THE URINE PODOCIN/CREATININE RATIO AS A NOVEL BIOMARKER
OF CARDIORENAL SYNDROME IN DOGS DUE TO DEGENERATIVE
MITRAL VALVE DISEASE
INTRODUCTION
The heart and kidney are involved in maintaining hemodynamic stability, which is crucial for life support. Dysfunction of one of these organs leads inevitable to the dysfunction of the other. For that reason, this phenomenon was termed the “cardiorenal syndrome” (CRS) (1). Previous studies have confirmed existence CRS in dogs with degenerative mitral valve disease (DMVD). The incidence of DMVD in dogs is estimated at 0.36%, which comprises 70% of the reported heart disease cases (2, 3). Chronic heart failure (CHF) caused by DMVD may result in a chronic decrease in renal cortical perfusion, and lead to congestion of the kidney tissue and its insufficiency (4). In dogs, kidney failure is classified using the IRIS grading system (5). This system does not take into account cardiac disease as a risk factor for chronic kidney disease (CKD), despite the fact that Martinelli et al. showed a direct correlation between the progression of heart failure and the degree of kidney failure (6). The prevalence of CKD in dogs suffering from CHF is estimated to range from 7.4% to 35.48%, based on the serum urea and creatinine levels (6, 7) while 70% of dogs with severe heart failure (grade IV according to NYHA) had kidney failure resulting in azotemia (8). It seems that kidney failure is underestimated in the course of heart disease as serum urea and creatinine seems to be not sufficient indicators of early kidney damage.
The detection of podocytes in urine may be a promising diagnostic test for the early detection of kidney damage. Podocytes are highly specialised glomerular epithelial cells involved in selective plasma filtration and the formation of primary urine. Pathological processes that occur in the kidneys cause podocyte tearing and excretion, elevating the urinary podocyte content (9-17). Due to the fact that podocytes do not regenerate, their loss is irreversible (18). It has been shown that a 20 – 40% loss of glomerular podocytes, i.e. approximately 100 – 200 podocytes/glomerulus, leads to glomerulosclerosis (16, 17). Numerous reports indicate that an early diagnosis of an elevated urinary podocyte excretion may facilitate the diagnosis of kidney disease in humans and animals (21). A quantitative urinary podocyte, albumin and protein analysis is strongly dependent on the degree of urine concentration (22, 20). Therefore, their levels are assessed relative to the urine creatinine concentration: the urine podocine/creatinine ratio (UPoC), the urine albumin/creatinine ratio (UAC) and the urine protein/creatinine ratio (UPC) (22, 23). This allows comparison of results despite differences in the urine concentration and it is particularly significant in the case of patients with CHF and CKD, who may have diluted urine due to the disease itself or medication.
The goal of the study was to assess the usefulness of commercial test to measure podocyturia in dogs. The second aim of this study was to test the hypothesis that UPoC is an early marker of kidney disease in dogs with DMVD, and that it is a more sensitive marker of kidney damage than serum urea, creatinine, symmetric dimethylarginine (SDMA) and cystatin C (Cyst C).
MATERIAL AND METHODS
Animals
The study included fifty dogs that were patients of the Department of Internal Diseases with the Clinic of Horses, Dogs and Cats, Faculty of Veterinary Medicine at the University of Life Sciences in Wroclaw. Based on the anamnesis, clinical examination and results of additional tests the dogs were divided into three groups: 1) the control group - 15 healthy dogs, 2) the heart group - 29 dogs with CHF and 3) the kidney group - 6 dogs with CKD.
There were two inclusion criteria in the control group: the absence of any signs of illness in the clinical examination (and in the last 6 months prior to the study) and normal results in all the laboratory tests.
Dogs included in the heart group had moderate symptoms of degenerative mitral valve disease, i.e. a stage C-chronic of the heart disease according to the ACVIM classification (23). Dogs in this groups had: degenerative changes on the mitral valve, hemodynamically significant mitral regurgitation, an enlarged left atrium and left ventricle (confirmed in an echocardiographic examination), mild clinical symptoms, e.g. weakness, reduced exercise tolerance, increased breathing after effort, restlessness or agitation while sleeping, sometimes coughing or gagging. Heart murmurs were detected in all the dogs in this group during the clinical examination. This dogs was at stable stage of heart disease and need home care (chronic) management of heart failure (25). All the dogs were treated with pimobendan 0.25 – 0.3 mg/kg PO q12 h, benazepril hydrochloride 0.25 mg/kg POq24 h, spironolactone 2 mg/kg POq24 h and torasemide 0.1 – 0.6 mg/kg POq24 h. Torasemide was used in relation to respiratory symptoms. The presence of abnormally high levels of serum creatinine > 1.4 mg/dl, 125 µmol/l was an exclusion criterion.
