DIFFERENT NUTRIENT INTAKE AND PREVALENCE OF GASTROINTESTINAL COMORBIDITIES IN WOMEN WITH ENDOMETRIOSIS
INTRODUCTION
With 4 – 30%, endometriosis presents one of the most frequent gynaecological diseases in women of the reproductive age (1-3), with an even higher prevalence in infertile women (4). It is characterized by the presence of estrogen depending endometrial tissue outside the cavum uteri (5). Thereby endometriosis manifests on different inner parts of the body, encompassing endometriosis genitalis interna (adenomyosis uteri), endometriosis genitalis externa (disease in the internal female genital tract) and endometriosis extragenitalis (disease elsewhere) (6).
Endometriosis is a multifactorial disease. Even though different theories about etiology and pathology exist (7, 8), the exact mechanisms have not yet been fully understood. Several parameters including immunologic aspects, inflammatory mediators (9), genetics or oxidative stress are discussed as influencing factors.
Endometriosis is often accompanied by different clinical manifestations expressed by various symptoms. A large proportion of the affected women suffer from pelvic pain, especially during menstruation (dysmenorrhea), as a leading symptom (10, 11). Further typical symptoms are dysuria (12), dyschezia (13) and dyspareunia (14, 15). Interestingly, no correlation between the degree of endometriosis and the severity of pain symptoms seems to exist (16, 18). Besides inflammation processes and the release of prostaglandins, a higher density of nerve-fibers in endometriotic lesions (19), as well as a generalized hyperalgesia (20) is discussed in pain pathogenesis. However, exact mechanisms are poorly understood at present.
In general, endometriosis is associated with a high morbidity. Above all, the presence of pelvic pain leads to a poor quality of life and mental health (21). So, next to the preservation of fertility, pain relief is one of the most important treatment goals. Additionally, also an association of colonic endometriosis or other endometriosis localizations (e.g. uterus) and endometrioid adenocarcinoma, which can be indicated by an impaired gamma-glutamyl transferase (GGT) (22), was described (23, 24). Therefore, different management options exist including analgesics, hormonal therapies or surgical methods (25). Apart from medical interventions, the nutrition has received more and more attention in the past years; not only as a modifiable risk factor, but also as a new therapeutic approach (26-28).
Gastrointestinal symptoms are frequent in endometriosis patients (29). Reasons for these symptoms are various, reaching from intestinal manifestation of endometric lesions to an elevated prevalence of food intolerances, allergies or irritable bowel syndrome. The diagnosis and treatment of diet-induced disorders, especially food intolerances or allergies, by nutritional interventions may help to improve overall well-being and symptoms like abdominal pain, bloating or nausea (30). Furthermore, several studies demonstrated an association between the pathophysiological mechanisms of endometriosis and the intake of certain nutrients. Pelvic pain was reduced after supplementation of different antioxidants e.g. vitamin C (31) and risk for endometriosis was increased by a high intake of trans-fats (32). Nevertheless, the knowledge is scarce about the actual nutrient intake of women with endometriosis. However, this knowledge is important for the detection of an under- or oversupply of special nutrients and a prerequisite for an adequate dietary intervention. Therefore, we aimed at determining the precise nutrient intake of endometriosis patients with the help of a validated food frequency questionnaire. A comparison with an age-matched control group of women without endometriosis should reveal differences in the nutrient intake. As possible influencing factors on nutrient intake, we also determined and compared the prevalence of comorbidities and gastrointestinal symptoms of these two patient groups.
MATERIALS AND METHODS
Patients and study design
This retrospective case-control study was conducted in women with a histological or clinical confirmed diagnosis of endometriosis (EM) between November 2013 and February 2015. Two thirds of the patients were recruited from the outpatient clinic or during their inpatient stay at the University Endometriosis Center for Franconia in Erlangen. External acquisition included endometriosis support groups, gynaecological practices and disease-related internet forums. The age-matched women of the control group were recruited from the circle of friends or colleagues. Exclusion criteria were a previous diagnosis of endometriosis or inflammatory bowel diseases.
For nutritional analysis the nutrient intake of all participants was recorded by a self-administered food frequency questionnaire (FFQ) of the past twelve months. Additionally, data about demographic and gynaecological features were acquired. Both questionnaires had to be filled in once.
All participants were informed about the background and procedure of the study and gave their written informed consent prior study inclusion. The study protocol was approved by the Ethics Committee of the Friedrich Alexander University Erlangen (FAU) and in accordance with the declaration of Helsinki.
Demographic and gynaecological data
A standardized questionnaire including questions on pregnancy history, previous use of hormonal contraceptives, medical history, family history and lifestyle was completed by the patients. A structured interview with trained medical personnel was done if any questions had not been fully answered. The version used in our study was adjusted for patients with chronic pain/endometriosis. It comprised questions about demographic features, the gynaecological medical history (preexisting diseases, diagnosis of endometriosis, hysterectomy, ovariectomy, tubal ligation, other surgeries and endometriosis-related surgeries, first-degree relatives with gynaecological disease, endometriosis-related medication and other therapeutic approaches), lifestyle, pregnancy and birth as well as endometriosis-related symptoms and physical limitations. Pain intensity was measured using a numeric rating-scale (NRS, 0 = no pain, 10 = worst possible pain). To evaluate comorbidities and gastrointestinal symptoms, questions about the presence of irritable bowel syndrome, allergies, intolerances and indigestions were added to the questionnaire. For control patients, endometriosis-related questions were excluded.
Dietary assessment
A self-administered FFQ was used for the retro-perspective evaluation of the nutrient intake in the past twelve months. Thereby FFQ was designed by the German Institute of Nutritional Research Potsdam-Rehbrucke. It consists of thirty-two pages encompassing 17 food groups and preparation types, respectively, including foods and beverages. Portion sizes were illustrated by pictures (e.g. a handful, a heaped table spoon, a whole banana, etc.) (33). There were different categories for consumption frequency depending on the respective food group, whereas seasonal fluctuations were considered as well.
All FFQs were sent to the German Institute of Nutritional Research Potsdam-Rehbruecke for evaluation. The daily intake of food groups and 135 nutrients, including carbohydrates, fat, protein, fibers, minerals and alcohol, fatty acids, amino acids, special carbohydrates, trace elements, vitamins and dietary fibers were calculated using the computer-assisted EPIC-Soft program. All data about nutrient content were based on the German Food Code (34). The daily intake of macronutrients, vitamins and minerals was compared with the D-A-CH reference values for nutrient intake of the German Nutrition Society (DGE), the Austrian Nutrition Society (OGE) and the Swiss Society for Nutrition (SGE).
