ANGIOTENSIN 1-7 FORMATION IN BREAST TISSUE IS ATTENUATED IN BREAST CANCER - A STUDY ON THE METABOLISM OF ANGIOTENSINOGEN IN BREAST CANCER CELL LINES
INTRODUCTION
The renin-angiotensin system (RAS) has long been recognized as an important regulator of systemic blood pressure and electrolyte homeostasis. However, our view of RAS has experienced remarkable change over the past decades, as new enzymes and products, as well as their local formation and action, have been described (1, 2).
Tissue RAS plays an important role in the development of various diseases. All components of classical RAS, such as angiotensinogen, specific convertases (e.g., angiotensin-converting enzyme (ACE)), and receptors for angiotensins, have been found in various types of cancer tissues. It has been demonstrated that some angiotensin peptides can contribute to the development and progression of tumor (3, 4). Angiotensin II (ANG II) stimulates cell proliferation and angiogenesis. It can also stimulate some other proangiogenic factors’ release, such as the vascular endothelial growth factor (5-7).
ANG II acts through the specific receptors angiotensin receptor type 1 (AT1R) and type 2 (AT2R), which are widely distributed in the epithelium. ANG II stimulates cell proliferation and angiogenesis via the AT1 receptor and induces apoptosis via the AT2 receptor. Many researchers indicate a significant increase in these receptors’ expressions in various types of cancers (breast, prostate, lung). According to a number of studies, the polymorphisms of ACE and AT1R have been associated with the risk of cancer. Because of the elevated expression of ACE and the AT1 receptor in cancer tissues, ACE inhibitors (ACE-I) and AT1R blockers (AT1R-B) appear to offer new opportunities in cancer therapy. The results obtained in animal models of cancer show that both ACE-I and AT1R-B inhibit tumor growth (5, 8-10).
In some pathological conditions, such as cancer, the activation of the AT2 receptor can oppose the proangiogenic action of ANG II through the AT1 receptor. Many researchers underline the possible beneficial effects of AT2R stimulation and the usefulness of the agonists of AT2R in cancer treatment. On the other hand, the results obtained from other studies indicate some proangiogenic AT2R activities (4).
The effects of ANG II on autocrine and paracrine signaling pathways are mediated by AT1R and inhibited by angiotensin 1-7 (ANG 1-7), a peptide produced from ANG II by the action of angiotensin-converting enzyme 2 (ACE2). ANG 1-7 was shown to play an opposite role to that of ANG II, showing antiproliferative effects and reducing fibrosis, angiogenesis, tumor volume, and weight (11-13). It has been demonstrated that overexpression of the ANG 1-7 receptor (mitochondrial assembly (MAS)), as well as the MAS receptor agonist AVE 0991, has multiple benefits of being orexigenic, anticachectic, and antitumorigenic, revealing AVE 0991 as a potential adjunct therapy for cancer (14).
Some studies demonstrated that ACE is overexpressed in laryngeal cancer and thus promotes cell proliferation. The upregulation of ACE is significantly influenced by tumor stage and lymph node metastasis. Patients with a high ACE expression have shorter overall survival compared with those with a low ACE expression. The ACE gene was also found to be an important factor in the prognosis of laryngeal cancer (15). Similarly, the upregulation of ACE expression has been observed in some types of breast cancers (3, 16).
The presence of the main RAS components both in normal breast tissue and in breast cancer tissue has been confirmed. There is strong evidence of the important role of the local RAS in breast cancer development, but studies have mostly been limited to crucial angiotensins, such as ANG II and ANG 1-7 (10).
In 2006, Nagata et al. (2) identified a pro-peptide hormone of the RAS, angiotensin 1-12 (ANG 1-12), in plasma and numerous rat tissues. The biological actions of this pro-peptide as a substrate for ANG II formation were demonstrated by showing that the administration of ANG 1-12 in isolated vessels produced a vasopressor response that could be blocked by both ACE-I and AT1R-B. ANG 1-12 has been detected in human atrial tissue (17). Ahmad et al. showed that ANG 1-12, not angiotensin I (ANG I), is a better substrate for ANG II formation by chymase in adult rats (18). This finding further suggests that this angiotensinogen-derived product is a previously unrecognized important precursor peptide to the RAS cascade (Fig. 1) (18-22). However, studies on the role and action of ANG 1-12 in cancer tissue remain limited.
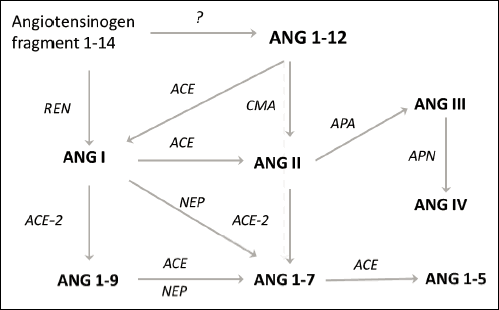 |
Fig. 1. Main pathways of angiotensinogen metabolism. Abbreviations: ACE, angiotensin-converting enzyme; ACE-2, angiotensin-converting enzyme type 2; APA, aminopeptidase A; APN, aminopeptidase N; CMA, chymase; NEP, neutral endopeptidase; REN, renin. |
Therefore, the main aim of the present study was to evaluate the ability of breast cancer cells to produce various metabolites of angiotensinogen, with a focus on two of them: ANG II and ANG 1-7. Additionally, the ability to form ANG 1-12 in healthy breast tissue and cancer cells was assessed.
MATERIALS AND METHODS
Chemicals
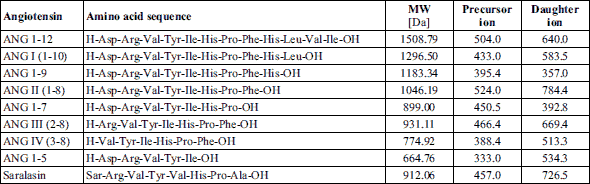
Angiotensin peptides, namely, human angiotensinogen fragment 1-14 (ANG 1-14), human angiotensin 1-12 (ANG 1-12), and angiotensins I (ANG I), II (ANG II), III (ANG III), IV (ANG IV), 1-9 (ANG 1-9), 1-7 (ANG 1-7), and 1-5 (ANG 1-5), were purchased from Bachem (USA). All reagents and solvents used in the liquid chromatography - tandem mass spectrometry (LC/MS/MS) analysis - formic acid (FA), trifluoroacetic acid (TFA) (both purchased from Fluka), and acetonitrile (ACN; from JT Baker) - were of LC/MS grade.
