CHARACTERIZATION OF CHANGES IN THE CONFIGURATION OF ACTION POTENTIALS IN THE MOUSE, GUINEA PIG, AND PIG SINOAURICULAR NODE AFTER APPLICATION OF CHANNEL BLOCKERS OF THE RAPID AND SLOW DELAYED RECTIFIER POTASSIUM CURRENTS
INTRODUCTION
Action potentials (APs) at the sarcolemma are generated by several outward and inward potassium, sodium, and calcium currents (1). Selective potassium channels regulate the maximum diastolic potential (MDP), AP duration (2, 3), refractory period, and spontaneous electrical activity (1, 4). The AP configuration in mammals varies between species depending on differences in the expression of specific ion channels. The delayed outward rectifier potassium current (IK) responsible for the repolarization phase consists of two components, rapid (IKr) and slow (IKs) (5). The role of the rapid component is usually evaluated using E-4031, a methanesulfonanilide class III antiarrhythmic drug that specifically blocks the corresponding potassium channels (6, 7). To assess the contribution of the slow component, chromanol 293B is used (5, 8). The application of E-4031 has been found to prolong the repolarization phase in sinoauricular (SA) cells of the rabbit (7) and mouse heart (9), leading to a twofold reduction of AP generation rate in 70% of SA node strips and a complete block of electrical activity in 30% of the strips. As observed in a number of studies, the main contribution to AP generation in the SA cells of pigs - animals with a heart rate of 60 – 70 min–1 - is made by the chromanol-sensitive slow component of the delayed rectifier potassium current (IKs). Experimental findings and numerical calculations using the ‘Kyoto’ model (1) have also lead to the conclusion that the main role is played by the slow component in large animals with a low APs generation rate (e.g., pigs) and by the rapid component (IKr) in animals with a high AP generation rate (> 300 min–1), such as mice (6, 10). However, the authors of this model hypothesize have not analyzed changes in APs caused by the application of IKr blocker E-4031 in the experiment.
We hypothesized that the repolarization phase in animals with a high cardiac output could not be reliably controlled by only one component of the delayed rectifier potassium current. To test this hypothesis experimentally, we performed a comparative study of AP sensitivity to E-4031 and chromanol 293B blockers in spontaneously contracting strips of SA tissue from mouse, guinea pig, and pig hearts. The results provide evidence that the rapid delayed rectifier potassium current (IKr) is the key component responsible for AP generation in the SA node of the pig heart.
MATERIALS AND METHODS
The study was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH) and the provisions of the Declaration of Helsinki on the proper care and treatment of laboratory animals. The experimental protocol was approved by the independent Ethics Committee of the Institute of Physiology, Kоmi Science Centre.
Male albino mice (nstrips= 11, body mass 30 ± 3 g) and guinea pigs of both sexes (nstrips = 12, body mass 650 ± 50 g) were from the Scientific Collection of Experimental Animals maintained at the Institute of Biology, Komi Science Centre (Syktyvkar, Russian Federation). Pigs (nstrips = 5, body mass 60 – 70 kg) were purchased from a farm near Syktyvkar.
Mice were sacrificed by instant cervical dislocation, guinea pigs and pigs were anaesthetized by Zoletil injection (15 mg/kg); the chest cavities were opened, and the hearts were excised to make SA preparations (strips). The SA node strips were placed with the subendocardial side facing up (11) in equilibrium bath solution (140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM MgSO4, 10 mM NaHCO3, 10 mM D-glucose; 5 mM HEPES, pH 7.4) at 31 ± 1°C, which was bubbled with a gas mixture of 95% O2 and 5% CO2. After 30- to 40-min incubation, APs of the strips in the control bath solution were recorded for 15 min; then chromanol 293B was added to a final concentration of 5 µM, and APs were recorded for 10 – 15 min until the steady state was reached. The strips were washed with the control solution until the initial AP rhythm was restored (for about 60 min), and the procedure was repeated with E-4031 (final concentration 1 µM).
Chemicals used in the study were from Sigma and AppliChem (Germany).
According to published data, these IKs and IKr blockers at the indicated concentrations show maximum specificity to their targets (7-9, 13, 14). The SA node strips were exposed to them for no more than 15 min, with AP recording starting on the 5th min.
To record APs, we used glass microelectrodes with long tips and initial resistance of 50 – 60 MΩ when filled with 2.7 M KCl, an Electro 705 amplifier (WPI, United States) with an operation range of 0 – 5 kHz, and an E14-140 analog-to-digital converter (L-Card, Russian Federation); the records were stored on a hard drive.
The following AP parameters were documented and analyzed: AP amplitude, mV; maximum diastolic potential (MDP), mV; threshold potential, mV; AP duration measured at 20% (APD20), 50% (APD50), 90% (APD90), and 100% repolarization (APD100), ms; duration of slow diastolic depolarization (SDD), ms; AP cycle length (APD100 + SDD), ms; spontaneous contraction rate, min–1; maximum upstroke velocity (dV/dtmax), V/s; plateau phase velocity (V2), V/s; velocity of the final repolarization phase (V3), V/s; and diastolic depolarization rate (DDR), mV/s.