Statistical analysis
Characteristic data are described as means ± standard deviation (SD) or in numbers (n) and percent (%). The data were checked by the Kolmogorow-Smirnow-test for normal distribution.
Differences between continuous variables of endometriosis patients and controls were determined using the Mann-Whitney-U-Test for non-parametric and the unpaired Student’s t-test for parametric data. Pearson’s chi-squared test was used for comparisons of categorical variables.
To determine the risk for comorbidities of study groups, the odds ratio was calculated and described as odds ratio (OR) and 95% confidence interval (95% CI).
A P-value of P < 0.05 was considered as significant.
Statistical analysis was performed using SPSS version 21 (IBM SPSS Statistics, Ehningen, Germany) and GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
Characteristics
A total of 208 participants (age 34.7 ± 7.9 years) were included in the study. Thereof 156 patients (age 35.5 ± 7.8 years) were diagnosed with endometriosis. Study participants showed no significant differences in age and body mass index (BMI), but with about one third, a greater proportion of EM patients showed a BMI over 25 kg/m² compared to the controls.
With regard to gynaecological characteristics, EM patients and controls showed similar menopausal statuses (approximately 90% premenopausal). Significant differences between the two study groups were found for cycle regularity (P = 0.005), bleeding duration (P = 0.011), amenorrhea over 6 months (P = 0.025), pregnancy (P = 0.003) and infertility (P = 0.001) (Table 1).
There were no significant differences in the family history regarding endometriosis, endometrial carcinoma or ovarian carcinoma between the EM and control group. A higher percentage of EM patients had surgical interventions. Thereby 9.0% and 8.3% of the EM patients had undergone hysterectomy (P = 0.019) and ovariectomy (P = 0.032), respectively.
For detailed patients’ characteristics see Table 1.

Endometriosis characteristics
Of the 156 endometriosis patients about 89.1% received a medical therapy. Most common treatments included analgesics and oral contraceptives or vaginal rings. The most frequent alternative medical treatments comprised homeopathy, osteopathy and acupuncture. Further therapeutics are listed in Table 2.
Almost all endometriosis patients showed disease-related symptoms (99.4%). EM patients mostly suffered from lower abdominal pain (89.1%) followed by dyschezia (48.7%), dyspareunia (46.2%) and dysuria (27.6%; Table 2). As a result, endometriosis impairs the daily life of patients to a great extent. Our study patients stated discomfort on 11.5 days and a reduced activity on 7.5 days a month. On average, they spent about 1.6 days per month in bed (Table 2).
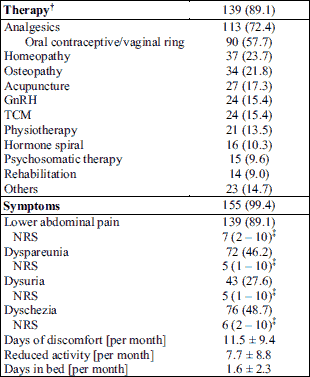
Prevalence IBS, food intolerances, allergies and gastrointestinal symptoms
With 12.8%, patients with endometriosis showed a higher prevalence as well as a higher a chance for IBS (OR = 3.68; 95% CI: 0.83 – 16.30) than controls (3.8%; P = 0.059).
Also the prevalence of self-reported food intolerances was significantly higher in EM patients compared to controls (Table 3). Significantly more EM patients reported intolerances to sorbitol (P = 0.031) and histamine (P = 0.045) or a gluten sensitivity (P = 0.025). Multiple food intolerances occurred in 5.8% of the control and in 11.5% of the EM group (OR = 2.13; 95% CI: 0.60 – 7.55).
The prevalence of various allergies was significant higher in the EM group (P = 0.001), too. About 57% of the EM patients and 31% of the controls stated to be allergic to any substances. Thereby a higher percentage of EM patients showed reactions against dust (P = 0.042) or other factors (P = 0.015) compared to the controls (Table 3). The risk of allergic diseases was elevated in the EM group (OR = 2.99; 95% CI: 1.53 – 5.83).
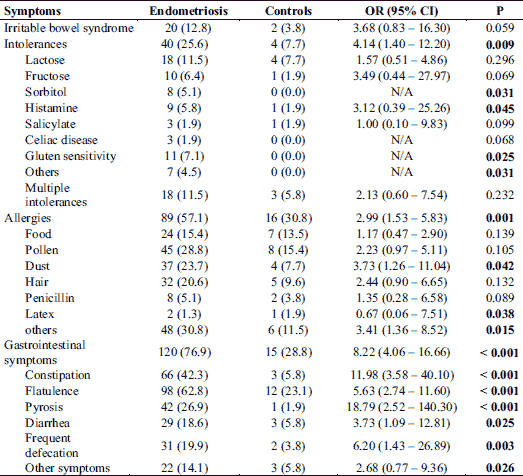
Since food intolerance, allergies and endometriosis itself are often associated with gastrointestinal symptoms, we also assessed the occurrence of gastrointestinal afflictions. About 77% of the EM group suffered from gastrointestinal symptoms, which was significantly higher compared to the control group (29%; P < 0.001). Also specific functional gastrointestinal disorders including constipation, flatulence, pyrosis, diarrhea or frequent defecation were significantly more frequent in endometriosis patients (Table 3). Overall the risk for gastrointestinal symptoms was elevated in the EM group (OR = 8.22; 95% CI: 4.06 – 16.66).
Because medical treatments and gynaecological surgeries may influence the prevalence of gastrointestinal symptoms, also the differences between EM patients with and without those treatments were analysed. No significance differences in the prevalence of gastrointestinal symptoms between EM-patients under treatment with analgesics and contraceptives and EM-patients without these medical treatments were observed (data not shown). However, EM patients with gonadotropin releasing hormone (GnRH) treatment showed a significant higher prevalence of constipation compared to patients without GnRH therapy (75.0% versus 37.4%; P = 0.040).