Cell cultures
Human breast cancer cell lines, namely, MCF-7 (adenocarcinoma), MDA-MB-231 (adenocarcinoma), and T-47D (ductal carcinoma), as well as healthy breast tissue cells, namely, the PCS-600 line (primary mammary epithelial cells from human breast), were obtained from the American Type Culture Collection (ATCC). The cancer cells were cultured in RPMI-1640 (GIBCO) medium supplemented with 10% fetal bovine serum (Sigma) and penicillin-streptomycin (GIBCO Pen-Strep), as well as with human recombinant insulin solution (MCF-7 line) or bovine insulin solution (T-47D line) (Sigma). The control cell line, PCS-600, was cultured in Mammary Epithelial Cell Basal Medium (ATCC) supplemented with the Mammary Epithelial Cell Growth Kit (ATCC) and antibiotics (penicillin and streptomycin). The cell lines were grown at 37°C in humidified atmosphere containing 5% CO2/95% air. The cells were passaged once or twice a week, dependently on their growth.
in vitro conversion of angiotensinogen fragments
To provide the optimal conditions for angiotensinogen conversion, a pilot experiment was carried out using an angiotensinogen fragment, 1-14 (ANG 1-14), as a substrate. First, the optimal time of incubation was selected; the cancer cell lines and the control line were incubated with ANG 1-14 (a final concentration of 3 µM) at 15, 30, 60, or 90 min. Second, the dependency of substrate concentration was assessed (three different concentrations of ANG 1-14: 0.1, 0.3, and 1 µM) in a 15 min incubation.
The main experiment was carried out at a substrate concentration of 1 µM and an incubation time of 15 min.
All incubations were conducted in the following manner: the cells (passage number between 3 and 10) were placed in 24-well plates (5 × 105 cells/well) and maintained overnight in the medium. Then, they were exposed to different angiotensinogen fragments (ANG 1-14, ANG 1-12, or ANG I) at a final concentration of 1 µM. After a 15 min incubation, the supernatants were collected and frozen at –80°C.
Sample purification for liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis
The supernatants were purified and concentrated using Ultra-Micro Spin C-18 columns (Harvard Apparatus, USA). Acidified samples with an internal standard (saralasin) were applied on the columns and centrifuged (2 min, 1000 × g). The columns were then washed with 300 µl of 0.1% TFA, and angiotensin peptides were eluted by centrifugation with 300 µl of 0.1% TFA in 40% acetonitrile. Sample eluates were lyophilized overnight, and dry residues were reconstituted in 500 µl of 0.1% FA for further LC/MS/MS analysis. The samples for the calibration curves of each examined peptide (mixture of standards in the culture medium) were prepared in the same manner as above.
LC/MS/MS analysis
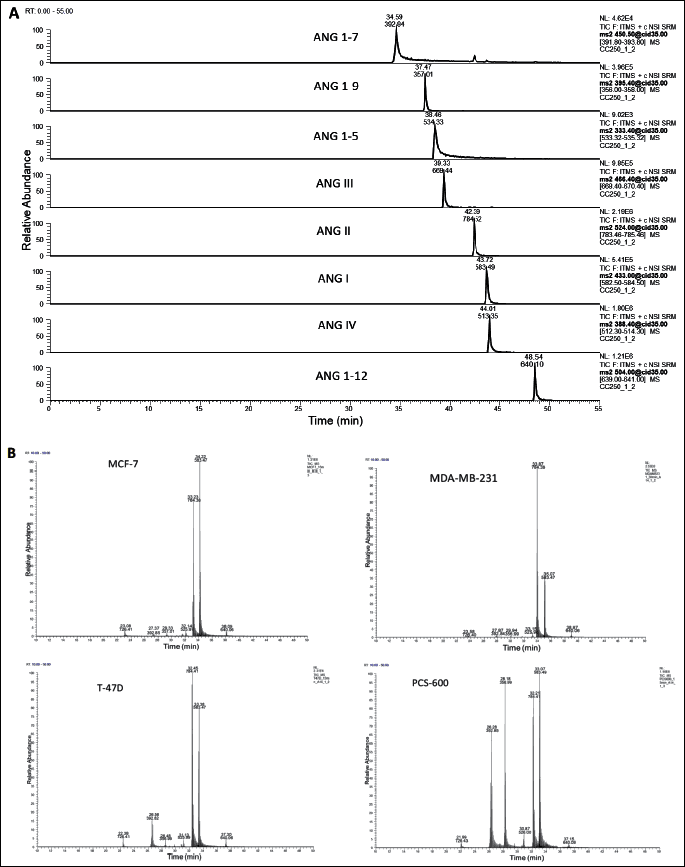
The separation of the angiotensin peptides was performed on a reversed-phase HPLC system (Dionex Ultimate 3000, Thermo Scientific USA) using an Acclaim® PepMap 100 nanoViper C18 column (250 mm × 75 µm ID, 3 µm particle size, 100 Å) with a guard column C18 PepMap 100 (5 mm × 300 µm, 5 µm, 100 Å) (Thermo). The mobile phase solvents were 0.1% FA in 2% ACN (phase A) and 0.1% FA in 80% ACN (phase B). The samples were injected onto a chromatographic column (oven temperature: 40°C) in a volume of 10 µl and separated at a flow rate of 300 nl/min with a linear gradient from 10% to 60% B over 42 min (the representative ion chromatograms of the standards and samples are presented in Fig. 2). Mass spectrometric detection was performed using a Velos Pro ion-trap mass spectrometer (Thermo Scientific, USA), with a nanospray flex ion source (source voltage: 1.5 kV; capillary temperature: 250°C, positive ion mode). For the detection, the selected reaction monitoring (SRM) mode was used (collision energy: 35%; maximum injection time: 100 ms, 2 µscans, isolation width: 1.0 amu) (the monitored ion pairs are presented in Table 1). The acquired data were analyzed with Xcalibur Software v.2.07.
