EFFECT OF OSTARINE (ENOBOSARM/GTX024), A SELECTIVE ANDROGEN RECEPTOR MODULATOR, ON ADIPOCYTE METABOLISM IN WISTAR RATS
INTRODUCTION
Testosterone and its metabolite dihydrotestosterone (DHT) are the most important androgens that are needed for the normal functioning of the reproductive system in males (1). Testosterone is mainly produced by the testes; however, it is also produced in smaller quantities by the adrenal cortex. Androgen synthesis is controlled by the hypothalamic-pituitary-gonadal (HPG) axis. In response to the hypothalamic signal, the pituitary gland produces two types of gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In the testis, FSH and LH mediate their actions via specific transmembrane receptors, FSH-R and LH-R. Predominantly, FSH-R is expressed in the Sertoli cells within the seminiferous tubules, whereas LH-R is expressed in the interstitial Leydig cells. FSH stimulates the maturation and growth of gonads and enhances spermatogenesis, whereas LH increases the activity of enzymes involved in steroidogenesis (1). Leydig cells synthesize testosterone, which is converted to DHT in many tissues by the enzyme 5-α-reductase. DHT shows a fivefold higher affinity to the androgen receptor (AR) than that of testosterone. The functions of testosterone are dependent on the developmental age of the person. First, it is necessary for the normal development of the gonads during the prenatal period. During this period, it also determines the development of the brain and the psychic sex and causes further maturation of the testes. During puberty, it shows an anabolic effect on the muscles and bones, increases spermatogenesis, and plays a special role in the development of sexual orangs. During maturity, it maintains normal spermatogenesis. Androgens affect the growth and development of the skeletal system and densification of bone (2). It has also been shown that testosterone is a strong regulator of adipose tissue metabolism (3). Therefore, testosterone deficiency is associated with conditions such as obesity, metabolic syndrome, sexual dysfunction, and increased cardiovascular risk in men (4, 5). On the other hand high level of androgens results in low-birth-weight offspring, hypergonadotropism and intrauterine growth retardation (6). These complications also include disturbances in the metabolism of the adipose tissue and may lead to the development of abdominal obesity (7-9).
However, treatment of androgen deficiency with the administration of exogenous testosterone has been shown to cause uncontrolled hypertrophy of the prostate gland which negatively affects the function of the testes leading to infertility (10). Therefore, pharmacological agents that show positive health benefits with low side effects are constantly being sought that will help in the treatment of androgen deficiency. Nonsteroidal SARMs are one of the promising therapies to improve physical function with fewer side effects.
Nonsteroidal SARMs and their series show selective androgenic activity while maintaining a beneficial anabolic effect in tissues such as bones and skeletal muscles (11). SARMs can be classified based on their ability to activate or inhibit transcription factors. SARMs, which are only, to a certain extent, agonists in androgenic tissues, can be used in the treatment of cancer and muscle-wasting diseases while maintaining the proper functioning of the testes and prostate (12). Interestingly, the role of these substances in the functioning of adipose tissue is unknown.
Enobosarm/ostarine/GTx024 is an AR agonist which has shown positive effects in humans. It has undergone preclinical proof of concept and toxicological testing in Phase I, II, and III clinical trials and has demonstrated positive effects in humans. It has been shown to stimulate the growth of lean muscle mass with a limited effect on the gonads (13). Many studies have shown that endogenous androgens regulate lipolysis and endocrine activity of white adipose tissue. However, there is no data about the effect of ostarine on the adipose tissue (14). At present, it has not been approved by the United States Food and Drug Administration (FDA), and its potential side effects are poorly known.
Therefore, we decided to investigate the effect of ostarine on the intensity of lipolysis and lipogenesis and on the secretion of adipokines from isolated rat adipocytes in vitro. Moreover, we examined the effect of ostarine on the expression level of leptin and adiponectin in mature rat adipocytes.
MATERIALS AND METHODS
Animals
Male Wistar rats were purchased from Brwinow, Warsaw, Poland. Animals were housed in standard conditions (12 h/12 h dark/light cycle, temperature 21°C ± 1°C).