Dogs were included in the kidney group based on the modified in 2017 IRIS staging of CKD (5). All the dogs had renal azotemia lasting more than three months with the serum creatinine leave > 1.4 mg/dl, 125 µmol/l. In addition, prerenal and postrenal causes of azotemia were excluded. Dogs in this stage were classified as stage 2 – 4 according to IRIS classification and received benazepril hydrochloride at a dose of 0.25 mg/kg because of chronic albuminuria and renal diet.
The exclusion criteria for all the groups were: pregnancy, lactation, periods of growth and convalescence, the presence of diseases that could significantly affect the blood flow in the kidneys, e.g., cancer, central nervous system diseases, the presence of acute inflammation process, food poisoning or an intake of medicines, such as glucocorticosteroids, fluid therapy that may have significantly changed the kidney blood flow. Dogs with endocrine, immunological diseases and acute respiratory failure were also excluded from the study.
All the procedures were performed in the morning. The examination was preceded by a 12-hour fasting period, and it was carried out without pharmacological or other restraint.
Clinical examination with measurements of arterial pressure
The standard clinical examination was performed with particular emphasis on the circulatory and urinary systems. The measurement of systolic arterial blood pressure (SAP) was performed on the common digital artery after a 20 min. rest period using model 811-B of the Doppler Flow Detector (Parks Medical Electronics Inc. USA). An average of 3 – 5 consecutive SAP measurements was recorded.
Hematological and biochemical blood test
Blood was collected from the v. saphena or v. cephalica antebrachii into 2 ml EDTA blood tubes. Blood collection was preceded by a 12-hour fasting period. The hematological examination was performed immediately after the blood collection using an IDEXX X LaserCyte (Japan) hematology analyzer and a Horiba ABC animal blood counter (USA). The following parameters were measured: concentration of haemoglobin (HGB), hematocrit (HT), red blood cell count (RBC), white blood cell count (WBC). The biochemical parameters were assessed in serum, after centrifugation using the TermoScientific Konelab Prime 30ISE (Finland) biochemical analyzer in the analytical laboratory of the Department of Internal Diseases of Horses, Dogs and Cats. The concentration of sodium (Na), potassium (K), ionized calcium (Ca2+), magnesium (Mg), iron (Fe), glucose, urea, creatinine, total protein, albumin, aspartate transaminase (AspAT), alanine transaminase (ALT), C-reactive protein (CRP) was also determined. The dogs were considered azotemic if the serum creatinine was >1.4 mg/dl, 125 µmol/l. The dogs were considered to have prerenal azotemia if they had increased serum urea concentrations without concomitant increased serum creatinine concentrations.
In addition, serum samples were transported to the IDEXX Ludwigsburg Germany Laboratories to determine concentrations of symmetric dimethylarginine (SDMA) and cystatin C (Cyst C). SDMA was determined using an enzyme immunoassay dedicated to dogs. Cyst C was determined by liquid chromatography with mass spectrometry - a nephelometric analysis dedicated to dogs. Reference values for urea, creatinine, Cyst C, SDMA that were used in this study have been published elsewhere (5, 26, 27).
Examination of urine
Morning urine was collected by the owner into a sterile container during spontaneous urination. The urine examination was carried out immediately after receiving the urine samples. A physicochemical examination of urine included an analysis of its colour, transparency, specific gravity, pH, protein, albumin, creatinine, glucose, blood, acetone and urobilinogen levels.
The urine sediment was obtained through centrifugation for 10 min at 2500 g. Low speed spinning prevented podocyte damage. Therefore, the cellular elements in the urine sediment were detected using a ZEISS Primotech microscope. Those included the number of epithelial cells, blood cells, casts and bacteria. A 0.25 ml urine sediment sample from each dog was frozen at –80ºC. After collecting all the urine samples, a standard MyBioSource.com canine podocin ELISA test (San Diego, California USA) were carried out according to the manufacturer’s protocol. First, each wells of the ELISA plate were treated with 75 µl PBS and 25 µl urine sediment and incubated at 37ºC for 30 min. Then the wells were washed with a washing buffer, and they were treated with a conjugate solution at RT for 1 hour. Then the wells were washed again and treated with 50 µl of a substrate solution for 1 hour. At the end of the incubation, the absorbance was measured at 450 nm by VICTOR2. The detection range (sensitivity) was 0.1 ng/ml.
At the beginning the podocine concentration was assessed in the supernatant and sediment of the urine samples in seven healthy dogs.
The results from heart group were compared with a negative control, consisting of healthy dogs, and a positive control, consisting of dogs with confirmed renal disease. As the results discriminate the three groups, it became known that the test could be used to determine podocin in dogs.
UPoC, UPC and UAC was calculated by dividing the podocin, protein, albumin concentration (mg/dL), and by the urine creatinine concentration (mg/dL). The result was a unitless ratio (dimensionless).