Additionally, some significant differences between EM patients with and without gynaecological surgeries were observed. Thereby endometriosis patients with hysterectomy showed a significant higher prevalence of constipation (78.6% versus 38.7%; P = 0.026), flatulence (100% versus 59.2%; P = 0.028) and diarrhea (42.9% versus 16.2%; P = 0.025) compared to those without hysterectomy. Furthermore, a higher prevalence of indigestions in general were observed in EM-patients with ovariectomy compared to EM patients without an ovariectomy (84.6% versus 76.2%; P = 0.002).
Macronutrients
The mean energy intake of endometriosis patients and controls was 2167 ± 914 kcal/day and 2232 ± 622 kcal/day, respectively. The analysis of macronutrient intake revealed no significant differences, except for a significant higher intake of organic acids in the control group (P = 0.006) (Table 4). The total food and energy intake were similar in both study groups. Also the daily ingestion of carbohydrates and fats showed no differences. However, a higher protein intake was observed for the control group (P = 0.073).
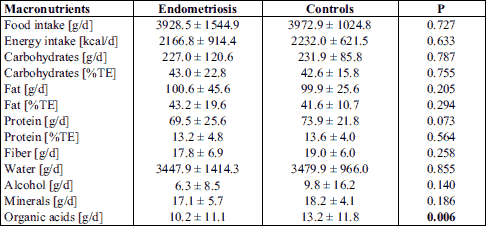
Regarding the D-A-CH reference values, patients with EM and controls showed a lower relative carbohydrate and protein intake (Fig. 1). Consequently, an elevated fat intake was observed. Alcohol consumption tended to be higher in the control group.

Carbohydrates
The analysis of the special carbohydrate intake revealed no relevant differences between the endometriosis patients and controls (Table 5). Only a significantly lower intake of the disaccharide maltose (P = 0.016) and the polysaccharide glycogen (P = 0.035) was observed in the EM group. The daily fiber intake was below the recommended D-A-CH level of 30 g per day in both study groups (Table 5).

Fats
The daily intake of fats and fatty compounds was comparable in both study groups (Table 5). Significant differences were observed for the fatty acids tetradecenoic acid (P = 0.041) and decosanoic acid (P = 0.018). The cholesterol intake of endometriosis patients and controls was above the recommended D-A-CH level of 300 mg per day, with a higher amount in the control group (Table 5).
Proteins
For daily protein and amino acid intake some significant differences between the study groups were observed (Table 5). The control group consumed significantly higher amounts of animal protein compared to the endometriosis patients (P = 0.041). The intake of vegetable protein was similar in both groups. Regarding the body weight, the endometriosis patients consumed 1.09 ± 0.44 g/kg b.w. protein, which was equal to the control group. Furthermore, the endometriosis patients showed a lower intake of the essential amino acids methionine (P = 0.046), lysine (P = 0.048), threonine (P = 0.046) and histidine (P = 0.049).
Vitamins, minerals and trace elements
Analysis of the daily vitamin intake revealed no significant differences in the ingestion of fat-soluble vitamins including vitamin A, D, E and K. In contrast, significant differences in the intake of water-soluble vitamin were observed with a significant lower ingestion of vitamin C (P = 0.031) and vitamin B12 (P = 0.008) in the EM patients compared to the control group (Table 5).
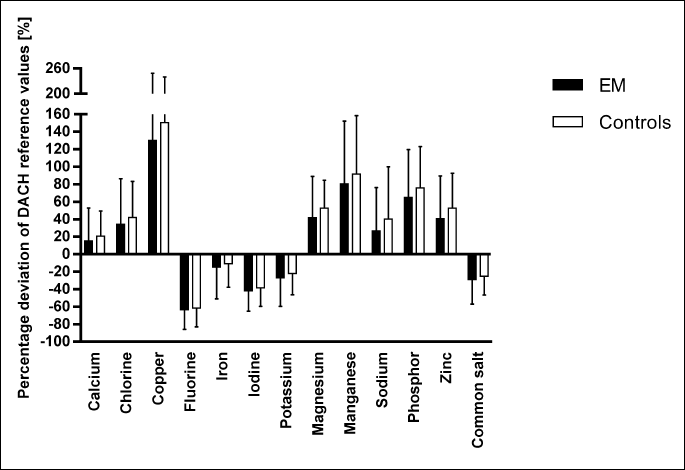
For the daily mineral intake only minor differences between the study groups were observed with a significantly lower intake of magnesium in the EM patients compared to the control group (P = 0.043) (Table 5).
Considering D-A-CH recommendations, the daily vitamin intake of endometriosis patients and controls reached or even exceeded the reference values (Fig. 1). However, both groups did not reach the recommended daily intake of vitamin D, pantothenic acid (vitamin B5) and vitamin B9 (folic acid) (Fig. 2). The daily intake of minerals mostly corresponded to the D-A-CH recommendations (Fig. 3). Nevertheless, the intake levels of fluorine, iron, iodine and potassium were below the D-A-CH recommended levels in both study groups.

Influence of gastrointestinal comorbidities, drugs and surgeries on nutrient intake within endometriosis patients
To assess the effect of gastrointestinal comorbidities, including food allergies and food intolerances, on nutrient intake of EM patients, we analysed the nutrient intake of EM patients with food allergies and intolerances in comparison to EM patients without those comorbidities.
Endometriosis patients with food allergies had a significantly lower intake of the fatty acid eicosadienoic acid compared to patients without a food allergy (0.03 ± 0.02 g/day versus 0.04 ± 0.04 g/day; P = 0.039). Furthermore, EM patients showed a significantly higher prevalence of intolerances against sorbitol, histamine, gluten and celiac disease compared to controls. Endometriosis patients with sorbitol intolerance showed significantly higher intake of polyols (1.11 ± 0.85 g/day versus 1.36 ± 0.73 g/day; P = 0.019) and a lower intake of alcohol (2.00 ± 2.18 g/day versus 5.12 ± 5.57 g/day; P = 0.038). Additionally, histamine intolerance was associated with a significantly reduced ingestion of calcium compared to patients without histamine intolerance (1.31 ± 0.33 g/day versus 1.14 ± 0.36 g/day; P = 0.05). Endometriosis patients with a self-reported gluten sensitivity showed a significantly higher intake of the oligo- or polysaccharide glycogen (0.23 ± 0.09 g/day versus 0.19 ± 0.11; P = 0.019) and non-absorbable oligosaccharides (1.11 ± 1.34 g/day versus 0.59 ± 0.91 g/day; P = 0.041). No significant differences in the nutrient intake of EM patients with and without celiac disease were observed.