Isolation of adipocytes
Adipocytes from rat epididymal fat depot were isolated based on the method described by Rodbell with some modifications (15). In brief, rats were sacrificed using the guillotine. Immediately after decapitation, epididymal fat tissue was dissected and transferred into a plastic beaker with phosphate-buffered saline (PBS). After removal of the blood vessels, the fat pads were cut into small pieces using scissors. Then, the tissue was digested in Krebs-Ringer bicarbonate buffer (118 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 24.8 mM NaHCO3) supplemented with 10 mM HEPES (KRBH), collagenase type II (3 mg/1 g tissue), 3% BSA, and 5 mmol/L glucose for 45 min at 37°C in a shaking water bath. Then, the cells were filtered through a nylon mesh (250 µm) and rinsed for four times with warm KRBH without collagenase. Adipocytes were counted using Burker-Turk chamber. All reagents were purchased from Sigma Aldrich.
Expression of androgen receptor
Isolated rat adipocytes (106 cells/mL) were incubated in KRBH in the presence of ostarine (0.1 and 1 µM) for 480 min in a shaking water bath maintained at 37°C. In addition, adipocytes were preincubated in the presence of specific inhibitors of AR - cyproterone acetate (1 µM) and flutamide (100 nM) - to investigate their effect on the expression of androgen receptor. The adipocytes were also incubated with testosterone (0.1 µM) as a natural ligand for AR.
Lipolysis
Isolated rat adipocytes (106 cells/mL) were incubated in KRBH in the presence of ostarine (0.001, 0.01, 0.1, 1, and 10 µM) for 120 and 480 min in a shaking water bath maintained at 37°C. Isoproterenol (1 µM) was used as a positive control. The adipocytes were incubated with testosterone (0.1 and 1 µM) as a natural ligand for AR. In addition, adipocytes were preincubated in the presence of specific inhibitors of the AR - cyproterone acetate (1 µM) and flutamide (100 nM) - to exclude other pathways of action of ostarine. The adipocytes were also incubated with testosterone (0.1 µM and 1 µM) as a natural ligand for AR. The intensity of lipolysis was measured as the concentration of glycerol released from adipocytes into the incubation medium using free glycerol reagent (Sigma Aldrich, Germany).
Leptin and adiponectin concentrations
The concentration of adiponectin (ADP) and leptin in the incubation medium of the isolated rat adipocytes was measured using species-specific enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) kits. ADP was measured by using Rat Adiponectin/Acrp30 ELISA kit (R&D Systems, United Kingdom). The leptin concentration was measured in the incubation medium by using Multi-Species Leptin RIA kit (EMD, Millipore, USA).
Lipogenesis
The intensity of lipogenesis in the isolated rat adipocytes was performed as described previously (16). Incorporation of [U-14C] glucose (PerkinElmer, Massachusetts, USA) into lipids was determined in order to assess the intensity of lipogenesis. Briefly, isolated mature adipocytes were transferred into polystyrene tubes (106 cells/tube) and incubated with ostarine (0.001, 0.01, 0.1, 1, and 10 µM) in KRBH. In addition, 500 nM (0.5 µM) of insulin was used as a positive control. After 120 min, Dole’s extraction mixture was added to stop the reaction. Subsequently, H2O and heptan were added, and the upper phase containing the lipid fraction was transferred to scintillation liquid for β-counting.
RNA isolation, cDNA synthesis, and real-time PCR
In this study, the expression of the genes encoding AR and adipokines (leptin and ADP) was determined in the isolated rat adipocytes. Total RNA was isolated from the rat adipocytes using Extrazol (DNA Gdansk, Poland) according to the manufacturer’s protocol. Then, cDNA was synthesized using 1 µg of RNA and High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, USA). Real-time polymerase chain reaction (PCR) was performed as described previously (17) by using Quant Studio Flex using SYBR Green PCR Master Mix (Applied Biosystem). GAPDH was used as the housekeeping gene. The total reaction volume was 10 µL which contained 3 µL of cDNA and 7 µL of Master Mix. Real-time PCR was conducted as follows: initial denaturation (0:15 min/95°C), followed by 40 cycles of denaturation (0:30 min/61.5°C) and annealing (0:15 min/72°C). After each cycle, melting curves were obtained to determine the specificity of the amplification process. The nucleotide sequence of primers is available upon request. Detection of the fluorescent product was set at the last step of each cycle.