Chest X-ray examination
Right lateral, left lateral and dorso-ventral thoracic X-ray images were obtained using a Gierth CHF200 (X-ray tube Toshiba, Japan) digital camera. The lung field, dilation of the pulmonary artery and vein, elevation of the distal part of trachea towards the spine and the presence of cardiomegaly were assessed (28). The size of the heart was assessed using the vertebral heart scale (VHS scale) (28).
Abdominal ultrasound examination
A standard abdominal ultrasound examination was carried out using the Hitachi Aloka F37 Japan machine with a 5 – 10 MHz microconvex and linear probe. Ultrasound examination allowed to exclude dogs with concurrent pathologies in the: adrenal glands, spleen, liver, intestines, pancreas, reproductive truck and lymphatics system. Additionally the careful examination of urinary bladder and kidneys was performed to exclude neoplastic changes (29).
Echocardiography
A standard chest parasternal echocardiography was performed with a simultaneous ECG recording using a Hitachi Aloka F37 (Japan) echocardiograph with a 5 – 7.5 MHz sector probe. The aorta diameter (Ao), left atrium size (LA), end-diastolic (RVIDd) internal diameter of the right ventricle, end-diastolic (LVIDd) and end-systolic (LVIDs) internal diameter of the left ventricle, thickness of the interventricular septum at end diastole (IVSd) and end systole (IVSs), end-diastolic (LVFWd) and end-systolic (LVFWs) thickness of the free wall of the left ventricle were measured. The shortening fraction (FS) of the left ventricle was calculated using the Teicholz formula. Mitral regurgitation was measured using the continuous wave Doppler technic from the left parasternal two- or four-chamber view. The normal echocardiographic value of the size of left ventricle and left atrium was based on Echocardiography: Principals of Interpretation by Bonagura et al. (30). Index dimension of left ventricle was based on ACVIM consensus guidelines (25).
Ethics approval and consent to participate
In accordance with the Experiments on Animals Act from January 15th 2015 (Journal of Laws of the Republic of Poland, 2015, item. 266), concerning the welfare of the animals used for research or teaching purposes, the provisions shall not apply to: 1) veterinary services as defined by the Act from December 18th 2003 concerning veterinary practices (Journal of Laws from 2004, No. 11, item 95 as amended in item 3), as well as agricultural activity, raising and breeding livestock according to the Animal Welfare Act, not designed to carry out medical procedures; 2) clinical veterinary studies carried out according to Article 37ah-37ak of the Act from September 6th 2001, Pharmaceutical Law (Journal of Laws from 2008, No. 45, item 271 as amended in item 4); 3) activity aimed at identifying animals; 4) capturing wild animals for biometric and systematic assessment; 5) veterinary procedures which to not cause pain, suffering, distress or permanent health impairment equal to or more invasive than the insertion of a needle. Hence, the study entitled “The urine podocin/creatinine ratio as a novel biomarker of cardiorenal syndrome in dogs due to degenerative mitral valve disease” does not require the approval of the Ethics Committee. All procedures were performed during the study with the owner consent.
Statistical analysis
Statistical analyses were performed using StatSoft Statistica PL 12.0 software. Data were expressed as mean and standard deviation (± SD) or median (depending on the distribution of variables) and IQ range. Data distributed normally was analysed using the Kolmogorov-Smirnov test, followed by the ANOVA and two-sample t-test. The U-Man test was used for non-normally distributed variables. Simple or multiple ordinal regression tests were used to describe the relationships between variables. A P value < 0.05 was considered statistically significant. The ROC curve was calculated by plotting the true against the false positive rate to establish the best cut off point discriminating the populations of patients. Multivariate logistic models were fitted using forward stepwise regression. Due to a limited number of observations, a maximum of two covariates were included in the model.
RESULTS
The results of the clinical, haematological, biochemical and urine analysis are summarized in Tables 1-5.
Dogs in heart group were statistically significant older and lighter than the healthy dogs. Systolic arterial pressure (SAP) was significantly lower in the control group compared to the kidney group (Table 1). In the heart group dogs older than 8 years had significantly higher blood pressure than younger dogs (162.0 ± 28.4 versus 137.5 ± 10.6).

ˆ statistically significant differences between the healthy and the heart group.
There were no statistically significant differences in the WBC and HT between groups, and all the values were within the reference range. However, dogs in control group had a significantly higher RBC than kidney group. The heart group had a significantly higher concentration of HGB than kidney group (Table 2).
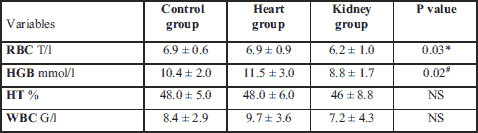
# statistically significant differences between the heart and the kidney group; NS, not statistically significant.
According to the study objectives, all the dogs in the control group had normal values of urea, creatinine, Cyst C and SDMA. All the dogs from the kidney group had elevated urea, creatinine, Cyst C and SDMA values. We found that all the dogs in the heart group had serum creatinine values in the reference range. Nine dogs had an elevated serum concentration of urea, seven dogs had an elevated serum concentration of SDMA, and five dogs had an elevated serum Cyst C level (Fig. 1B and 1C). The dogs in kidney group had statistically significant higher aspartate transaminase than in the control group. The dogs in the heart group had lower iron and chloride blood serum concentrations and an elevated alanine transaminase activity and level of C-reactive protein (CRP) compared to the healthy dogs (Table 3).