EM patients had a high prevalence of hysterectomy and ovariectomy. Within the EM group, patients with hysterectomy ingested a significantly higher amount of the unsaturated fatty acid erucic acid (0.41 ± 0.15 g/day versus 0.33 ± 0.78 g/day; P = 0.028) compared to EM patients without hysterectomy. Moreover, ovariectomy has a prominent influence on nutrient intake in the EM group. Especially the intake of certain carbohydrates seems to be altered. Thereby EM patients with ovariectomy showed a significant lower intake of mannitol (0.04 ± 0.17 g/day versus 0.06 ± 0.03 g/day; 0.17 g/day versus P = 0.008), polyols (0.94 ± 0.35 g/day versus 1.95 ± 2.53 g/day; P = 0.022), sorbitol (0.88 ± 0.33 g/day versus 1.87 ± 2.52 g/day; P = 0.023), disaccharides (57.10 ± 38.77 g/day versus 78.99 ± 53.09 g/day; P = 0.017), sucrose (47.65 ± 39.16 g/day versus 66.12 ± 50.78 g/day; P = 0.023), monosaccharides (37.87 ± 43.15 g/day versus 54.92 ± 50.88 g/day; P = 0.011), fructose (19.72 ± 20.33 g/day versus 31.49 ± 32.78 g/day; P = 0.009) and glucose (17.80 ± 22.82 g/day versus 22.97 ± 18.77 g/day; P = 0.019). Additionally EM patients with ovariectomy ingested significantly minor levels of the vitamins pantothenic acid (3.54 ± 0.80 mg/day versus 4.53 ± 1.72 mg/day; P = 0.013), vitamin B6 (1.13 ± 0.28 mg/day versus 1.45 ± 0.61 mg/day; P = 0.032), vitamin B7 (0.03 ± 0.01 mg/day versus 0.04 ± 0.01 mg/day; P = 0.029) and vitamin C (84.40 ± 36.40 mg/day versus 142.80 ± 106.68 mg/day; P = 0.009) and micronutrients copper (1.84 ± 0.75 mg/day versus 2.34 ± 1.21 mg/day; P = 0.037) and potassium (2.24 ± 0.62 g/day versus 2.98 ± 1.33 g/day; P = 0.014) as well as a lower intake of organic acids (6.10 ± 6.48 g/day versus 10.57 ± 1.40 g/day; P = 0.005).
DISCUSSION
This study provides a detailed and differentiated analysis of the nutrient intake with regard to existing comorbidities and gastrointestinal symptoms of endometriosis patients. Even though investigations about the correlation of nutrition and endometriosis were made, only certain macronutrients (e.g. fats) and micronutrients (35, 36) or food groups (e.g. fruits and vegetables, red meat) (37, 38) were evaluated (32). Limited data and different study results questions the supposed association between nutrition und endometriosis (32).
With the acquisition of almost all macro- and micronutrients differences, we were able to reveal some interesting differences in the nutrient intake as well as in the occurrence of partly gastrointestinal-mediated diseases or symptoms in women with endometriosis, including food intolerances, allergies or irritable bowel syndrome (IBS), offering data for new preventive or therapeutic options.
Previous studies already showed an association between endometriosis and other diseases, especially autoimmune or endocrine disorders (39). In our study we observed a significant relationship between women with endometriosis and the occurrence of allergic diseases. In comparison to the controls, they had an almost three times higher chance to suffer from allergies. These findings were also confirmed by other studies (40, 41). The elevated coexistence of both, allergies and autoimmune diseases (P = 0.0274) in women with endometriosis, may indicate an involvement of the immune system in the pathogenesis and progress of endometriosis (41). Different studies observed alterations in the immune function of endometriosis patients including an elevated activation of macrophages and neutrophils or dendritic cells inducing the activation of adaptive immunity, a diminished cytotoxicity of natural killer cells (NK) and increased levels of inflammatory cytokines (42). However, the role of the dysregulated immune system in the pathogenesis of endometriosis has not yet been sufficiently researched.
The incidence of intestinal endometriosis varies between approximately 3.8 – 37% (43). Hence, gastrointestinal symptoms are not uncommon in endometriosis patents. In our study about 77% of the endometriosis patients stated to suffer from gastrointestinal symptoms. In particular, constipation and flatulence were common in these women. This is in line with other findings, which reported gastrointestinal symptoms in even 85% of women with endometriosis (30). Thereby symptoms were independent of menstruation or localization of endometriosis, whereas another study showed an association between the bowel proximity and gastrointestinal symptoms (44). An actual cause-effect-relationship between symptoms and localization is therefore still unclear. Compared to this, other localizations seem to affect specific organ systems. For example, bladder endometriosis seems to be associated with urinary incontinence (45, 46), like stress urinary incontinence, which is a common problem in pre- and postmenopausal women and associated with a lower premenopausal ERa gene expression (47).
However, besides disease localization, therapeutic treatments and surgeries may also influence gastrointestinal symptoms. In this study hysterectomy and ovariectomy impaired indigestions, especially constipation, flatulence and diarrhea in endometriosis patients. Former studies reported a higher prevalence of constipation after hysterectomy as well (48, 49), whereas others found no association of hysterectomy and de novo or deteriorating constipation in women (50). Additionally, the treatment with GnRH increased the prevalence of constipation in endometriosis patients and thus confirmed data from Ek et al. (30), who also observed a higher prevalence of gastrointestinal symptoms in endometriosis patients, especially during treatment with GnRH analogs. Since its presence in the gastrointestinal tract (51), GnRH may affect the gastrointestinal function and motility causing constipation and other gastrointestinal symptoms in treated women (30, 52).