Western blot
Western blot analyses were performed as previously described (18). Briefly, total protein was isolated using RIPA buffer containing: 25 mM Tris-HCl; pH 7.6, 150 mM NaCl, 5 mM EDTA, 1% NP-40 or 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS. Buffer was supplemented with protease and phosphatase inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein was mixed with Laemmli buffer and β mercaptoethanol. The next step was denaturation followed by electrophoresis in polyacrylamide gel (60 min, 125 V). Subsequently, the proteins were transferred onto PVDF membranes (Bio-Rad system). Each membrane was blocked for 1 hour using 3% BSA in TBST and incubated overnight with primary monoclonal mouse antibodies (AR receptor Santa Cruz cat. no sc-7305 1:500, β-actin cat. no 3700 1:5000). The next day, each membrane was washed three times in TBST, and placed in secondary antibody (anti-mouse) for 1 hour in room temperature. Signals were visualized using Amersham ECL prime Western Blotting Detection Reagent (GE Healthcare Life Sciences, UK) on VersaDoc system (BioRad, USA).
Statistical analysis
Statistical analysis was performed as previously described (17). We used one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparison test to analyze the data obtained. The results are presented as the arithmetic mean ± standard error of mean (SEM) (GraphPad Prism, GraphPad Software, Inc., USA). Statistical significance was defined as *P < 0.05, **P < 0.01, or ***P < 0.001.
RESULTS
Ostarine stimulates androgen receptor expression
First, to confirm the effect of ostarine on the adipocyte metabolism via AR, we investigated its effect on the AR mRNA and protein expression. The adipocytes were preincubated with specific inhibitors of AR (flutamide and cyproterone acetate) for 30 min, and then ostarine and testosterone were added to the incubation medium. The cells were incubated for up to 8 hours. According to the results, the addition of ostarine (1 µM) into the incubation medium upregulated the AR mRNA expression (P < 0.05); ostarine at 0.1 µM concentration did not show any effect (Fig. 1a). In addition, in our study, we used testosterone (1 µM) as a natural ligand of AR. According to the results, testosterone increased the level of AR mRNA expression. Moreover, specific antagonists of AR were used to investigate the effect on the expression of ostarine receptor. After preincubation with flutamide and cyproterone acetate, we did not observe any effect of ostarine on the AR mRNA expression (Fig. 1a). Western blot analysis showed that ostarine at a concentration of 0.1 µM (P < 0.01) and 1 µM (P < 0.05) increased AR expression at the protein level, whereas testosterone had this effect only at a concentration of 1 µM (P < 0.05). After preincubation with flutamide and cyproterone acetate, we did not observe any effect of testosterone and ostarine on AR protein level (Fig. 1b).
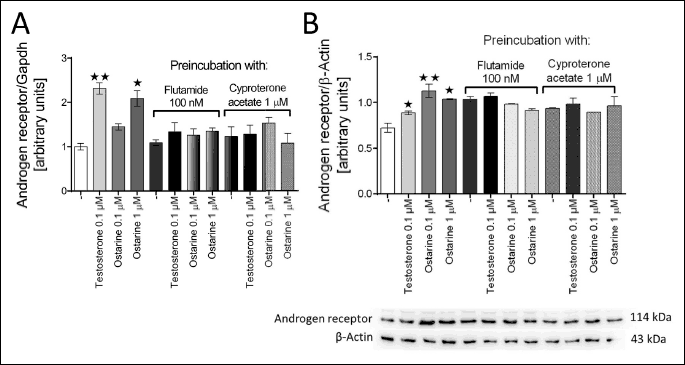
A real-time PCR was performed; relative expression of AR was normalized to the housekeeping gene GAPDH. Western Blot was performed; relative production of AR protein was normalized to the reference protein β-actin. The blot show representative results of Western blot analysis of androgen receptor and β-actin staining. Figure shows mean ± standard error of mean (SEM). Statistically significant differences are represented as *P < 0.05 and **P < 0.01 versus corresponding control.
Ostarine stimulates lipolysis
Next, we investigated the effect of ostarine on lipolysis in the isolated rat adipocytes after 120 and 480 min of incubation. In this study, we used isoproterenol (10 µM) as the positive control and testosterone (0.1 and 1 µM) as the natural AR agonist. We found that 1 µM ostarine, as well as 1 µM of testosterone, stimulated lipolysis after 120 min of incubation (P < 0.05) (Fig. 2a), whereas, after 480 min, there was an increase in the concentration of glycerol in the incubation medium of cells exposed to 0.1 and 1 µM of ostarine and 0.1 µM of testosterone (Fig. 2b). The use of AR antagonists has shown that ostarine stimulates lipolysis via AR (1 µM cyproterone acetate and 100 nM flutamide).