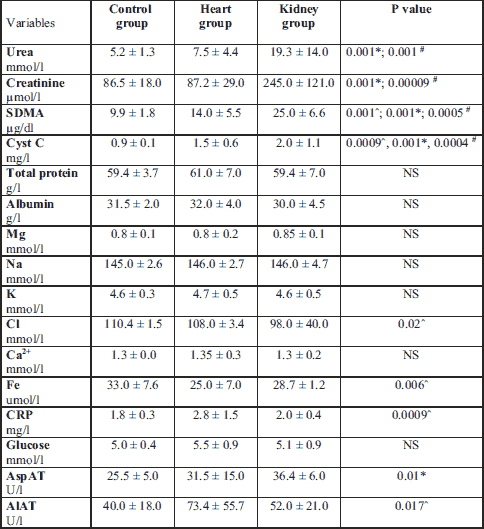
# statistically significant differences between the heart and the kidney group; ˆ statistically significant differences between the healthy and the heart group; NS, not statistically significant.
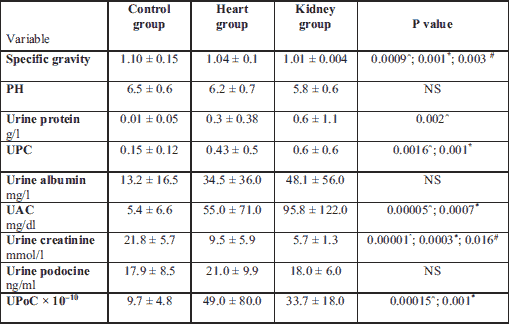
The urine specific gravity and urine creatinine were significantly lower in the kidney and heart groups compared to the control group, and these parameters were lowest in the kidney group.
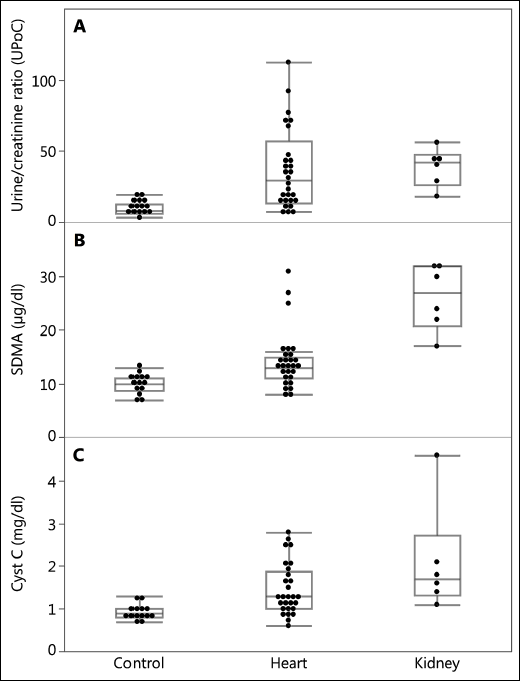 |
Fig. 1. The level of (A): UPoC, (B): SDMA and (C): Cyst C in the control group, heart group and the kidney group of dogs. |
The podocin concentration in the urinary sediment was 2.8 times higher than in the supernatant.
There was no statistically differences in urine albumin concentration and urine podocine concentration, but urine protein was statistically higher in kidney than in control group. UPC, UAC and UPoC were significantly higher in the heart and the kidney groups. Urine protein was elevated in the heart group in comparison to the healthy dogs (Table 4).
Mean ± standard deviation. UAC, urine albumin/creatinine ratio; UPC, urine protein/creatinine ratio; UPoC, urine podocine/creatinine ratio.
The maximum UPoC in the control group was 20.00 × 10–10, while the minimum UPoC in dogs from the kidney group was 29.14 × 10–10. The minimum UPoC in the heart group was min 5.08 × 10–10 and maximum 92.28 × 10–10 (Fig. 1A). In dogs in the heart group there were younger than 8 years the average level of UAC and UPC was slightly (insignificant) lower than in older dogs (UAC: 33.20 ± 33.24 versus 57.2 ± 74.29; UPC: 0.30 ± 0.26 versus 0.40 ± 0.54). In addition, higher UPoC were found in dogs with higher SAP (dogs in heart and kidney group).
The ROC curve revealed an optimal cut off of the UPoC at < 12.93 × 10–10 P-value < 0.0001 and showed an excellent discrimination level between healthy dogs and the heart group, AUC = 0.8138 (Fig. 2A). UPoC was a better predictor factor than podocine itself.