Real-time RT-PCR
The cancer cells and the normal breast tissue cells (passage numbers 3 – 10) were seeded in 6-well plates (1 × 106 cells/well) and maintained overnight in the medium. RNA was extracted from the cells using the Maxwell 16 Cell LEV Total RNA Purification Kit (Promega, Madison USA) on a Maxwell instrument (Promega, Wisconsin, Madison, USA). After quantity and quality evaluation, the RNA concentration was normalized to 15 ng/µl. Reverse transcription was done with the High-Capacity Reverse Transcription Kit (Life Technologies, USA). According to the manufacturer’s protocol, qPCR reaction was performed on a 96-well plate with TaqMan primers and probes (Life Technologies, Carlsbad, California, USA) on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, California, USA). TaqMan Fast Advanced Master Mix and commercially available TaqMan Gene Expression Assays (Applied Biosystems) for human ACE (Hs00174179_m1, NM_00789.3), ACE2 (Hs01085333_m1, NM_021804.2), AGTR2 (Hs02621316_s1, NM_000686.4), and MAS1 (Hs00267157_s1, NM_002377.2), GoTaq qPCR Master MIX (Promega), and QuantiTect Primer Assays (Quiagen) for human AGTR1-Hs_AGTR1_va.1_SG (QT01036812, NM_032049) were used according to the manufacturers’ instructions. The normalization was performed using the geometric mean of the two housekeeping genes, GAPDH (Hs99999905_m1, NM_002046.4) and HPRT1 (Hs01003267_m1, NM_000194.2; QT00059066, NM_000194). Endogenous control genes were selected on the basis of the pilot experiment. Relative expression was calculated with the comparative Ct method (2–ΔΔCt) using DataAssist (Applied Biosystems).
Western blot procedure
The cells (passage numbers 3 – 10) were placed in 6-well plates (2 × 106 cells/well) and maintained overnight in the medium. The samples of the cancer cells and control cells were homogenized in 2% sodium dodecyl sulfate (SDS) homogenizing buffer containing 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, protease, and phosphatase inhibitor cocktails (Sigma Aldrich, USA). The homogenates were boiled at 95°C for 10 min, followed by centrifugation at 10,000 rpm for 10 min at 4°C before the supernatants were finally collected. The protein concentrations in the supernatant were determined using the BCA assay with bovine serum albumin (Sigma-Aldrich, Germany) as a standard, and then normalized. The normalized samples were then mixed with sample loading buffer (125 mM Tris-HCl, pH 6.8; 4% SDS, 20% glycerol, 10% 14.3 M mercaptoethanol, 2 mM EDTA, bromophenol blue) in a ratio of 1:1 and subsequently boiled for 5 min. For the Western blot analysis, the protein samples were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membranes (Merck-Millipore, Immobilon-P; 0.45 µm). The gel was run at 100 V until it reached the end of the gel. The proteins were transferred onto a polyvinylidene fluoride (PVDF; Bio-Rad) membrane using a MiniTrans-Blot Cell (Bio-Rad) and transfer buffer (0.30% Tris-Base, 1.44% glycine, 20% absolute methanol) at 110 V at a low temperature (with ice) for 60 min. The protein standard of molecular weights was used (Bio-Rad Laboratories, Precision Plus Protein Dual Xtra Standards). The PVDF membrane was blocked for 1 hour at room temperature with a blocking solution. The blocking buffer for the membranes contained 5% milk in tris-buffered saline and TBS (20 mM/l of Tris-HCl, pH 7.5, 150 mM/l NaCl) with 0.1% Tween 20 (200 µl/100 ml TBS). Then, the membranes were incubated overnight with a monoclonal rabbit 1:800 dilution of a primary anti-angiotensin II type 1 receptor antibody ([EPR3873] (ab124734); USA), a 1:800 dilution of an anti-angiotensin II type 2 receptor antibody ([EPR3876] (ab92445); USA), and a 1:1000 dilution of an anti-GAPDH antibody (Santa Cruz Biotechnology Inc. sc32233 #), followed by incubation in 1:6,000 dilution of a horseradish-peroxidase-conjugated anti-rabbit/anti-mouse secondary antibody (Santa Cruz Biotechnology Inc. sc-2030#/sc-2060#). The protein bands were visualized using an enhanced chemiluminescence reagent (WesternBright™ Quantum Chemiluminescent HRP Substrate Kit [Advansta corporation]). The protein amount was normalized to glyceraldehyde 3-phosphate dehydrogenase level in the same sample. The quantitative analysis of specific bands was performed with G-Box Syngene using Genesys densitometry software (GeneTools version 4.03; Synoptic Ltd; Cambridge, England).
Statistical analysis
The concentrations of the angiotensin peptides were expressed as pg/ml of the medium. All values placed in the figures and in the text are expressed as mean ± SD of n observations. Peptide levels were compared using one-way ANOVA with Dunnett’s post hoc test. Student’s t-test was used in the analysis of real-time RT-PCR data. The results obtained in Western blot analysis were compared using one-way ANOVA with Dunnett’s post hoc test. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 5.0.
RESULTS
Optimization of in vitro experiment conditions
The incubation of the cancer and control cells with ANG 1-14, ANG 1-12, and ANG I at different times showed that the optimal time for the experiment was 15 min, as the highest production of all analyzed metabolites (ANG 1-12, ANG I, ANG II, ANG III, ANG IV, ANG 1-9, ANG 1-7, and ANG 1-5) was achieved with this time. A time-dependent decrease in the production of analyzed peptides was observed in all cell lines. For further experiments, a time of 15 min was chosen (the best pronounced presence of all metabolites was observed during this time of incubation).
The metabolism of angiotensinogen fragment ANG 1-14 in both cancer and normal cells was also dependent on substrate concentration.