Ostarine inhibits lipogenesis
Furthermore, we examined the effect of ostarine on the biotransformation of glucose to lipids in the isolated rat adipocytes. In contrast to the results of lipolysis assay, we found that the greatest effect was obtained at low concentrations of ostarine (0.01 µM, P < 0.01) and (0.001 µM, P < 0.05). Ostarine added to the medium at the highest concentration also inhibited the rate of lipogenesis (10 µM, P < 0.05). According to our results, both the tested concentrations of testosterone statistically decreased the intensity of lipogenesis after 120 min of incubation (0.1 µM, P < 0.01 and 1 µM, P < 0.05) (Fig. 3).
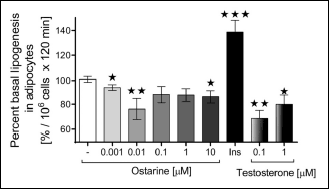 |
Fig. 3. Effect of ostarine on the intensity of lipogenesis as measured by the incorporation of C14 glucose into lipids in rat adipocytes. Insulin (10 nM) was used as a positive control. Moreover, testosterone was used as a natural ligand for androgen receptor (AR). Incubation lasted for 120 min. All the experiments were repeated at least thrice. Results are shown as a percentage of basal lipogenesis (set to 100%). Figure shows mean ± standard error of mean (SEM). Statistically significant differences are represented as *P < 0.05, **P < 0.01, and ***P < 0.001 versus corresponding control. |
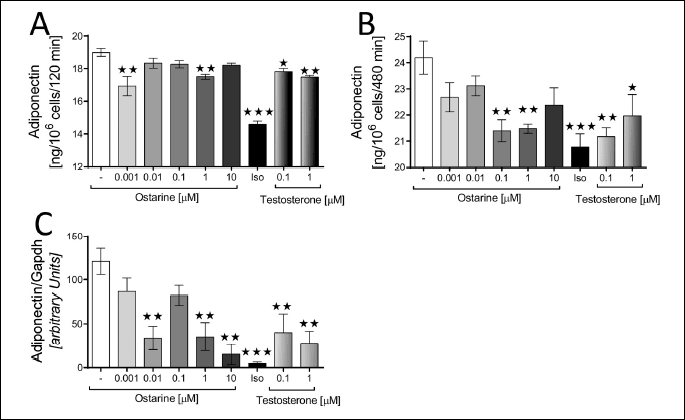
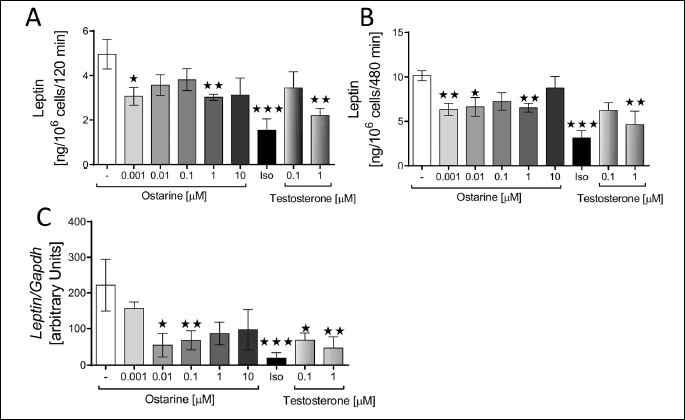
Ostarine modulates the expression and secretion of adiponectin and leptin
In the last part of the experiment, we investigated the effect of ostarine on mRNA expression and secretion of ADP and leptin in the isolated rat adipocytes.
According to our results, the leptin gene (Ob) expression in the adipocytes was downregulated by ostarine at concentrations of 0.01 (P < 0.1) and 0.1 µM (P < 0.05) and by testosterone at concentrations of 100 nM (P < 0.1) and 1 µM (P < 0.05) (Fig. 5c). A similar effect was demonstrated for the ADP gene. Except for ostarine at concentrations of 0.01 and 0.1 µM, all other tested concentrations of both of ostarine and testosterone downregulated the expression of ADP gene (P < 0.05) (Fig. 4c).
Next, we examined the effect of ostarine on the secretion of leptin and ADP in the isolated rat adipocytes. After 120 min of incubation, ostarine decreased the secretion of leptin at concentrations of 0.001 (P < 0.1) and 1 µM (P < 0.05) and testosterone at 1 µM (P < 0.05). After 480 min of incubation, ostarine at 0.01 µM concentration also decreased the secretion of leptin (P < 0.1). (Fig. 5a and 5b).
