenc D, Ilic S, et al. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol 2017 (in press).
- Stupnisek M, Franjic S, Drmic D, et al. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb Res 2012; 129: 652-659.
- Sikiric P, Seiwerth S, Grabarevic Z, et al. Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. J Physiol (Paris) 1997; 91: 113-122.
- Mason JC. NSAIDs and the oesophagus. Eur J Gastroenterol Hepatol 1999; 11: 369-373.
- Cao WB, Harnett KM, Chen Q, Jain MK, Behar J, Biancani P. Group I secreted PLA2 and arachidonic acid metabolites in the maintenance of cat LES tone. Am J Physiol 1999; 277: G585-G598.
- Cagossi M, Salgarello M, Patrignani P, Salgarello G. Effects of various prostaglandin synthesis inhibitors on the tone of the lower oesophageal sphincter in man. Eur J Clin Pharmacol 1992; 43: 303-305.
- Daniel EE, Crankshaw J, Sarna S. Prostaglandins and tetrodotoxin-insensitive relaxation of opossum lower esophageal sphincter. Am J Physiol 1979; 236: E153-E172.
- Daniel EE, Sarna S, Waterfall W, Crankshaw J. Role of endogenous prostaglandins in regulating the tone of opossum lower esophageal sphincter in vivo. Prostaglandins 1979; 17: 641-648.
- Eastwood GL, Beck BD, Castell DO, Brown FC, Fletcher JR. Beneficial effect of indomethacin on acid-induced esophagitis in cats. Dig Dis Sci 1981; 26: 601-608.
- El Sayah M, Calixto JB. Study of the mechanisms involved in the bradykinin-induced contraction of the pig iris sphincter muscle in vitro. Eur J Pharmacol 2003; 458: 175-181.
- Mokka RE, Punto L, Kairaluoma MI, Larmi TK. Indomethacin and canine lower esophageal sphincter pressure. Am J Gastroenterol 1977; 67: 366-369.
- Pantoja JL, Defilippi C, Valenzuela JE, Csendes A. Nonsteroidal antiinflammatory drugs: effect on pyloric sphincter and duodenogastric reflux. Dig Dis Sci 1979; 24: 217-220.
- Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 2009; 17: 275-342.
- Robert A. Cytoprotection by prostaglandins. Gastroenterology 1979; 77: 761-767.
- Djakovic Z, Djakovic I, Cesarec V, et al. Esophagogastric anastomosis in rats: improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J Gastroenterol 2016; 22: 9127-9140.
- Skorjanec S, Kokot A, Drmic D, et al. Duodenocutaneous fistula in rats as a model for "wound healing-therapy" in ulcer healing: the effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J Physiol Pharmacol 2015; 66: 581-590.
- Barisic I, Balenovic D, Klicek R, et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept 2013; 181: 50-66.
- Cesarec V, Becejac T, Misic M, et al. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol 2013; 701: 203-212.
- Petrovic I, Dobric I, Drmic D, et al. BPC 157 therapy to detriment sphincters failure-esophagitis-pancreatitis in rat and acute pancreatitis patients low sphincters pressure. J Physiol Pharmacol 2011; 62: 527-534.
- Dobric I, Drvis P, Petrovic I, et al. Prolonged esophagitis after primary dysfunction of the pyloric sphincter in the rat and therapeutic potential of the gastric pentadecapeptide BPC 157. J Pharmacol Sci 2007; 104: 7-18.
- Petrovic I, Dobric I, Drvis P, et al. An experimental model of prolonged esophagitis with sphincter failure in the rat and the therapeutic potential of gastric pentadecapeptide BPC 157. J Pharmacol Sci 2006; 102: 269-277.
- Sikiric P, Seiwerth S, Mise S, et al. Corticosteroid-impairment of healing and gastric pentadecapeptide BPC- 157 creams in burned mice. Burns 2003; 29: 323-334.
- Grgic T, Grgic D, Drmic D, et al. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur J Pharmacol 2016; 780: 1-7.
- Baric M, Sever AZ, Vuletic LB, et al. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci 2016; 148: 63-70.
- Klicek R, Sever M, Radic B, et al. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system. J Pharmacol Sci 2008; 108: 7-17.
- Skorjanec S, Dolovski Z, Kocman I, et al. Therapy for unhealed gastrocutaneous fistulas in rats as a model for analogous healing of persistent skin wounds and persistent gastric ulcers: stable gastric pentadecapeptide BPC 157, atropine, ranitidine, and omeprazole. Dig Dis Sci 2009; 54: 46-56.
- Tkalcevic VI, Cuzic S, Brajsa K, et al. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol 2007; 570: 212-221.
- Hsieh MJ, Liu HT, Wang CN, et al. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl) 2017; 95: 323-333.
- Huang T, Zhang K, Sun L, et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther 2015; 9: 2485-2499.
- Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014; 19: 19066-19077.
- Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985) 2011; 110: 774-780.
- Sikiric P, Seiwerth S, Grabarevic Z, et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesion induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promoters and gut peptides. Life Sci 1994; 54: PL63-PL68.
- Hrelec M, Klicek R, Brcic L, et al. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J Physiol Pharmacol 2009; 60 (Suppl. 7): 161-165.
- Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J Physiol Pharmacol 2009; 60: 191-196.
- Sikiric P, Separovic J, Anic T, et al. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J Physiol Paris 1999; 93: 479-485.
- Sikiric P, Seiwerth S, Brcic L, et al. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, Pliva, Croatia). Full and distended stomach and vascular response. Inflammopharmacology 2006; 14: 214-221.
- Jandric I, Vrcic H, Jandric Balen M, et al. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med Sci Monit Basic Res 2013; 19: 93-102.
- Kokot A, Zlatar M, Stupnisek M, et al. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol 2016; 771: 211-219.
- Tsuji S, Miyoshi H, Tomita T, et al. Celecoxib, a cyclooxygenase-2 inhibitor, improved upper gastrointestinal lesions in rheumatoid arthritis patients as assessed by endoscopic evaluation. Mod Rheumatol 2012; 22: 353-362.
- Boban Blagaic A, Blagaic V, Mirt M, et al. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur J Pharmacol 2005; 512: 173-179.
- Tohyama Y, Sikiric P, Diksic M. Effects of pentadecapeptide BPC 157 on regional serotonin synthesis in the rat brain: alpha-methyl-L-tryptophan autoradiographic measurements. Life Sci 2004; 76: 345-357.
- Bilic I, Zoricic I, Anic T, et al. Haloperidol-stomach lesions attenuation by pentadecapeptide BPC 157, omeprazole, bromocriptine, but not atropine, lansoprazole, pantoprazole, ranitidine, cimetidine and misoprostol in mice. Life Sci 2001; 68: 1905-1912.
- Jelovac N, Sikiric P, Rucman R, et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. Eur J Pharmacol 1999; 379: 19-31.
- Sikiric P, Marovic A, Matoz W, et al. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson's disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J Physiol (Paris) 1999; 93: 505-512.
- Sikiric P, Separovic J, Buljat G, et al. Gastric mucosal lesions induced by complete dopamine system failure in rats. The effects of dopamine agents, ranitidine, atropine, omeprazole and pentadecapeptide BPC 157. J Physiol (Paris) 2000; 94: 105-110.
- Sikiric P, Seiwerth S, Rucman R, et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr Neuropharmacol 2016; 14: 857-865.
A c c e p t e d : April 28, 2017