Conversion of angiotensinogen fragments in breast tissue
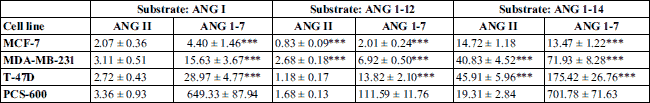
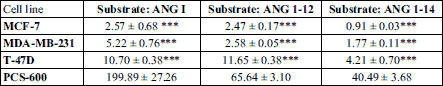
To evaluate angiotensinogen metabolism in breast tissue, three substrates were used: ANG I, ANG 1-12, and ANG 1-14. Regardless of the substrate used for the incubation, the main metabolite was ANG 1-7 both in normal breast tissue cells and in cancer cells. The production of ANG II from ANG I or ANG 1-12 was marginal. When ANG 1-14 was used as a substrate, the formation of ANG II was higher. When the shortest substrate (ANG I) was used, the main metabolite was ANG 1-7; its production was 20 – 40 times higher in the control cells than in the cancer cells. The production of ANG II from ANG I did not significantly differ between cell lines (Table 2). The degradation of angiotensinogen fragments was rapid and efficient in healthy tissue, as well as in two of three cancer cell lines (T-47D and MDA-MB-231). In the case of the third cancer cell line, MCF-7, the degradation of the substrate was weakly pronounced (Fig. 3).
The pattern of metabolites differed between cell lines. Healthy tissue was especially able to produce ANG 1-7. The ANG I/ACE/ANG II pathway was poorly pronounced (with a formation level of approximately 2%). The alternative pathway, ANG 1-9/ACE2/ANG 1-7, was much more efficient, as more than 50% of the total metabolites consisted of ANG 1-7; the ANG 1-9 content was approximately 35%. The degradation of angiotensinogen in cancer cells was less efficient, as the levels of metabolites were significantly lower than those of the control cells. However, the main metabolite was also ANG 1-7. The formation of ANG 1-12 from ANG 1-14 was marginal in all studied cell lines (Fig. 4).
MCF-7 cells produced lower levels of metabolites than did the other cell types (Fig. 3). In this line, the action of degrading enzymes was less efficient, as a higher level of unconverted substrate was observed. The levels of formatted ANG 1-7 and ANG II were comparable (Tables 2 and 3), suggesting that the ACE-dependent pathway in MCF-7 lines was also important.
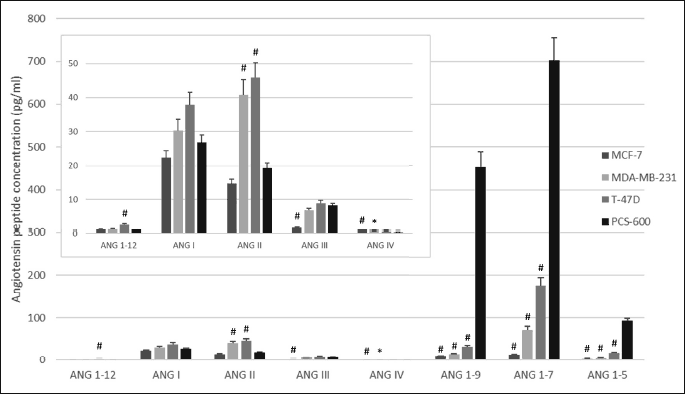
*P < 0.05; #P < 0.001; one-way ANOVA test; F(3,38).
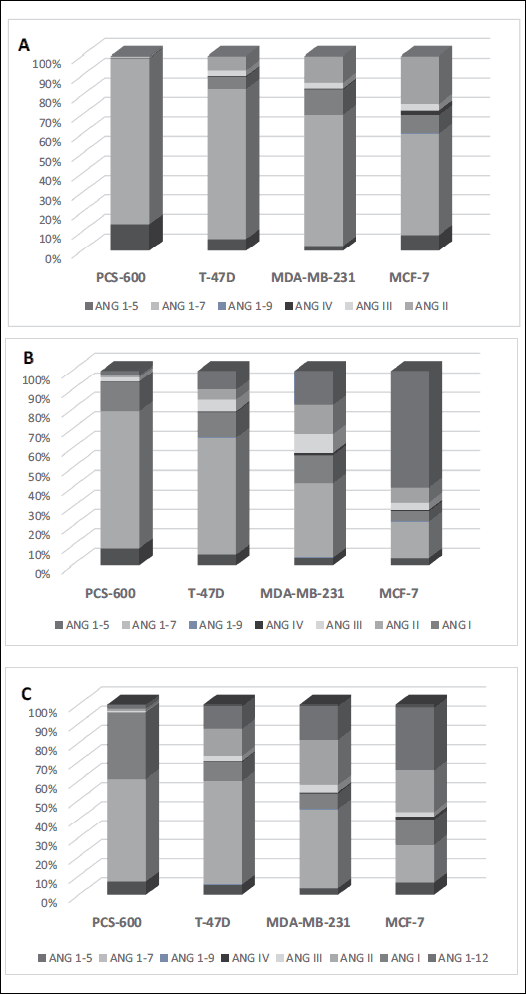 |
Fig. 4. Relative formation of angiotensinogen metabolites (expressed as % of sum of all analyzed angiotensins) in the cancer cells and the control cells in terms of substrate used: A, ANG I; B, ANG 1-12; C, ANG 1-14. |
Expression of renin-angiotensin system components in cancer cells
The RT-PCR measurements confirmed the presence of the mRNA of different RAS components - enzymes (ACE, ACE-2) and receptors (AT1R, AT2R, MAS) - both in healthy breast tissue cells and cancer cells. The results obtained from the Western blot analysis showed the presence of AT1R and AT2R proteins in all cell lines.
In the cancer cells, the expression of the mRNA of ACE differed between cell lines. ACE’s relative expression was significantly higher in T-47D cells than in the control cells, whereas in MDA-MB-231 and MCF-7 cells, the expression of ACE was a few times lower than that in the control (Fig. 5). Different results were achieved for ACE-2 expression: it was slightly higher in the MDA-MB-231 line and markedly higher in the two other lines compared with the control cells. An especially high expression was observed in the MCF-7 line (Fig. 5).