Next, we examined the effect of ostarine on the secretion of ADP. Similar to leptin, the secretion of ADP decreased in the presence of ostarine. After 120 min of incubation with 0.001 and 1 µM ostarine, ADP content decreased in the culture medium (P < 0.05). Similarly, testosterone also decreased the secretion of ADP at both the tested concentrations: 100 nM (P < 0.1) and 1 µM (P < 0.05). After 480 min of incubation, significant differences were observed for ostarine at 0.1 and 1 µM concentrations (P < 0.05) and for testosterone at 100 nM (P < 0.05) and 1 µM (P < 0.1) concentrations (Fig. 4a and 4b).
DISCUSSION
Regulation of lipid metabolism by steroid hormones is well known; however, the effect of synthetic AR agonists on the functions of adipocytes is poorly understood. Therefore, we decided to investigate the effect of ostarine on lipid processes and endocrine function of rat adipocytes. In this study, for the first time, we have shown that ostarine, a SARM, is a potent regulator of adipose tissue metabolism. We also found that ostarine increased lipolysis, decreased lipogenesis, downregulated the expression of leptin and ADP mRNA, and decreased the secretion of ADP and leptin in adipocytes.
As previously mentioned, ostarine is a selective agonist of AR; therefore, in the first part of the experiment, we decided to investigate its effect on the expression of AR at the gene and protein level. We found that ostarine (1 µM), as well as testosterone, upregulated the AR mRNA and protein expression in isolated rat adipocytes, whereas the use of pharmacological blockers downregulated its expression. In our study this effect was confirmed at the protein level as well. Previous studies have shown that AR is expressed in rat and mice adipose tissue (19, 20). Moreover, using 10T1/2 Pluripotent Cells and rat ventral prostate explants, it has been shown that the addition of testosterone upregulated the expression of AR mRNA and protein level (21, 22). To demonstrate the action of ostarine through the AR, we used selective AR blockers (flutamide and cyproterone acetate). We did not find any effect of ostarine on the expression of AR after the pretreatment of adipocytes with the aforementioned blockers. Therefore, we can conclude that ostarine upregulates AR expression in rat adipocytes. It seems surprising that effective doses of ostarine and testosterone are relatively high around 0.1 or 1 µM. This phenomenon could be explained by relatively low activity of androgen receptor in fat tissue of male rodents. Dart et al. in 2013 showed using mice model that androgen receptor activity in fat tissue is more visible in female mice. In male mice androgen receptor activity is relatively low. Moreover, it is difficult to compare results obtained on isolated adipocytes with the whole fat tissue containing other type of cells like e.g. macrophages, fibroblasts, vascular endothelial cells or others tissues (23).
Lipid metabolism in adipose tissue takes places via two main processes: lipid accumulation (lipogenesis) and lipid degradation (lipolysis). Therefore, we decided to investigate the effect of ostarine on these two processes. According to our results, ostarine, as well as testosterone (in these studies, treated as a natural AR receptor agonist - control), stimulated the process of lipolysis. Moreover, the effect was abolished by adding selective AR blockers (flutamide and cyproterone acetate). Our results also confirm that ostarine works via AR (with reference to Fig. 1). Lipolysis is mostly regulated by adrenoceptors. Activation of β-adrenergic receptors stimulates lipolysis, whereas activation of α2-adrenergic receptors inhibits lipolysis. The balance between activation of α2- and β-adrenergic receptors determines the overall level of lipolysis in adipose tissue (24, 25). This mechanism seems to be extremely important in the context of the activity of testosterone and other AR modulators. Previous studies have shown that testosterone increases the intensity of lipolysis and the number of β-adrenoceptors in male rat adipocytes (26), which can also explain the mechanism of ostarine action in isolated adipocytes. Unfortunately, there are no direct results showing the effect of ostarine on the activity of β-adrenergic receptors; therefore, this seems to be an interesting topic for a future study. Due to the presence of AR, as well as the enzymes involved in androgen metabolism in adipocytes, fat tissue appears to be an important regulator of energy homeostasis in response to androgens (3). The fact that androgen deficiency predisposes to obesity and metabolic disorders, for example, insulin resistance, is well understood (27, 28). Furthermore, decreased levels of serum testosterone and increased quantity of adipose tissue are known to be associated with each other (29). Under experimental conditions, testosterone stimulates catecholamine-induced lipolysis depending on its concentration. This is consistent with our results which show that testosterone has a stronger effect on isoproterenol-stimulated lipolysis. It is noteworthy that this effect was confirmed by using an aromatase inhibitor, which excludes the possible action of estrogens produced by aromatase (30).