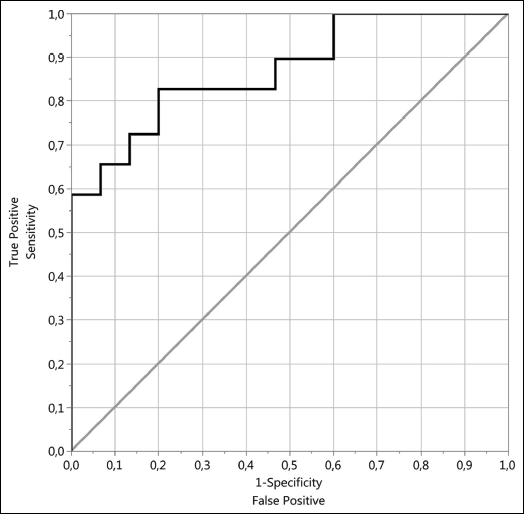 |
Fig. 2. Heart group predictors: (A) ROC curve of UPoC and (B) ROC curve of urine podocine (ng/ml). |
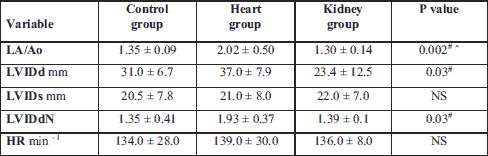
The heart rate was the lowest in healthy dogs and the highest in the cardiac group but the heart rate did not differ significantly between the investigated groups. The left atrium-to-aorta ratio was higher in the heart group, which was in accordance with the authors’ predictions. Left ventricular chamber size normalized to the body weight was statistically higher in heart group than in kidney (Table 5).
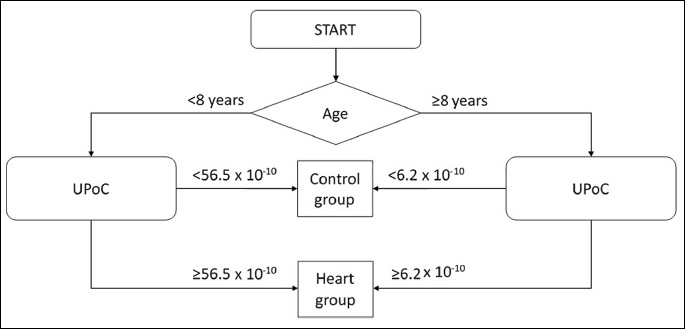
The multivariate logistic regression (Fig. 3) showed that the UPoC cut-off point in dogs with heart failure above 8 years of age exceeded 6.2 × 10–10, while it exceeded 56.5 × 10–10 in dogs younger than 8 years old and was used differentiate the control group from the heart group.
DISCUSSION
The detection of renal failure prior to the occurrence of azotemia is of high clinical importance as the clinical signs of renal failure, such as an elevated level of serum urea and creatinine, are present only in an advanced stage of the kidney damage. Similarly to humans, heart failure in dogs disturbs the kidney function leading to azotemia. Forty to sixty % of human CHF patients have CKD and coexistence these two disease worsen their prognoses (31, 32).
CKD can be attributed to multiple etiologies including congenital disorders (e.g., renal dysplasia, polycystic kidney disease,) glomerulopathies, which can be secondary to acquired systemic conditions (e.g., neoplasia or infection) and others (such as heart disease, diabetes mellitus, periodontal or infectious diseases) (5, 6).
We were particularly interested to study dogs suffering from chronic DMVD as this is the most frequent reason of heart failure in dogs. Dogs with moderate symptoms of DMVD, i.e. a C-chronic stage of the heart disease according to the ACVIM classification, were included in the study, as most veterinary patients present with this stage of the disease. The development of the kidneys injury is detected until now usually by serum creatinine, SDMA or UPC and UAC (33). To emphasize the differences between veterinary patients and people with renal injury secondary to heart disorders the consensus statement proposed cardiovascular-renal disorders (CvRD) in domestic dogs and cats (however in practice the CRS is still used (7).
However, we wanted to investigate the possibility of detecting kidney damage before the azotemia appears, so we enrolled dogs with DMVD in the study if their serum urea and creatinine concentrations remained within the reference values. We believe that this knowledge is crucial, both from a scientific and clinical point of view. In the present study, we used the common blood serum markers of early kidney injury, such as serum Cyst C, serum SDMA, urine UAC, urine UPC and a novel marker of podocyturia (UPoC), to diagnose asymptomatic kidney failure. We chose to determine the ratio of podocin/urine creatinine ratio as this method avoids the impractical and unfeasible 24-hour collection of urine by the dog owners. The value of this method has been previously confirmed clinically and scientifically (34). The correction using creatinine was proposed to avoid the influence of diuretics on the concentration of podocytes in urine samples.