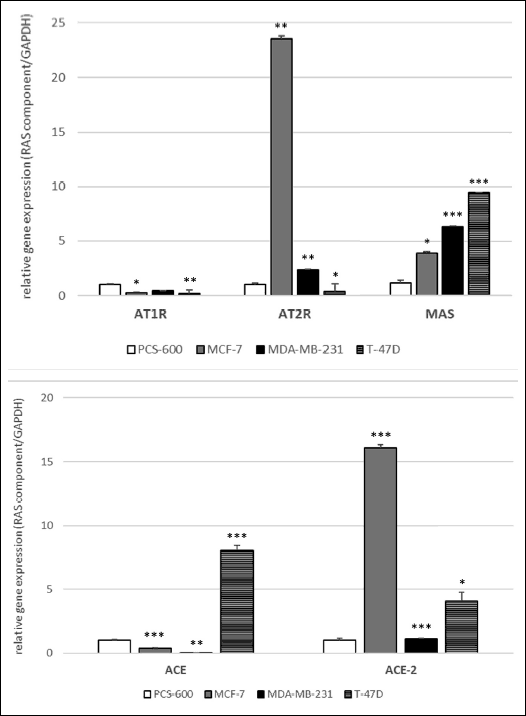 |
Fig. 5. Relative expression of the mRNA of some RAS components - receptors: AT1, AT2, MAS (A), and enzymes ACE, ACE-2 (B) in the breast cancer cells. *P < 0.05; **P < 0.01; ***P < 0.001; Student’s t-test. |
In all the cancer cell lines, the expression of the MAS receptor was significantly higher compared with that in the control cells (Fig. 5). The expression of the mRNA of the AT1 receptor was significantly lower in all the studied cancer cells lines compared with that in the control. The expression of the AT2 receptor was also decreased in T-47D cells, but it was higher in MDA-MB-231 and MCF-7 (Fig. 5). However, the expression of receptor proteins (AT1R and AT2R), measured using the Western blot technique, was found to be significantly higher in all cancer cells (GAPDH used as a loading control showed a constant expression both in the control cells and in all cancer cell lines) (Fig. 6).
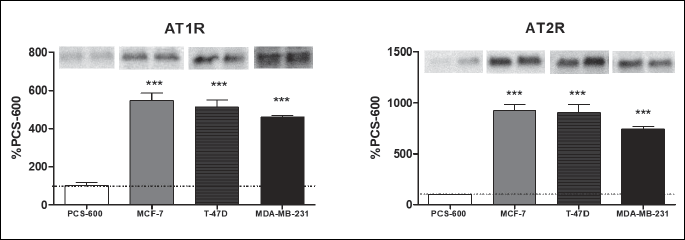
***P < 0.001; one-way ANOVA test; F(3,18).
DISCUSSION
In this study, we described the metabolism of angiotensinogen in three different cancer cell lines. Recent papers indicate the importance of the local RAS in the development and progression of different cancer types. It is known that ANG II can play an important role in the physiological processes occurring in healthy breast tissue and that ANG II action can be altered in breast cancer. The AT1 and AT2 receptors, as well as other crucial RAS components (angiotensinogen, prorenin, ACE), are detected in both normal and cancer breast tissues, but in disease, some dysregulation of RAS is observed, particularly in invasive carcinoma (7, 16, 23).
In our study, one control cell line (PCS-600, normal breast epithelial cell line) and three cancer cell lines with a different expression of estrogen and progesterone receptors have been selected. Two of them represent hormone-dependent breast cancer, MCF-7 (adenocarcinoma) and T47D (invasive ductal cancer), and one hormone-independent cell line, MDA-MB-231 (adenocarcinoma). The selected cell lines are a well-established model for the investigation of breast cancer pathophysiological processes, as well as for testing the possibilities of its treatment (24-26).
The obtained results showed that regardless of the length of the angiotensinogen fragment, the main product of degradation was ANG 1-7. In normal cells, the production was 20 – 100 higher than that in cancer cells (Fig. 3). ANG 1-7 is a beneficial angiotensin; its action counterbalances the effects of ‘bad’ ANG II; moreover, it shows antiproliferative effects and inhibitory action in some types of tumors in animal studies (27, 28). ANG 1-7 was shown to induce proliferation in astrocytoma, but it inhibits proliferation in lung cancer cells through a non-AT1R and non-AT2R mechanism. Harmer et al. (2002) found ACE-2 expressed in most human cell lines and tissues examined, including those of the breast, indicating that ANG 1-7 can be produced in most tissues (29).
In normal breast cells, the metabolism of angiotensinogen was rapid and efficient; the obtained results suggest the dominant pathway connected to the production of ANG 1-7. The comparison of the main metabolites’ content in terms of the substrate used indicates that the longer the substrate, the lower the ANG 1-7 production, expressed as a percentage of the sum of the produced metabolites: 85%, 70%, and 54% for ANG I, ANG 1-12, and ANG 1-14 as a substrate (Fig. 4).
Our results suggest that the prevalence of ANG 1-7 (and also ANG 1-9) forms some kind of protection from the disadvantageous action of ANG II and ANG III via the AT1 receptor. This balance was found to be dysregulated in cancer cells (Fig. 3). Moreover, the formation of ANG II in cancer cells is much higher than that in normal tissue, indicating the higher activity of ‘classical’ RAS (30). The protective role of ANG 1-7 was also observed in another study on MDA-MB-231 cells. ANG II was found to stimulate the proliferation, invasion, and migration of cancer cells (27).
It is known that ANG III and partially ANG IV can mimic the action of ANG II acting through the AT1 and AT2 receptors. ANG IV acts mainly through its specific receptor, AT4R, which is identified as an insulin-regulated aminopeptidase (IRAP) and plays a significant role in memory and learning processes. IRAP is an enzyme that breaks down such substrates as oxitocin, vasopresin, and ANG III. ANG IV inhibits the enzymatic activity of IRAP and indirectly inhibits the cleavage of ANG III (31, 32). Some studies indicate the role of aminopeptidases in cancer progression. In human and rat mammary tissue in cancer condition, the higher activity of APA and the lower activity of APN were detected. It was stated that in normal breast tissue, the conversion of ANG II to ANG III is slow, whereas in further steps, the degradation of ANG III to ANG IV is rapid (33). In our experiment, the concentrations of the produced ANG III and ANG IV in cancer cells were similar to those observed in the control cells despite the significantly higher formation of ANG II, suggesting some decrease in aminopeptidase activity. Moreover, the low production of ANG IV can potentiate ANG III degradation by IRAP and attenuate the further formation of ANG IV from ANG III.