It is known that the increase in muscle mass is associated with better use of energy stores deposited in adipose tissue. Besides many substances produced by muscles and adipose tissue like irisin (31), adiponectin or resistin (32), androgens produced mainly by gonads have a significant impact on the functioning of both these tissues (33). Testosterone differentiates pluripotent mesenchymal stem cells into a myogenic line and simultaneously inhibits the differentiation of mesenchymal stem cells into the adipogenic line in vitro (21). It is believed that one of the mechanisms responsible for this effect is the inhibition of the expression of adipogenic genes (PPARγ and CEBP/α) by testosterone (21, 34). Moreover, testosterone increases the insulin sensitivity of skeletal muscles, which promotes the metabolic status of the skeletal muscle (35). Androgen deficiency leads to the intensification of lipogenesis and the reduction in the triglyceride turnover (36). In our study, we performed de novo analysis of lipogenesis based on the conversion of glucose to lipids. We have demonstrated a significant inhibitory effect of testosterone, which is consistent with the literature regarding androgen (37). The molecular mechanism of this response is associated with the activation of the AR (38). However, no detailed studies have been conducted on the effect of ostarine on adipose tissue. Dalton et al. investigated the possible participation of ostarine in lipid metabolism conducting a Phase II clinical trial with 120 healthy elderly men and postmenopausal women. According to their results, there was a significant decrease in the total fat mass in the body of the participants. In addition, there was a decrease in the concentration of high-density lipoprotein (HDL) without affecting the concentration of low-density lipoprotein (LDL). Dalton et al.’s study was conducted for a period of 3 months with the administration of ostarine. However, there are no long-term studies describing the use of ostarine and its possible side effects. Dalton et al. reported that the effect of 3-month treatment with ostarine did not cause any adverse effect on the ratio of HDL/LDL, but it important to note that this may have an impact on cardiovascular health (39). In addition, there was a decrease in the concentration of HDL without affecting the concentration of LDL. A decrease in the concentration of blood glucose and a decrease in insulin resistance was also observed (39). In this study, we have demonstrated that ostarine shows testosterone-like effects with respect to lipolysis and lipogenesis. Due to the fact that ostarine is not a substrate for aromatase (40), it seems to be a better substitute for traditional androgen therapy because it does not cause side effects associated with the conversion of steroidal androgens to estrogens, thereby avoiding possible side effects caused due to the usage of aromatase inhibitors (41).
An important role of adipose tissue is the production of adipokines, i.e. leptin, ADP, and resistin. Their secretion is closely related to the amount and distribution of adipose tissue. e.g. high leptin level is associated with obesity and type 2 diabetes (42). There are multiple effects of androgens on the adipose tissue, they regulate the intensity of lipolysis and lipogenesis and they affect the expression of genes and proliferation of adipocytes (3). The relationship between androgens and adipose tissue is bi-directional. It has been shown that testosterone deficiency causes deposition of adipose tissue. Both leptin and ADP can inhibit the HPG axis in the case of obesity (43). However, it turns out that low testosterone levels may contribute to the development of leptin resistance and increase its plasma concentration. In in vivo studies, it has been shown that testosterone supplementation reduces the level of leptin, which is associated with the loss of fat mass (44). The results of in vitro test on human cells (33) and the results of this study confirm that testosterone is directly involved in the regulation of leptin expression. A similar effect was observed in a previous study conducted on rat adipocytes. In that, activation of AR via testosterone and DHT decreased the expression of the leptin gene (45). However, an experiment conducted on murine AR-knockout model reveal resistance to leptin, confirming the relationship between AR, androgen, and leptin (46). Unfortunately, the mechanism of action of leptin in regulation of AR remains unknown. In this study, we have demonstrated that the nonsteroidal SARMs also reduce the secretion of leptin from rat adipocytes. High levels of leptin in the blood accompanying obesity can cause HPG axis suppression. Testosterone treatment can restore the proper functioning of the HPG axis and contribute to weight loss (43). During 2000 – 2011, the use of hormone replacement therapy increased by threefold (47). However, hormone replacement therapy is associated with the development of hypertension which in turn increases the risk of cardiovascular complications, e.g. stroke (48). Ostarine seems to be a promising alternative to treat androgen deficiency.