The reviewed literature suggests that podocin can be analysed in the urinary supernatant (9) and sediment (10-14). Recent human literature suggests that podocin should be evaluated in the urinary sediment (10-14). Hence, we carried out a preliminary examination to assess and compare the podocin concentration in the urinary supernatant and sediment in seven urinary samples from dogs. We found that the podocin concentration was almost three times higher in the urinary sediment than in the supernatant. For that reason we chose to evaluate podocin in the urinary sediment. In order to avoid damage of the podocyte cellular membrane and the transfer of small membrane fragments into the supernatant, the samples were centrifuged at a relatively low speed. The podocyte cellular basement membrane may be damaged upon freezing at –80ºC, leading to a release of podocin. However, in this study, we evaluated the full sample volume (0.25 ml), minimising the impact of this phenomenon on the results.
The currently used classification of kidney insufficiency in veterinary medicine include SDMA values (5). However, Lorin and Choi found that there is no increase in SDMA in dogs with CHF, contrary to the reports in humans (31, 32). This is consistent with our findings, where only one in four dogs with DMVD had increased SDMA levels, which means that there was no increase in SDMA in up to 72.4% of the dogs. The difference in the SDMA elevation recorded in humans and dogs is most likely caused by the differences in the etiology of heart disease. In humans, ischemic heart failure is mainly associated with an impaired systolic function. In dogs with DMVD for a long period (including class C-chronic ) systolic function (ejection fraction) is preserved.
The diagnosis of CRS poses a clinical challenge, as 40 – 60% cardiologic patients develop CKD (35, 36). It is currently believed that in dogs with CHF the CKD prevalence ranges from 7.4% to 35.48% based on the serum urea and creatinine levels (6). Based on the UPoC results, we found that podocyturia in dogs with CHF and DMVD exceeding 12.93 × 10–10 indicated renal failure and an onset of the CRS. The prevalence of CKD in dogs with CHF was significantly higher than initially assumed, and amounted to 79.3%. This signifies that approximately eight out of ten dogs with CHF develop CRS. In addition, age substantially affected the UPoC value until an age of 8 years was reached. Based on the UPoC in dogs younger than eight years old suffering from DMVD, the UPoC value was significantly higher and exceeded 56.5 × 10–10, while the UPoC value in dogs older than eight years suffering from DMVD markedly lower and exceeds 6.2 × 10–10 (Fig. 3). The rate of podocyte loss varied between individuals and is affected by drugs prescribed by veterinarians. Hence, it is crucial to carry out urinary podocyte counts in those patients, in order to minimise kidney damage and prevent progressive heart failure. Performing urinary podocyte counts may be a useful tool in the long-term management of DMVD. You cannot clearly confirm what was the reason for the reduction of the podocytes lost in urine in older dogs with heart disease. The most likely, we consider dogs younger than 8 years are in the earlier, active stage of glomerular injury - stage connected with elevated podocytes loss. It contrast to the humans, it seems in dogs podocyturia is independent of systemic blood pressure because the older dogs had higher blood pressure than younger. There was no differences between UAC and UPC levels, despite younger dogs in the heart group had slightly lower average UAC and UPC than older one.
The huge difference in the incidence of CKD between humans and dogs may partly be caused by an underestimation of dogs with CKD due to an inaccurate diagnosis of canine early kidney damage, as shown in our study. Podocyturia may be the first detectable indicator of kidney failure as it appears as a consequence of damage of the glomerular basement membrane. In humans, a loss of more than 20% of the podocytes from the glomerular basement membrane leads to a serum creatinine increase and causes permanent, irreversible damage and progression of CKD (19, 20).
For that reason, the first aim of this study was to assess whether a commercially available podocin test may be used in dogs. We found that dogs with a documented kidney disease and uremia (that also had increased SDMA and Cyst C levels) had significantly increased UPoC levels. The second phase of the study found that podocyturia was present in dogs with DMVD without uraemia or increased SDMA and Cyst C levels. We found that dogs with DMVD + normal SDMA values + UPoC values exceeding the 12.93 × 10–10 had significantly higher serum creatinine levels (despite the levels still reminds within normal limits < 1.4 mg/dl, 125 µmol/l) and lower urine creatinine values compared to remained dogs with cardiac disease. This confirms that the UPoC concentration correlates with the degree of kidney damage. In the group with increased UPoC values, the average values of SDMA that were above the reference value (11.7 µg/dl) were almost doubled (20.1 µg/dl). Between the groups with elevated and physiological level of SDMA, there was a three-fold difference in the UPoC values (87.3 × 10–10 versus 31.8 × 10–10). Figs. 1A, 1B and 1C illustrate that UPoC increases faster in the CRS (heart group) than Cyst C and SDMA. However, SDMA and Cyst C are higher in dogs that have kidney disease.