Besides disease-related and therapy-related effects, other causes are supposed for gastrointestinal symptoms. These include an altered bowel function in consequence of a local prostaglandin release and inflammatory processes by lesions and cycle irritations of the rectal wall or mechanic obstructions (30, 53), but also a coexistence of irritable bowel syndrome and endometriosis is often discussed (54). In a study of Seaman et al. (53), women with endometriosis seem to have a 3.5 times higher chance for IBS compared to healthy controls, which is also in line with our findings. Another study demonstrated that 35% of the investigated IBS patients had the diagnosis of endometriosis (28). The overlapping of endometriosis and IBS impedes on the one hand an accurate diagnosis, but on the other hand offers new therapeutic options for patients suffering from both diseases. Especially targeted dietary interventions could be a promising tool. Here, a FODMAP-(fermentable oligo-, di-, monosaccharides and polyols)-low diet may exert a positive influence on gastrointestinal symptoms and pain (28). FODMAPs are a common reason of various food intolerances (e.g. for lactose, fructose or sorbitol). In our study the chance for intolerances was about four-times higher in endometriosis patients compared to controls and they were also more prone for multiple intolerances. Besides lactose and fructose intolerance, especially sorbitol, histamine and gluten intolerances were described. Data about the prevalence of food intolerances in endometriosis patients are scarce and especially recent studies are missing. A former study of Lamb et al. described a significantly higher risk of endometriosis patients for food sensitivities (RR 3.21; P = 0.035) (55) and another study found a higher risk for food intolerances (RR 2.10; P < 0.02) (56). In a more recent Italian study, a higher prevalence of celiac disease among women with endometriosis compared to women without endometriosis was observed (2.2% versus 0.8%; P = 0.265), even though this trend was not significant (57). With 1.9%, the proportion of endometriosis patients with celiac disease was similar in our study. A further study showed an association between endometriosis and prior celiac disease (58). As possible reasons for this association, celiac disease mediated inflammatory processes and other etiological factors are discussed (58). Also changes of the autonomic nervous system activity were observed in both, endometriosis (59) and celiac disease (60).
Besides celiac disease, we also observed a significantly higher proportion of gluten-sensitive women with endometriosis compared to controls, which may explain the positive effects of a gluten-free diet in the management of pelvic pain in these patients (61, 62). Additionally, endometriosis patients of our study showed a higher chance for histamine intolerance. Even though studies about the prevalence of histamine intolerance in endometriosis patients are lacking, a recent study demonstrated higher histamine levels in the peripheral blood of patients with external genital endometriosis, which was related to pain intensity (63). A recent study also observed an altered intestinal bacterial composition in patients with proven histamine intolerance (64), which may also contribute to a higher prevalence of gastrointestinal symptoms, especially bloating, in endometriosis patients.
However, more studies are needed to investigate the prevalence of food intolerance in relationship to endometriosis, which might also be helpful in the management of pain and gastrointestinal symptoms, e.g. through dietary interventions. Considering the described gastrointestinal symptoms and food intolerances, endometriosis patients probably have a different nutritional behavior compared to women without endometriosis. Several studies observed an increased risk of endometriosis by an altered intake of specific nutrients or foods like fatty acids or fruits and vegetables. In our study we observed differences of the nutrient intake between women with endometriosis and controls. Therefore, medical treatments, especially in form of hysterectomy and ovariectomy, as well as co-existing food intolerances, seem to influence this intake within the EM group additionally.
Regarding the macronutrient intake and the D-A-CH recommendations, a higher fat intake was observed in both study groups. A prospective study of Missmer et al. (35) found no association between total fat intake and endometriosis risk. However, specific fatty acids seem to influence the risk for endometriosis. A high intake of long-chain omega-3 fatty acids decreases the risk (35, 36), whereas a high intake of trans-unsaturated fat seems to favor the development of endometriosis (35). Nevertheless, we found no relevant differences in the intake of fatty acids. The intake of omega-3 fatty acids like linoleic acid or docosahexaenoic acid did not differ between endometriosis patients and controls. However, both groups showed an unfavorable ratio of the essential n-6 and n-3 fatty acids linolic acid (LA) and α-linolenic acid (ALA), which was above the recommended ratio of 1 – 5:1 (65). An unbalanced composition of these fatty acids may promote inflammatory processes and can be associated with increased menstrual pain, autoimmune and hormonal disorders (66, 67).
Furthermore, murine studies showed a significant influence of an elevated fat intake on endometriosis lesions with an increase in proinflammatory cytokines and oxidative stress (68, 69). Since inflammation processes play a major role in the pathogenesis and progression of endometriosis, a regulation or monitoring of quantitative and qualitative fat intake might be recommended for disease treatment. In addition to fat intake, women with endometriosis showed a significant lower intake of organic acids compared to controls. Organic acids, like citric or sorbic acid, are often used as additives in animal feeds or in many foods and beverages (70). They have anti-oxidative and antimicrobial properties and also showed anti-inflammatory effects in animal experiments (71). Thus, these organic acids may also have beneficial effects on endometriosis. Comparatively high concentrations of organic acids are naturally found in fruits and vegetables. Therefore, a low intake of organic acid may be related to a reduced intake of these foods. However, we did not examine the fruit and vegetable intake of endometriosis, but a recent study of Harris et al. (37) showed a lower endometriosis risk for women with high fruit consumption, especially citrus fruits. In view of the micronutrients, EM patients of our study consumed significantly less magnesium than the controls. A prospective cohort study observed an inverse relationship between the magnesium intake and the risk for endometriosis (RR = 0.86, 95% CI: 0.73 – 1.01; P = 0.007) (72). A high magnesium intake seems to be associated with lower levels of pro-inflammatory markers and endothelial dysfunction in postmenopausal women (73). Since fallopian tubes move more spasmodically in endometriosis patients, the relaxing effect of magnesium on smooth muscles found in tubes and uteri (74) may be beneficial in endometriosis. Remarkable was also an increased ingestion of copper in both, endometriosis patients and controls. This might be of interest, since copper belongs to the metalloestrogens (75). Higher urinary and serous copper levels were found in women with endometriosis (76, 77). Furthermore, the detected association between copper, ceruloplasmin and oxidative stress markers suggests their potential role of oxidative stress in the etiopathogenesis of endometriosis (77). Nevertheless, the influence of a high dietary copper intake on endometriosis is unclear, since controls showed an even higher intake. Future prospective association studies may clarify the effect of the binding of metalloestrogens on estrogen receptors as well as their influence on oxidative stress and inflammation on the onset and progress of endometriosis.