Statistical analysis
The data from each experiment (n = 8 – 11 cells) were expressed as the mean ± standard deviation (M ± SD) and processed statistically with Microsoft Office Excel and PowerGraph Professional version 3.3 (Russian Federation) using the Wilcoxon’s paired t-test and Mann-Whitney U-test. Differences were considered significant at P < 0.05.
RESULTS
Characterization of action potentials in mouse, guinea-pig and pig sinoauricular node cells
The SA node is a heterogeneous structure. To level of the effect of its heterogeneity on AP parameters, we standardized the SA strips with respect to their length and tension strength and used microelectrode AP recording to identify the area containing cells with the lowest upstroke velocity dV/dtmax. This parameter in mouse strips averaged 3.2 ± 0.6 V/s (nstrips = 5), which was characteristic of true pacemaker cells (9, 10). The SA node cells with comparable dV/dtmax parameters were also identified in guinea pig and pig strips:1.8 ± 0.7 and 2.1 ± 0.9 V/s, with AP generation rates being 138 ± 20 min–1 (nstrips = 10) and 54 ± 7 min–1 (nstrips = 5), respectively (Tables 1 and 2; Fig. 1A, 1E and 1I).
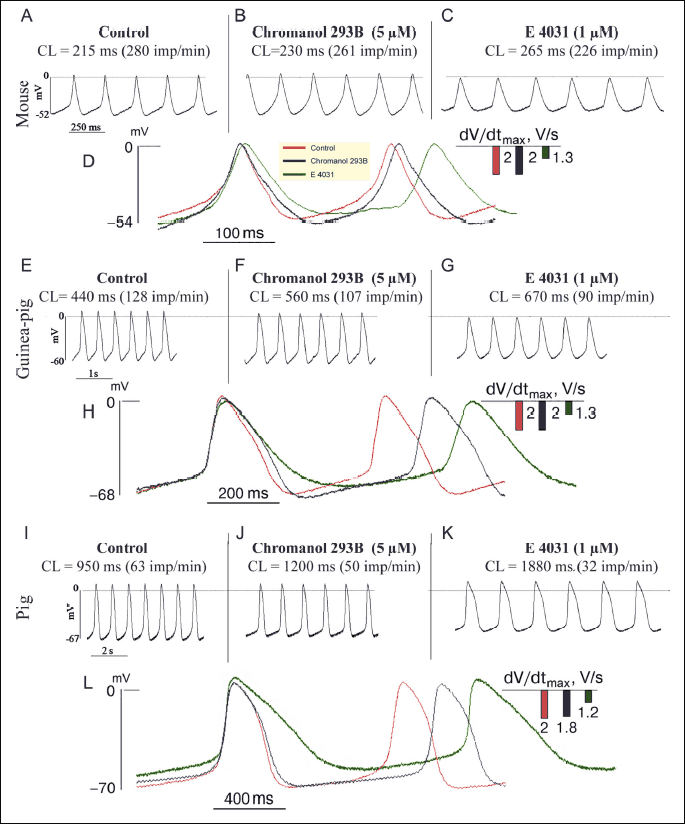
Effects of blocker chromanol 293B
The exposure to IKs blocker chromanol 293B (5 µM) caused in mouse SA cells a reduction of AP generation rate by 3 – 9% (on average by 5.3%) due to a 30% increase in both APD50 and APD90,with the SDD duration decreasing by 10 – 15%. In guinea pig and pig strips, the APs generation rate in SA pacemaker cells was reduced by 16% and 18%, respectively, compared to the control values (Table 1, Fig. 1I-1L).
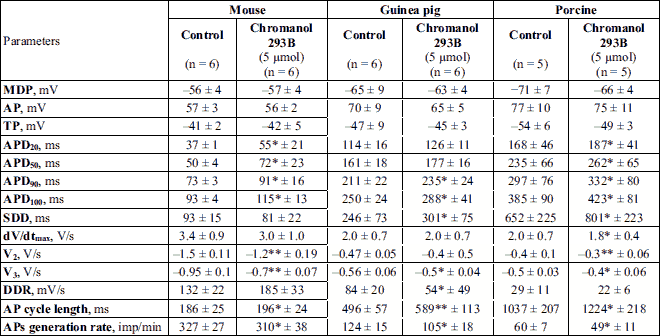
* P < 0.05; ** P < 0.01 significantly compared to controls.
Effects of blocker E-4031
After the exposure to IKr blocker E-4031 (1 µM), mouse SA node cells showed a 15% decrease in AP amplitude and a 25% increase in APD90 duration; the velocity of final repolarization phase (V3) decreased by 50%, and DDR by 14%. As a consequence, the AP generation rate was reduced by an average of 24%, compared to the control (Table 2, Fig. 1A-1D). In spontaneously contracting SA strips of guinea pigs, the E-4031 exposure resulted in sarcolemma depolarization by approximately 10 mV, a 41% increase in APD90, and a 34% decrease in DDR. Consequently, the APs generation rate was reduced by 26%, compared to the control (Table 2, Fig. 1E-1H). In pig SA strips, the IKr blocker caused a statistically significant 36% reduction of the AP generation rate due to a twofold increase in APD90 (Table 2, Fig. 1I, 1K-1L).