There was no significant difference in the podocyte count between the studied groups (Table 4), and the ROC analysis confirmed that the podocyte count did not enable good discrimination between groups (sensivity 0.79, specificity 0.53, AUC 0.6) (Fig. 2B). However, significant differences in the urine specific gravity were found between the groups. Both study groups of ill dogs had significantly less concentrated urine. A lower creatinine concentration in those dogs was associated with an increased diurnal urine output.
As mentioned above in our study dogs in the heart group received diuretics (torasemide), because all of them need it (all of dogs were in stable but symptomatic stage of heart disease). For that reason we cannot stopped the treatment. In order to minimise the effect of this factor on the results, podocyturia was evaluated with respect to the urine concentration. It is assumed that creatinine is a good marker of the concentration of urine, hence, parameters which strongly depend on the urine concentration, are corrected based on the urine/creatinine ratio.
However, urine creatinine excretion is affected by several non-renal factors e.g. it is lower in human and animals with a lower muscle mass and a reduced muscle activity (32). This was also confirmed in our study as we found a lower urine creatinine concentration in older (less active) animals with a low body mass as well as in dogs treated with diuretics.
The ROC analysis of the UPoC revealed a significantly higher discriminant value, a sensitivity of 0.83, a specificity of 0.2 and an AUC of 0.87 (Fig. 2A).
As a result, the analysis of UPoC is considered a primary non-invasive method enabling the differentiation of an active kidney disease from inactive damage. In order to determine the utility of podocyturia as a clinical marker of an active disease process, further research into canine podocyturia is needed. Painless, non-invasive sampling that does not require special animal preparation is a key advantage of this test. This method may allow: the detection of kidney damage prior to the occurrence of clinical signs, enable an assessment of the degree of kidney damage and allow the identification of the pathological processes occurring in the kidneys, without the need for a kidney biopsy. The latter is of particular clinical value as it enables a preliminary evaluation of the pathological process in patients unable to undergo a kidney biopsy. Moreover, the test is time and cost-effective due to the presence of a commercially available ELISA kit.
The main limitation of this study was a lack of owner consent to a kidney biopsy. Carrying out a kidney biopsy would have enabled us to assess the degree of renal damage should be based upon histopathological findings or GFR (37). Ichii et al. in his publication used rabbit antihuman podocin antibodies (SIGMA) to estimate podocin positive reactions in light microscopy of renal histopathologic examinations of canine renal corpuscles. To our knowledge our experiment is the first report showing the assessment of the canine ELISA test to estimate podocin in canine urine in early stage of kidney injury (CRS) and compare with CKD and healthy dogs.
In conclusion our data suggest that a commercially available ELISA test may be used to assess podocyturia in dogs. DMVD leads to kidney failure, which may be diagnosed using the UPoC marker prior to an increase in the concentration of serum SDMA, Cyst C, urea and creatinine and urine UAC and UPC. An UPoC increase above 12.93 × 10–10 indicates glomerular damage in dogs suffering from chronic heart failure. Based on UPoC, 79.3% of dogs with C-chronic stage of DMVD developed CRS.
List of abbreviations: ACVIM, American College of Veterinary Internal Medicine; AUC, area under the ROC curve; CHF, chronic heart failure; CKD, chronic kidney disease; CRS, cardiorenal syndrome; Cyst C, cystatin C; DMVD, degenerative mitral valve disease; ROC, receiver operating characteristic curve; SAP, systolic arterial pressure; SDMA, symmetric dimethylarginine; UAC, urine albumin/creatinine ratio; UPC, urine protein/creatinine ratio; UPoC, urine podocine/creatinine ratio.
Funding: No financial support was provided for this project.
Conflict of interests: None declared.
REFERENCES
- Orvalho JS, Cowgill LD. Cardiorenal syndrome: diagnosis and management. Vet Clin North Am Small Anim Pract 2017; 47: 1083-1102.
- Mattin MJ, Boswood A, Church DB, et al. Prevalence of and risk factors for degenerative mitral valve disease in dogs attending primary-care veterinary practices in England. J Vet Intern Med 2015; 29: 847-854.
- Kim HT, Han SM, Song WJ, et al. Retrospective study of degenerative mitral valve disease in small-breed dogs: survival and prognostic variables. J Vet Sci 2017; 18: 369-376.
- Lubas A, Ryczek R, Kade G, Niemczyk S. Renal perfusion index reflects cardiac systolic. Med Sci Monit 2015; 21: 1089-1096.
- IRIS. Available: www.iris-kidney.com/pdf/3_staging-of-ckd.pdf.
- Martinelli E, Locatelli C, Bassis S, et al. Preliminary investigation of cardiovascular-renal disorders in dogs with chronic mitral valve disease. J Vet Intern Med 2016; 30: 1612-1618.
- Jung HB, Kang MH, Park HM. Evaluation of serum neutrophil gelatinase-associated lipocalin as a novel biomarker of cardiorenal syndrome in dogs. J Vet Diagn Invest 2018; 30: 386-391.