The vitamin intake of endometriosis patients partly differed from controls. Exceptional was a significantly lower vitamin C and vitamin B12 intake in the endometriosis group, which was more distinct in endometriosis patients with ovariectomy. Vitamin C is known of its anti-oxidative and anti-inflammatory, but also anti-angiogenic properties (78). Since oxidative stress is discussed in the etiology of chronic pelvic pain and endometriosis (67, 79), several studies investigated the effect of antioxidants, including vitamin C, on endometriosis. Animal studies showed a suppressive effect of an intravenous vitamin C supplementation on the regression and induction of endometric lesions (80) and of an oral vitamin C supplementation on the growth and volume of endometric cysts (81). A human study of Santanam et al. (31) suggested a reduction of chronic pelvic pain in women with endometriosis after a supplementation of vitamin C and E. In view of these findings, a dietary supplementation of these micronutrients seems to be effective in the prevention and treatment of endometriosis, especially by reducing oxidative stress (79). As there was a decreased intake of vitamin C in our endometriosis patients, a nutritional intervention and counseling for increasing the vitamin C intake (orally or through supplementation) could help to reduce symptoms in these patients.
Our endometriosis patients also showed a significant lower intake of vitamin B12. Since vitamin B12 is primarily included in animal products, the lower intake of animal protein in the EM patients could be responsible for this observation. Additionally, EM patients failed to reach the recommended daily intake of 300 µg folate. In a prospective cohort study of Darling et al. (82) a high intake of folate was associated with a 21% lower risk for endometriosis. Since B-vitamins are assumed to have positive effects on endometriosis and inflammation processes in general (83, 84), the relevance and influence of a lower vitamin B12 or folate intake needs further investigations. Furthermore, both study groups did not reach the D-A-CH recommended daily intake of vitamin D. Vitamin D plays different roles in immune regulation. Anastasi et al. (85) observed significantly lower 25-OH vitamin D serum levels in women with endometriosis with a positive correlation of insufficient vitamin levels with pelvic pain. Since vitamin D has different anti-inflammatory, immunomodulatory and anti-proliferative functions (86), an insufficient intake is probably associated with inflammatory or autoimmune diseases (87). Thus, a high vitamin D intake seems to be beneficial in endometriosis. But whereas a large prospective cohort study showed an inverse association of predicted plasma 25-OH vitamin D levels and endometriosis (72), other studies found no associations (88, 89). When considering the inconsistent findings, the effect of a low vitamin D intake or blood levels on the risk or disease process is still unclear.
In summary, vitamin and mineral intake seems to be important for women with endometriosis and different therapies, including gynaecological surgeries and medications, may exert an additional effect on that intake. Here, patients after ovariectomy showed a minor intake of micronutrients, especially of vitamin C, B-vitamins, potassium or cooper. However, future studies investigating the nutrient intake of women before and after ovariectomy, could help to detect some causal relationships.
Also self-reported food intolerances and food allergies affected the nutrient intake of endometriosis patients, but in a lower extent. Interestingly, endometriosis patients with sorbitol intolerance showed a significantly increased intake of overall polyols, which seems to be contradictory, since these patients often do not tolerate others polyols, e.g. mannitol or xylitol. However, mean overall intake of polyols was low in all study groups making a possible negative effect on gastrointestinal symptoms unlikely. Other observations of an altered nutrient intake of endometriosis patients with food intolerances, like a reduced calcium intake in histamine intolerant patients or an increased intake of glycogen and non-absorbable oligosaccharides in gluten-sensitive patients, are observed for the first time and needs further investigations in future studies.
Altogether major part of mentioned micronutrients is found in sufficient amounts in a mixed diet, including fruits and vegetables, dairy products, fish and meat. Especially the anti-oxidative properties may exert positive effects on the endometriosis, since women with endometriosis are suggested to have an increased oxidative stress (27, 79). However, occasional dietary intake may be insufficient to ensure beneficial effects of these nutrients. Mier-Cabrera et al. (27) showed a reduction of peripheral oxidative stress markers and an increase of antioxidant markers in women with endometriosis after an anti-oxidative vitamin and mineral supplementation. Thus, dietary supplements may present an interesting approach in regard to the nutritional therapy of endometriosis patients.
Overall study results indicate a possible association of endometriosis and gastrointestinal disorders. The increased prevalence of food intolerances and irritable bowel syndrome suggest dietary interventions as a promising approach for the treatment of abdominal symptoms including pelvic pain and digestive disorders in this patient group. The altered intake of certain nutrients may also suggest the dietary supplementation of e.g. vitamin C or magnesium to reduce disease burden and oxidative stress in the affected women. In future, controlled and randomized intervention studies are necessary to investigate the effect of certain nutrients on endometriosis with regard of co-existing gastrointestinal disorders and therapy side effects.
M. Schink and P.C. Konturek contributed equally to this work.
Acknowledgements: We acknowledge the support by the H.W. and J. Hector Foundation for funding our research.
Conflict of interests: None declared.
REFERENCES
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004; 18: 177-200.
- Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol 1997; 7: 267-741.
- Bulun SE. Endometriosis. New Engl J Med 2009; 360: 268-279.
- Burghaus S, Fehm T, Fasching PA, et al. The International Endometriosis Evaluation Program (IEEP Study) - A systematic study for physicians, researchers and patients. Geburtshilfe Frauenheilkd 2016; 76: 875-881.
- Ulrich U, Buchweitz O, Greb R, et al. Interdisciplinary S2k Guidelines for the Diagnosis and Treatment of Endometriosis: Short Version - AWMF Registry No. 015-045, August 2013. Geburtshilfe Frauenheilkd 2013; 73: 890-898.
- Halis G, Mechsner S, Ebert AD. The diagnosis and treatment of deep infiltrating endometriosis. Dtsch Arztebl Int 2010; 107: 446-55; quiz 56.
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10: 261-275.
- Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 2012; 39: 535-549.
- Kaur KK, Allahbadia G. An update on pathophysiology and medical management of endometriosis. Adv Reprod Sci 2016; 4: 53-73.
- Bungum HF, Vestergaard C, Knudsen UB. Endometriosis and type 1 allergies/immediate type hypersensitivity: a systematic review. Eur J Obstet Gynecol Reprod Biol 2014; 179: 209-215.
- DiVasta AD, Vitonis AF, Laufer MR, Missmer SA. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am J Obstet Gynecol 2018; 324.e1-e11. doi: 10.1016/j.ajog.2017.12.007
- Ballester M, Santulli P, Bazot M, Coutant C, Rouzier R, Darai E. Preoperative evaluation of posterior deep-infiltrating endometriosis demonstrates a relationship with urinary dysfunction and parametrial involvement. J Minim Invasive Gynecol 2011; 18: 36-42.