ADP is negatively correlated with the amount of adipose tissue (49). It is known that the secretion of ADP depends on many factors, including sex. It has been found that androgens decrease the concentration of ADP (50). Our results are consistent with the results obtained by other researchers. Testosterone, as well as a strong steroidal agonist trenbolone, decrease the concentration of adiponectin in blood serum (50). The involvement of androgens in the regulation of adiponectin secretion has also been demonstrated in human studies. In men with hypogonadism, an increased level of adiponectin has been shown. Hormone replacement therapy with testosterone can decrease in the level of adipokine (51). Interestingly, in human studies, it has been shown that testosterone independently decreases leptin secretion (43). This effect has been found in other studies (52) and in ours. However, the same study showed that testosterone has no independent effect on adiponectin secretion because this effect coexisted with the change in body fat tissue mass (43). Our studies have shown that testosterone and ostarine both have an effect on the secretion of adiponectin at the gene level. However, it has been shown that the low level of circulating ADP precedes the occurrence of insulin resistance and type II diabetes and increases the risk of myocardial infarction (50). Our results show that ostarine downregulates the expression of ADP. Thus, our results show that ostarine, as well as testosterone, has a modulating effect on the expression of adipokines, confirming the hypothesis that ostarine shows genomic activity.
The results of this study demonstrate that ostarine stimulates ARs in rat adipocytes and modulates the expression of genes coding adipokine synthesis. Therefore, we can conclude that stimulation of AR is an important factor in carbohydrate-lipid homeostasis. The use of SARMs may be an alternative therapeutic solution for metabolic complications in patients with androgen deficiency.
Authors’ contributions: NL and PAK designed the study, obtained the data, and wrote the manuscript. EPO, contributed to the study design; performed the experiments; edited, supported, and critically revised the manuscript; and contributed to the Discussion section. JB researched data. LN critically revised the manuscript and contributed to the discussion. All authors approve for the publication of the final version of this manuscript.
Acknowledgments: This research was partially supported by the grant no. 507.558.14 of the Young Researcher Program of the Faculty of Veterinary Medicine and Animal Science Poznan University of Life Sciences, Poland financed by the Polish Ministry of Science and Higher Education and by the statutory funding no. 508.558.01 of the Faculty of Veterinary Medicine and Animal Science Poznan University of Life Sciences, Poland; Department of Animal Physiology and Biochemistry.
Conflict of interests: None declared.
REFERENCES
- Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: role of FSH and LH/ testosterone. Spermatogenesis 2014; 4: e996025. doi: 10.1080/21565562.2014.996025
- Clarke B, Khosla S. Androgens and bone. Steroids 2009; 74: 296-305.
- O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol 2014; 143: 277-284.
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011; 34: 1669-1675.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93: 1834-1840.
- Davis GK, Newsome AD, Ojeda NB, Alexander BT. Effects of intrauterine growth restriction and female sex on future blood pressure and cardiovascular disease. Curr Hypertens Rep 2017; 19: 13. doi: 10.1007/s11906-017-0712-7
- Traish A, Toselli P, Jeong S, Kim N. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficienc. J Androl 2005; 26: 242-248.
- Kemnitz JW, Goy RW, Keesey RE. Effects of gonadectomy on hypothalamic obesity in male and female rats. Int J Obes 1977; 1: 259-270.
- Ferenc K, Pietrzak P, Wierzbicka M, et al. Alterations in the liver of intrauterine growth retarded piglets may predispose to development of insulin resistance and obesity in later life. J Physiol Pharmacol 2018; 69: 211-218.
- Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert Opin Pharmacother 2014; 15: 1247-1264.
- Narayanan R, Mohler ML, Bohl CE, Miller DD, Dalton JT. Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal 2008; 6: E010. doi: 10.1621/nrs.06010
- Bhasin S, Jasuja R. Selective androgen receptor modulators (SARMs) as function promoting therapies. Curr Opin Clin Nutr Metab Care 2009; 12: 232-240.
- Crawford J, Prado CMM, Johnston MA, et al. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr Oncol Rep 2016; 18: 37. doi: 10.1007/s11912-016-0522-0
- Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol 2009; 301: 97-103.
- Rodbell M. Metabolism of isolated fat cells. 1. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964; 239: 375-380.
- Pruszynska-Oszmalek E, Kolodziejski PA, Sassek M, Sliwowska JH. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 2017; 56: 54-64.