In all the studied cancer cell lines, the mRNA expression of the receptor for ANG 1-7 (MAS) was significantly higher than that in the normal breast cells. This finding is similar to the results obtained by other authors. Luo et al. indicated changes in MAS1 receptor expression regarding the severity of neoplasm lesions; in benign tissue, the expression of MAS1 receptor was higher, but in invasive ductal carcinoma, the expression was attenuated (34). The lowering of MAS1 expression was related to disease progression, such as tumor growth or metastasis. The presence of the ANG 1-7/MAS receptor pathway was confirmed in benign and malignant breast cancer tissues. It suggests that substances with MAS receptor agonist activity might be useful in breast cancer therapy (35, 36).
It is known that ANG II facilitates breast cancer cell migration and metastasis. The direct exposure of breast cancer cells to ANG II contributes to increased tumor-endothelial cell adhesion, trans-endothelial migration, and motility, and it accelerates metastatic progression in an experimental mouse model in vivo. ANG II is a potent vasoactive peptide that can both be released in the bloodstream and generated locally by endothelial, stromal, and/or cancer cells (16, 35, 37, 38). Rodrigues-Ferreira et al. proposed that the autocrine or paracrine effects of ANG II, either present in the circulation or in the microenvironment of secondary tissue, may trigger an activating signal facilitating the dissemination and establishment of micrometastases in target organs (35).
According to recent findings, ANG II can be produced not only as a result of the degradation of ANG I by ACE but in a manner that is independent of ACE and renin activity (18-20, 22). Alternative pathways of ANG II formation (e.g., from ANG 1-12) are believed to also contribute to overall protumorigenic action. However, data on ANG 1-12’s role in cancer development and progression are lacking. Interestingly, the formation of ANG 1-12 from ANG 1-14 fragment in our experiment was low, but it was two to four times higher in cancer cells than in the control (statistical significance was reached in the T-47D cell line). Taken together, with the higher production of ANG II (and other peptides of the ACE-dependent pathway), these results point to the possible prevalence of classical RAS in breast cancer.
Analyzing the relative production of angiotensin peptides from ANG 1-12 (percentage content of metabolites) regarding the cell line used in our experiment, significant differences in conversion were observed. Healthy breast tissue cells were able to produce mostly ANG 1-7 (approximately 70%), whereas the production of ANG I (2.24%) and ANG II (1.06%) seemed marginal. In T-47D cancer cells, the formation of ANG 1-7 was lower (60.06%), but it was still the main metabolite. MDA-MB-231 and MCF-7 cells produced significantly less ANG 1-7 (38.32% and 18.75%, respectively). Moreover, the formation of ANG I and ANG II was stronger pronounced in cancer cells (ANG I: 9.74%, 17.61%, and 59.70%, ANG II: 5.13%, 14.84%, and 7.74%), and the lower the level of produced ANG 1-7, the higher the relative amount of these two angiotensins (Fig. 4). A similar observation was made in an experiment with ANG 1-14 used as the substrate.
The formation of ANG 1-12 was marginal both in the cancer and control cells. ANG 1-12 is regarded as an alternative, ACE-independent substrate to ANG II formation. The important role of this non-canonical pathway in the cardiovascular system is emphasized, especially in the context of the development of some diseases (arterial hypertension) and resistance to drugs acting through the ANG I/ACE/ANG II pathway (e.g., ACE inhibitors). Interestingly, some recent studies indicated that ACE is a main enzyme degrading ANG 1-12 in the circulation of hypertensive and normotensive rats (39).
ANG II can be cleaved by ACE-related carboxypeptidase (ACE-2) into ANG 1-7. This enzyme is also involved in the formation of ANG 1-9 directly from ANG I (Fig. 1). Recent studies indicate the beneficial role of ACE-2 in the inhibition of breast cancer angiogenesis. The authors found that in cancer tissue, the level of ACE-2 was significantly lower than that in normal breast tissue; moreover, the lower level of ACE-2 was related to a poor prognosis (7). The basal expression of ACE-2 in the MCF-7 lines was found to be much higher than that in other cancer cell lines, whereas in the MDA-MB-231 lines, the expression was lower. In our experiment, similar observations were made; the expression of ACE-2 in MCF-7 cells was almost 20 times higher than that in the control cells (in T-47D, it was only four times higher), whereas in MDA-MB-231 cells, it was comparable to the control. Zhang et al. concluded that the expression of ACE-2 is related to the metastatic ability of cancer cells; the MDA-MB-231 line is regarded as having high metastatic potency, whereas MCF-7 is weak (7).
Herein we demonstrated the prevalence of the ACE-2-dependent pathway of angiotensinogen metabolism in normal breast tissue. In cancer cells, we observed a stronger pronounced classical ACE-dependent pathway, especially in MCF-7, despite the very high ACE-2 expression. Interestingly, the mRNA expression of ACE both in the MCF-7 line and the MDA-MB-231 line was significantly lower than that in the PCS-600 line. Only T-47D cells showed a clearly higher ACE expression.
ACE was shown to be present both in normal and cancer breast tissues, mainly localized in the secretory epithelium. This result suggests that it is possible for ANG II to be formed directly in breast tissue (16). The presence of ACE was detected both in benign and malignant breast cancer tissues (16). In an animal study, expressions of the AT1 receptor and ACE mRNA were reported to increase in chemically induced carcinoma of the rat mammary gland (40).