- Seracchioli R, Mabrouk M, Guerrini M, et al. Dyschezia and posterior deep infiltrating endometriosis: analysis of 360 cases. J Minim Invasive Gynecol 2008; 15: 695-699.
- Berker B, Seval M. Problems with the diagnosis of endometriosis. Womens Health 2015; 11: 597-601.
- Tripoli TM, Sato H, Sartori MG, de Araujo FF, Girao MJ, Schor E. Evaluation of quality of life and sexual satisfaction in women suffering from chronic pelvic pain with or without endometriosis. J Sex Med 2011; 8: 497-503.
- Renner SP, Rix S, Boosz A, et al. Preoperative pain and recurrence risk in patients with peritoneal endometriosis. Gynecol Endocrinol 2010; 26: 230-235.
- Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril 1996; 65: 299-304.
- Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod 2015; 30: 2427-2438.
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol 2017; 209: 14-24.
- He W, Liu X, Zhang Y, Guo SW. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod Sci 2010; 17: 1099-111.
- Facchin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015; 36: 135-141.
- Pitynski K, Ozimek T, Galuszka N, et al. Association of the immunohistochemical detection of gamma-glutamyl transferase expression with clinicopathological findings in postmenopausal women with endometrioid adenocarcinoma of the uterus. J Physiol Pharmacol 2016; 67: 395-402.
- Palla VV, Karaolanis G, Bliona T, Katafigiotis I, Anastasiou I, Hassiakos D. Endometrioid adenocarcinoma arising from colon endometriosis. SAGE Open Med Case Rep 2017; 5: 2050313x17745204. doi: 10.1177/2050313X17745204
- Park HM, Lee SS, Eom DW, Kang GH, Yi SW, Sohn WS. Endometrioid adenocarcinoma arising from endometriosis of the uterine cervix: a case report. J Korean Med Sci 2009; 24: 767-771.
- Ulrich U, Buchweitz O, Greb R, et al. National German Guideline (S2k): Guideline for the Diagnosis and Treatment of Endometriosis: Long Version - AWMF Registry No. 015-045. Geburtshilfe Frauenheilkd 2014; 74: 1104-1118.
- Halpern G, Schor E, Kopelman A. Nutritional aspects related to endometriosis. Rev Assoc Med Bras (1992) 2015; 61: 519-523.
- Mier-Cabrera J, Aburto-Soto T, Burrola-Mendez S, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol 2009; 7: 54. doi: 10.1186/1477-7827-7-54
- Moore JS, Gibson PR, Perry RE, Burgell RE. Endometriosis in patients with irritable bowel syndrome: specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol 2017; 57: 201-205.
- Maroun P, Cooper MJ, Reid GD, Keirse MJ. Relevance of gastrointestinal symptoms in endometriosis. Aust NZJ Obstet Gynaecol 2009; 49: 411-414.
- Ek M, Roth B, Ekstrom P, Valentin L, Bengtsson M, Ohlsson B. Gastrointestinal symptoms among endometriosis patients - a case-cohort study. BMC Womens Health 2015; 15: 59. doi: 10.1186/s12905-015-0213-2
- Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl Res 2013; 161: 189-195.
- Parazzini F, Vigano P, Candiani M, Fedele L. Diet and endometriosis risk: a literature review. Reprod Biomed Online 2013; 26: 323-336.
- Bohlscheid-Thomas SH, Hoting I, Boeing H, Wahrendorf J. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26 (Suppl. 1): S59-S70.
- Dehne LI, Klemm C, Henseler G, Hermann-Kunz E. The German Food Code and Nutrient Data Base (BLS II.2). Eur J Epidemiol 1999; 15: 355-359.
- Missmer SA, Chavarro JE, Malspeis S, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod 2010; 25: 1528-1535.
- Savaris AL, do Amaral VF. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol 2011; 158: 314-318.
- Harris HR, Eke AC, Chavarro JE, Missmer SA. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod 2018; 33: 715-727.
- Parazzini F, Chiaffarino F, Surace M, et al. Selected food intake and risk of endometriosis. Hum Reprod 2004; 19: 1755-1759.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002; 17: 2715-2724.
- Matalliotakis I, Cakmak H, Matalliotakis M, Kappou D, Arici A. High rate of allergies among women with endometriosis. J Obstet Gynaecol 2012; 32: 291-293.
- Caserta D, Mallozzi M, Pulcinelli FM, Mossa B, Moscarini M. Endometriosis allergic or autoimmune disease: pathogenetic aspects-a case control study. Clin Exp Obstet Gynecol 2016; 43: 354-357.
- Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int 2015; 2015: 795976. doi: 10.1155/2015/795976
- Young S, Burns MK, DiFrancesco L, Nezhat A, Nezhat C. Diagnostic and treatment guidelines for gastrointestinal and genitourinary endometriosis. J Turk Ger Gynecol Assoc 2017; 18: 200-209.
- Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Breart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril 2002; 78: 719-726.
- Leone Roberti Maggiore U, Ferrero S, Salvatore S. Urinary incontinence and bladder endometriosis: conservative management. Int Urogynecol J 2015; 26: 159-162.
- Leone Roberti Maggiore U, Ferrero S, Candiani M, Somigliana E, Vigano P, Vercellini P. Bladder endometriosis: a systematic review of pathogenesis, diagnosis, treatment, impact on fertility, and risk of malignant transformation. Eur Urol 2017; 71: 790-807.
- Adamiak-Godlewska A, Tarkowski R, Winkler I, et al. Stress urinary incontinent women, the influence of age and hormonal status on estrogen receptor alpha and beta gene expression and protein immunoexpression in paraurethral tissues. J Physiol Pharmacol 2018; 69: 53-59.
- Heaton KW, Parker D, Cripps H. Bowel function and irritable bowel symptoms after hysterectomy and cholecystectomy - a population based study. Gut 1993; 34: 1108-1111.
- Prior A, Stanley KM, Smith AR, Read NW. Relation between hysterectomy and the irritable bowel: a prospective study. Gut 1992; 33: 814-817.
- Altman D, Zetterstrom J, Lopez A, Pollack J, Nordenstam J, Mellgren A. Effect of hysterectomy on bowel function. Dis Colon Rectum 2004; 47: 502-508. Discussion 508-509.