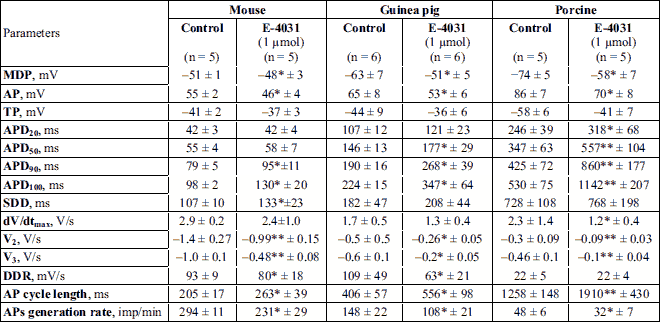
* P < 0.05; ** P < 0.01 significantly compared to controls.
DISCUSSION
Thus, we have shown for the first time that pig SA node cells are sensitive to E-4031, a selective IKr blocker. According to our data, the contribution of this component increases from 24 to 36% in the series “mouse-guinea pig-pig”. This result suggests that the rapid component of the delayed rectifier potassium current makes the main contribution to AP generation frequency in the pig SA node cells. In contradiction to the “Kyoto” model (1, 10), it is not determinative in any of these species. The role of the slow component of the delayed rectifier potassium current in AP generation in the SA node cells is still a point of discussion. In our opinion, both IK currents contribute to the maintenance of reliable AP generation in the SA node cells. The results of this study can be used for modeling the electrical activity of mammalian SA node cells and developing new pharmacological compounds for treatment of heart arrhythmias.
Acknowledgements: This study was supported by the Ural Division, Russian Academy of Sciences (projects no. AAAA-A17-117012310152-2 and no. AAAA-A18-118012290365-2 (2018-2020) and the Russian Foundation for Basic Research (project no.18-34-00654, MAG).
Conflicts of interests: None declared.
REFERENCES
- Sarai N, Matsuoka S, Kuratomi S, Ono K, Noma Aki. Role of individual ionic current systems in the SA node hypothesized by a model study. Jap J Physiol 2003; 53: 125-134.
- Kistamas K, Szentandrassy N, Hegyi B, et al. Changes in intracellular calcium concentration influence beat-to-beat variability of action potential duration in canine ventricular myocytes. J Physiol Pharmacol 2015; 66: 73-81.
- Horvath B, Szentandrassy N, Veress R, et al. Effect of the intracellular calcium concentration chelator BAPTA acetoxy-methylester on action potential duration in canine ventricular myocytes. J Physiol Pharmacol 2018; 69: 99-107.
- Yang KC, Nerbonne JM. Mechanisms contributing to myocardial potassium channel diversity, regulation and remodeling. Trends Cardiovasc Med 2016; 26: 209-218.
- Sanguinetti ML, Jurkiewiez NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 1990; 96: 195-215.
- Ono K, Shibata S, Iijima T. Properties of the delayed rectifier potassium current in porcine sino-atrial node cells. J Physiol 2000; 524: 51-62.
- Verheijck EE, van Ginneken AC, Bourier J, Bouman LN. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res 1995; 76: 607-615.
- Busch AE, Suessbrich H, Waldegger S, et al. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflugers Arch 1996; 432: 1094-1096.
- Clark RB, Mangoni ME, Lueger A, Couette B, Nargeot J, Giles WR. A rapidly activating delayed rectifier K+ current regulates pacemaker activity in adult mouse sinoatrial node cells. Am J Physiol Heart Circ Physiol 2004; 286: 1757-1766.
- Ono K, Shibata S, Iijima T. Pacemaker mechanism of porcine sino-atrial node cells. Smooth Muscle Res 2003; 39: 195-204.
- Golovko VA, Gonotkov MA, Lebedeva EA. Effects of 4-aminopyridine on action potentials generation in mouse sinoauricular node strips. Physiol Rep 2015; 3: e12447. doi.org/10.14814/phy2.12447
- Gonotkov M.A, Golovko VA. Aminopyridine lengthened the plateau phase of action potentials in mouse sinoatrial node cells. Bull Exp Biol Med 2013; 156: 4-6.
- Matsuura H, Ehara N, Ding W, Omatsu-Kanbe M, Isono T. Rapidly and slowly activating components of delayed rectifier K(+) current in guinea-pig sino-atrial node pacemaker cells. J Physiol 2002; 540: 815-830.
- Sun ZQ, Thomas GP, Antzelevitch C. Chromanol 293B inhibits slowly activating delayed rectifier and transient outward currents in canine left ventricular myocytes. J Cardiovasc Electrophysiol 2001; 12: 472-478.
A c c e p t e d : June 28, 2019