The AT1 receptor has been shown to be expressed both at the mRNA and protein levels in normal and malignant breast tissues. Functional studies indicated that the overexpression of AT1R in breast cancer cells promotes cell invasion in vitro and tumor growth in vivo in the absence of any stimulation by the AT1R agonist ANG II. All these effects were dose-dependently inhibited by the AT1R antagonist losartan, confirming the specific role of the AT1 receptor. In our study, the expression of the mRNA of the AT1 receptor was lower in the cancer cells than in the control line (Fig. 5). Interestingly, the results obtained from the Western blot analysis showed the statistically significant higher expression of the receptor’s protein in all cancer lines. This finding suggests that in cancer cells, the activity of specific polymerases could be elevated, which results in the higher formation of receptor proteins despite the relatively low expression of mRNA. Therefore, the action of ANG II might be stronger pronounced in cancer cells, independent of the relatively low production of this peptide.
Numerous studies emphasize the meaning of the hormonal status of breast cancer in its prognosis and the possibility of treatment in the context of RAS dysregulation. It has been said that triple-negative breast cancer is a worse prognosis than positive breast cancer (30, 41). Herr et al. (2019) concluded that in hormone-positive tumor tissue, RAS acts more in an anti-angiogenic manner because of the influence of the beneficial ANG 1-7/MAS pathway (30).
A study on the role of ANG II in the regulation of angiogenesis-associated genes in receptor-negative and -positive breast cancer confirmed that ANG II is involved in tumor angiogenesis regulation via AT1R, especially in receptor-negative breast cancer (41). A meta-analysis of breast cancer profiling data sets showed the overexpression of the AT1 receptor gene in 10 – 20% of invasive breast tumors that are estrogen receptor positive (ER+) and human epidermal receptor 2 (HER2) negative. Analysis of the data obtained from HER2- breast tumor patients identified a high AT1R expression as a marker of resistance to anthracycline-based neoadjuvant chemotherapy. The most recent studies indicated a high level of AT1R as a potential predictive marker of bevacizumab response in breast tumors (35, 36, 42).
The cancer cell lines used in the present study were both hormone negative and positive: the triple-negative, hormone independent (ER–/PR–/Her2–) line MDA-MB-231 and the two positive (hormone-dependent) (ER+/PR+/–/Her2–) lines MF-7 and T-47D. According to a recent study, T-47D cells seem to be more sensitive to progesterone (PR+) than the MCF-7 line (PR–) is (43). As mentioned above, the lower expression of mRNA but the higher expression of protein for AT1R was detected in all cancer cell lines. However, in the triple-negative line MDA-MB-231, a lower expression of ACE and ACE-2 was observed compared with the control and hormone-dependent lines. Moreover, in the T-47D line (ER+ and PR+), the expression of both ACE and ACE-2 was significantly elevated; in the MCF-7 line, only the ACE-2 expression was higher compared with the control (Fig. 5). The expression of AT2R was elevated in MDA-MB-231 and MCF-7 cells, whereas it was negative in T-47D.
The AT2 receptor for ANG II plays an important role in counteracting ANG II effects by AT1R; it can produce antitumoral effects in the presence of ANG II at a high concentration. On the other hand, protumoral activity was observed at a low ANG II concentration (34). In our study, the formation of ANG II was higher in cancer cells, but the ANG II concentration was only twofold higher than that in normal tissue. Significantly, a higher mRNA expression of AT2R in two of three cancer cell lines (MCF-7 and MDA-MB-231) was detected. The results obtained from the Western blot analysis showed the significantly higher AT2 receptor protein content in all the studied cancer cell lines. This finding might suggest that drugs acting via the AT2 receptor could be useful in the inhibition of disease progression, but this needs further investigations.
In summary, we demonstrated that cancer cells can produce numerous angiotensin peptide metabolites from angiotensinogen. The metabolism of angiotensinogen differed between various types of breast cancer cells. The obtained results indicate the greater importance of the classical pathway - ANG I/ACE/ANG II - in breast cancer cells. The production of ANG 1-12 seems to be marginal in breast tissue, but the obtained results suggest some tendency for the greater formation of this peptide in cancer cells. To the best of our knowledge, this is the first report describing the metabolism of angiotensinogen in breast cancer cells.
In the control cells, the prevalence of ANG 1-7 and ANG 1-9 production was observed. In cancer cells, the production of ANG 1-7 was significantly lower, whereas the expression of the MAS receptor was a few times higher than that in the control cells. These findings suggest that substances with MAS receptor agonist activity could be useful in the treatment of breast cancer. Confirming the effectiveness of MAS receptor agonists requires further investigations.
Acknowledgements: This study was supported by the Polish National Center of Science grant DEC-2013/09/B/NZ4/02580. The authors would like to thank Ms. Renata Budzynska and Ms. Jolanta Reyman for their excellent technical support.
Conflict of interests: None declared.
REFERENCES
- Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med (Berl) 2008; 86: 663-671.
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible componentof the renin-angiotensin system. Biochem Biophys Res Commun 2006; 350: 1026-1031.
- Vinson GP, Barker S, Puddefoot JR. The renin-angiotensin system in the breast and breast cancer. Endocr Relat Cancer 2012; 19: R1-R19.
- Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 2005; 16: 293-299.
- Goldstein B, Trivedi M, Speth RC. Alterations in gene expression of components of the renin-angiotensin system and its related enzymes in lung cancer. Lung Cancer Int 2017; 2017: 6914976. doi: 10.1155/2017/6914976
- Zarychta E, Rhone P, Bielawski K, et al. Elevated plasma levels of tissue factor as a valuable diagnostic biomarker with relevant efficacy for prediction of breast cancer morbidity. J Physiol Pharmacol 2018; 69: 921-931.
- Zhang Q, Lu S, Li T, et al. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res 2019; 38: 173. doi: 10.1186/s13046-019-1156-5
- Huang MM, Guo AB, Sun JF, Chen XL, Yin ZY. Angiotensin II promotes the progression of human gastric cancer. Mol Med Rep 2014; 9: 1056-1060.
- Ishikane S, Takahashi-Yanaga F. The role of angiotensin II in cancer metastasis: potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem Pharmacol 2018; 151: 96-103.