- Nicolle AP, Chetboul V, Allerheiligen T, et al. Azotemia and glomerular filtration rate in dogs with chronic valvular disease. J Vet Intern Med 2007; 21: 943-949.
- Hara M, Yamagata K, Tomino Y, Saito A, et al. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 2012; 55: 2913-2919.
- Nakamura T, Ushiyama C, Suzuki S, et al. The urinary podocyte as a marker for the differential diagnosis of idiopathic focal glomerulosclerosis and minimal-change nephrotic syndrome. Am J Nephrol 2000; 20: 175-179.
- Nakamura T, Ushiyama C, Suzuki S, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 2000; 15: 1379-1383.
- Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 2001; 89: 342-347.
- Hara M, Yanagihara T, Takada T, et al. Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol 1998; 18: 35-41.
- Le Jemtel TH, Rajapreyar I, Selby MG, et al. Direct evidence of podocyte damage in cardiorenal syndrome type 2: preliminary evidence. Cardiorenal Med 2015; 5: 125-134.
- Hashim AA, Helmy MM, Mouneir SM. Cysteinyl leukotrienes predominantly mediate cisplatin-induced acute renal damage in male rats. J Physiol Pharmacol 2018; 69: 779-787.
- Lajdova I, Oksa A, Horvathova M, Spustova V. Expression of purinergic P2X7 receptors in subpopulations of peripheral blood mononuclear cells in early-stage of chronic kidney disease. J Physiol Pharmacol 2017; 68: 779-785.
- Kasztan M, Jankowski M. Involvement of P2 receptors in regulation of glomerular permeability to albumin by extracellular nucleotides of intra-/extra-glomerular origins. J Physiol Pharmacol 2016; 67: 177-183.
- Wagner N, Wagner KD, Xing Y, Scholz H, Schedl A. The major podocyte protein nephrin is transcriptionally activated by the Wilms' tumor suppressor WT1. J Am Soc Nephrol 2004; 15: 3044-3051.
- Puelles VG, Bertram JF. Counting glomeruli and podocytes: rationale and methodologies. Curr Opin Nephrol Hypertens 2015; 24: 224-230.
- Sato S, Yanagihara T, Ghazizadeh M, et al. Correlation of autophagy type in podocytes with histopathological diagnosis of IgA nephropathy. Pathobiology 2009; 76: 221-226.
- Perez-Hernandez J, Olivares MD, Forner MJ, Chaves FJ, Cortes R, Redon J. Urinary dedifferentiated podocytes as a non-invasive biomarker of lupus nephritis. Nephrol Dial Transplant 2016; 31: 780-789.
- Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 2003; 285: 40-48.
- Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev 2011; 32: 97-102.
- Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009; 23: 1142-1150.
- Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med 2019; 33: 1127-1140.
- Dahlem DP, Neiger R, Schweighauser A, et al. Plasma symmetric dimethylarginine concentration in dogs with acute kidney injury and chronic kidney disease. J Vet Intern Med 2017; 31: 799-804.
- Miyagawa Y1, Takemura N, Hirose H. Evaluation of the measurement of serum cystatin C by an enzyme-linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J Vet Med Sci 2009; 71: 1169-1176.
- Buchanan JW. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract 2000; 30: 379-393.
- Hansen KL, Nielsen MB, Ewertsen C. Ultrasonography of the kidney: a pictorial review. Diagnostics (Basel) 2015; 23: E2. doi: 10.3390/diagnostics6010002
- Bonagura JD, O'Grady MR, Herring DS. Echocardiography. principles of interpretation. Vet Clin North Am Small Anim Pract 1985; 15: 1177-1194.
- Lorin J, Guilland JC, Stamboul K, et al. Increased symmetric dimethylarginine level is associated with worse hospital outcomes through altered left ventricular ejection fraction in patients with acute myocardial infarction. PLoS One 2017; 12: e0169979. doi: 10.1371/journal.pone.016997
- Choi BS, Moon HS, Seo SH, Hyun C. Evaluation of serum cystatin-C and symmetric dimethylarginine concentrations in dogs with heart failure from chronic mitral valvular insufficiency. J Vet Med Sci 2017; 79: 41-46.
- Carter CE, Katz R, Kramer H, et al. Influence of urine creatinine concentrations on the relation of albumin-creatinine ratio with cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2013; 62: 722-729.
- Lee C, Morris DL, Dieter PA. Validating and optimizing spot sampling of urine to estimate urine output with creatinine as a marker in dairy cows. J Dairy Sci 2018; 31: 22-32.
- Adams KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209-216.
- Shiba N, Shimokawa H. Chronic kidney disease and heart failure-bidirectional close link and common therapeutic goal. J Cardiol 2011; 57: 8-17.
- Ichii O, Yabuki A, Sasaki N, et al. Pathological correlations between podocyte injuries and renal functions in canine and feline chronic kidney diseases. Histol Histopathol 2011; 26: 1243-1255.
A c c e p t e d : April 29, 2019