- Ohlsson B. Gonadotropin-releasing hormone and its role in the enteric nervous system. Front Endocrinol (Lausanne) 2017; 8: 110. doi: 10.3389/fendo.2017.00110
- Hammar O, Ohlsson B, Veress B, Alm R, Fredrikson GN, Montgomery A. Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol 2012; 47: 1165-1173.
- Seaman HE, Ballard KD, Wright JT, de Vries CS. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: findings from a national case-control study - part 2. BJOG 2008; 115: 1392-1396.
- Vigano D, Zara F, Usai P. Irritable bowel syndrome and endometriosis: new insights for old diseases. Dig Liver Dis 2017; 50: 213-219.
- Lamb K, Nichols TR. Endometriosis: a comparison of associated disease histories. Am J Preventive Med 1986; 2: 324-329.
- Nichols T, Lamb K, Arkins J. The association of atopic diseases with endometriosis. Ann Allergy. 1987; 59: 360-363.
- Santoro L, Campo S, D’Onofrio F, et al. Looking for celiac disease in Italian women with endometriosis: a case control study. Biomed Res Int 2014; 2014: 236821. doi: 10.1155/2014/236821
- Stephansson O, Falconer H, Ludvigsson JF. Risk of endometriosis in 11,000 women with celiac disease. Hum Reprod 2011; 26: 2896-2901.
- Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update 2014; 20: 737-747.
- Przybylska-Felus M, Furgala A, Zwolinska-Wcislo M, et al. Disturbances of autonomic nervous system activity and diminished response to stress in patients with celiac disease. J Physiol Pharmacol 2014; 65: 833-841.
- Marziali M, Venza M, Lazzaro S, Lazzaro A, Micossi C, Stolfi VM. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir 2012; 67: 499-504.
- Marziali M, Capozzolo T. Role of gluten-free diet in the management of chronic pelvic pain of deep infiltranting endometriosis. J Minim Invasive Gynecol 2015; 22 (S6): S51-S52.
- Orazov MR, Radzinskiy VY, Khamoshina MB, et al. Histamine metabolism disorder in pathogenesis of chronic pelvic pain in patients with external genital endometriosis [in Russian]. Patol Fiziol Eksp Ter 2017; 61: 56-60.
- Schink M, Konturek PC, Tietz E, et al. Microbial patterns in patients with histamine intolerance. J Physiol Pharmacol 2018; 69: 579-593.
- Simopoulos AP. The omega-6/omega-3 fatty acid ratio: health implications. Oleagineux, Corps Gras, Lipides 2010; 17: 267-275.
- Netsu S, Konno R, Odagiri K, Soma M, Fujiwara H, Suzuki M. Oral eicosapentaenoic acid supplementation as possible therapy for endometriosis. Fertil Steril 2008; 90: 1496-1502.
- Sesti F, Capozzolo T, Pietropolli A, Collalti M, Bollea MR, Piccione E. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev 2011; 24: 31-38.
- Heard ME, Melnyk SB, Simmen FA, Yang Y, Pabona JM, Simmen RC. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology 2016; 157: 2870-2882.
- Wang B, Sun J, Ma Y, Wu G, Shi Y, Le G. Increased oxidative stress and the apoptosis of regulatory T cells in obese mice but not resistant mice in response to a high-fat diet. Cell Immunol 2014; 288: 39-46.
- Quitmann H, Fan R, Czermak P. Acidic organic compounds in beverage, food, and feed production. Adv Biochem Eng Biotechnol 2014; 143: 91-141.
- Grilli E, Tugnoli B, Passey JL, Stahl CH, Piva A, Moeser AJ. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet Res 2015; 11: 96. doi: 10.1186/s12917-015-0410-0
- Harris HR, Chavarro JE, Malspeis S, Willett WC, Missmer SA. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol 2013; 177: 420-430.
- Chacko SA, Song Y, Nathan L, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010; 33: 304-310.
- D’Angelo EK, Singer HA, Rembold CM. Magnesium relaxes arterial smooth muscle by decreasing intracellular Ca2+ without changing intracellular Mg2+. J Clin Invest 1992; 89: 1988-1994.
- Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol 2006; 26: 191-197.
- Pollack AZ, Louis GM, Chen Z, et al. Trace elements and endometriosis: the ENDO study. Reprod Toxicol 2013; 42: 41-48.
- Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur Rev Med Pharmacol Sci 2013; 17: 1472-1478.
- Mikirova NA, Ichim TE, Riordan NH. Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med 2008; 6: 50. doi: 10.1186/1479-5876-6-50
- Harlev A, Gupta S, Agarwal A. Targeting oxidative stress to treat endometriosis. Expert Opin Ther Targets 2015; 19: 1447-1464.
- Erten OU, Ensari TA, Dilbaz B, et al. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan J Obstet Gynecol 2016; 55: 251-257.
- Durak Y, Kokcu A, Kefeli M, Bildircin D, Celik H, Alper T. Effect of vitamin C on the growth of experimentally induced endometriotic cysts. J Obstet Gynaecol Res 2013; 39: 1253-1258.
- Darling AM, Chavarro JE, Malspeis S, Harris HR, Missmer SA. A prospective cohort study of Vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr 2013; 5: 17-26.
- Proctor ML, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2001; CD002124.
- Mazur-Bialy AI, Pochec E, Plytycz B. Immunomodulatory effect of riboflavin deficiency and enrichment - reversible pathological response versus silencing of inflammatory activation. J Physiol Pharmacol 2015; 66: 793-802.
- Anastasi E, Fuggetta E, De Vito C, et al. Low levels of 25-OH vitamin D in women with endometriosis and associated pelvic pain. Clin Chem Lab Med 2017; 55: e282-e284.
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006; 92: 4-8.
- Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease? a systematic review. Semin Arthritis Rheum 2011; 40: 512-531. e8.
- Somigliana E, Panina-Bordignon P, Murone S, Di Lucia P, Vercellini P, Vigano P. Vitamin D reserve is higher in women with endometriosis. Hum Reprod 2007; 22: 2273-2278.
- Almassinokiani F, Khodaverdi S, Solaymani-dodaran M, Akbari P, Pazouki A. Effects of vitamin D on endometriosis-related pain: a double-blind clinical trial. Med Sci Monit 2016; 22: 4960-4966.
A c c e p t e d : April 29, 2019