- Puddefoot JR, Udeozo UK, Barker S, Vinson GP. The role of angiotensin II in the regulation of breast cancer cell adhesion and invasion. Endocr Relat Cancer 2006; 13: 895-903.
- Hinsley EE, de Oliveira CE, Hunt S, Coletta RD, Lambert DW. Angiotensin 1-7 inhibits angiotensin II-stimulated head and neck cancer progression. Eur J Oral Sci 2017; 125: 247-257.
- Itcho K, Oki K, Kobuke K, Ohno H, Yoneda M, Hattori N. Angiotensin 1-7 suppresses angiotensin II mediated aldosterone production via JAK/STAT signaling inhibition. J Steroid Biochem Mol Biol 2019; 185: 137-141.
- Li X, Wang X, Xie J, Liang B, Wu J. Suppression of angiotensin-(1-7) on the disruption of blood-brain barrier in rat of brain glioma. Pathol Oncol Res 2019; 25: 429-435.
- Murphy KT, Hossain MI, Swiderski K, et al. Mas receptor activation slows tumor growth and attenuates muscle wasting in cancer. Cancer Res 2019; 79: 706-719.
- Han CD, Ge WS. Up-regulation of angiotensin-converting enzyme (ACE) enhances cell proliferation and predicts poor prognosis in laryngeal cancer. Med Sci Monit 2016; 22: 4132-4138.
- Tahmasebi M, Barker S, Puddefoot JR, Vinson GP. Localisation of renin-angiotensin system (RAS) components in breast. Br J Cancer 2006; 95: 67-74.
- Nagata S, Varagic J, Kon ND, et al. Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. Ther Adv Cardiovasc Dis 2015; 9: 168-180.
- Ahmad S, Varagic J, VonCannon JL, et al. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1-12) metabolizing enzyme. Biochem Biophys Res Commun 2016; 478: 559-564.
- Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One 2011; 6: e28501. doi: 10.1371/journal.pone.0028501
- Ahmad S, Varagic J, Groban L, et al. Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep 2014; 16: 429. doi: 10.1007/s11906-014-0429-9
- Chan KH, Chen YH, Zhang Y, Wong YH, Dun NJ. Angiotensin-[1-12] interacts with angiotensin type I receptors. Neuropharmacology 2014; 81: 267-273.
- Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 2008; 294: H2242-H2247.
- Vinson GP. Why isn’t the angiotensin type 1 receptor a target in cancer? Oncotarget 2017; 8: 18618-18619.
- Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 2017; 8: 3131-3141.
- Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res 2011; 13: 215. doi: 10.1186/bcr2889
- Lehmann TP, Kujawski J, Kruk J, Czaja K, Bernard MK, Jagodzinski PP. Cell-specific cytotoxic effect of pyrazole derivatives on breast cancer cell lines MCF7 and MDA-MB-231. J Physiol Pharmacol 2017; 68: 201-207.
- Cambados N, Walther T, Nahmod K, et al. Angiotensin-(1-7) counteracts the transforming effects triggered by angiotensin II in breast cancer cells. Oncotarget 2017; 8: 88475-88487.
- Yu C, Tang W, Wang Y, et al. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett 2016; 376: 268-277.
- Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002; 532: 107-110.
- Herr D, Sauer C, Holzheu I, et al. Role of renin-angiotensin-system in human breast cancer cells: is there a difference in regulation of angiogenesis between hormone-receptor positive and negative breast cancer cells? Geburtshilfe Frauenheilkd 2019; 79: 626-634.
- Chai SY, Fernando R, Peck G, et al. The angiotensin IV/AT4 receptor. Cell Mol Life Sci 2004; 61: 2728-2737.
- Slamkova M, Zorad S, Krskova K. Alternative renin-angiotensin system pathways in adipose tissue and their role in the pathogenesis of obesity. Endocr Regul 2016; 50: 229-240.
- del Pilar Carrera M, Ramirez-Exposito MJ, Mayas MD, Garcia MJ, Martinez-Martos JM. Mammary renin-angiotensin system-regulating aminopeptidase activities are modified in rats with breast cancer. Tumour Biol 2010; 31: 583-588.
- Luo Y, Tanabe E, Kitayoshi M, et al. Expression of MAS1 in breast cancer. Cancer Sci 2015; 106: 1240-1248.
- Rodrigues-Ferreira S, Nahmias C. G-protein coupled receptors of the renin-angiotensin system: new targets against breast cancer? Front Pharmacol 2015; 6: 24. doi: 10.3389/fphar.2015.00024
- Singh A, Nunes JJ, Ateeq B. Role and therapeutic potential of G-protein coupled receptors in breast cancer progression and metastases. Eur J Pharmacol 2015; 763: 178-183.
- Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, et al. Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One 2012; 7: e35667. doi: 10.1371/journal.pone.0035667
- Zhao Y, Chen X, Cai L, Yang Y, Sui G, Fu S. Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF-7 breast cancer cells survival via PI3-kinase/Akt pathway. J Cell Physiol 2010; 225: 168-173.
- Moniwa N, Varagic J, Simington SW, et al. Primacy of angiotensin converting enzyme in angiotensin-(1-12) metabolism. Am J Physiol Heart Circ Physiol 2013; 305: H644-H650.
- Tybitanclova K, Macejova D, Liska J, Brtko J, Zorad S. AT1 receptor and ACE mRNA are increased in chemically induced carcinoma of rat mammary gland. Mol Cell Endocrinol 2005; 244: 42-46.
- Herr D, Rodewald M, Fraser HM, et al. Potential role of renin-angiotensin-system for tumor angiogenesis in receptor negative breast cancer. Gynecol Oncol 2008; 109: 418-425.
- Arrieta O, Villarreal-Garza C, Vizcaino G, et al. Association between AT1 and AT2 angiotensin II receptor expression with cell proliferation and angiogenesis in operable breast cancer. Tumour Biol 2015; 36: 5627-5634.
- Yu S, Kim T, Yoo KH, Kang K. The T47D cell line is an ideal experimental model to elucidate the progesterone-specific effects of a luminal A subtype of breast cancer. Biochem Biophys Res Commun 2017; 486: 752-758.
A c c e p t e d : August 28, 2019