- Kolodziejski PA, Pruszynska-Oszmalek E, Micker M, et al. Spexin: a novel regulator of adipogenesis and fat tissue metabolism. Biochim Biophys Acta Mol Cell Biol Lipids 2018; 1863: 1228-1236.
- Kolodziejski PA, Pruszynska-Oszmalek E, Strowski MZ, Nowak KW. Long-term obestatin treatment of mice type 2 diabetes increases insulin sensitivity and improves liver function. Endocrine 2017; 56: 538-550.
- Dieudonne MN, Pecquery R, Leneveu MC, Jaubert AM, Giudicelli Y. Androgen receptors in cultured rat adipose precursor cells during proliferation and differentiation: regional specificities and regulation by testosterone. Endocrine 1995; 3: 537-541.
- McInnes KJ, Smith LB, Hunger NI, Saunders PTK, Andrew R, Walker BR. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes 2012; 61: 1072-1081.
- Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003; 144: 5081-508.
- Carvalho-Dias E, Miranda A, Martinho O, et al. Serotonin regulates prostate growth through androgen receptor modulation. Sci Rep 2017; 7: 1-11.
- Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One 2013; 8: 1-16.
- Wahrenberg H, Lonnqvist F, Hellmer J, Arner P. Importance of beta-adrenoceptor function in fat cells for lipid mobilization. Eur J Clin Invest 1992; 22: 412-429.
- Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidemia. Diabetes Metab 1998; 24: 418-420.
- Xu X, de Pergola G, Bjorntorp P. Testosterone increases lipolysis and the number of b-adrenoceptors in male rat adipocytes. Endocrinology 1991; 128: 379-382.
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev 2015; 16: 581-606.
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009; 5: 673-681.
- Khaw K, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 1992; 2: 675-682.
- Xu X, de Pergola G, Bjorntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology 1990; 126: 1229-1234.
- Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J Physiol Pharmacol 2017; 68: 243-251.
- Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol 2005; 186: 457-465.
- Mammi C, Calanchini M, Antelmi A, et al. Androgens and adipose tissue in males: a complex and reciprocal interplay. Int J Endocrinol 2012; 2012: 789653. doi: 10.1155/2012/789653
- Sato H, Sugai H, Kurosaki H, et al. The effect of sex hormones on peroxisome proliferator-activated. Biol Pharm Bull 2013; 36: 564-573.
- Elahi D, Hayes FJ, Pitteloud N, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005; 28: 1636-1642.
- Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 1996; 20: 291-302.
- Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity and diabetes in males and females. Obesity (Silver Spring) 2015; 23: 713-719.
- Yu IC, Lin HY, Sparks JD, Yeh S, Chang C. Androgen receptor roles in insulin resistance and obesity in males: the linkage of androgen-deprivation therapy to metabolic syndrome. Diabetes 2014; 63: 3180-3188.
- Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle 2011; 2: 153-161.
- Srinath R, Dobs A. Enobosarm (GTx-024, S-22): a potential treatment for cachexia. Future Oncol 2014; 10: 187-194.
- Ronde W, Jong F. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol 2011; 9: 93-100.
- Kaczmarek P, Skrzypski M, Pruszynska-Oszmalek E, et al. Chronic orexin-a (Hypocretin-1) treatment of type 2 diabetic rats improves glucose control and beta-cell functions. J Physiol Pharmacol 2017; 68: 669-681.
- Ng M, Fui T, Hoermann R, Grossmann M. Effect of testosterone treatment on adipokines and gut hormones in obese men on a hypocaloric diet. J Endocr Soc 2017; 1: 302-312.
- Machinal-Quelin F, Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine 2002; 18: 179-184.
- Machinal F, Dieudonne M, Leneveu M, Pecquery R, Giudicelli Y. in vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. 2014; 140: 1567-1574.
- Lin H, Xu Q, Yeh S, Wang R, Sparks JD. Mice lacking androgen receptor. Diabetes 2005; 54; 1717-1725.
- Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013; 173: 1465-146.
- Lusetti M, Licata M, Silingardi E, Reggiani Bonetti L, Palmiere C. Pathological changes in anabolic androgenic steroid users. J Forensic Leg Med 2015; 33: 101-104.
- Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 2003; 88: 4823-4831.
- Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of androgens on circulating adiponectin in male and female rodents. PLoS One 2012; 7: e47315. doi: 10.1371/journal.pone.0047315
- Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004; 60: 500-507.
- Wabitsch M, Blum WF, Muche R, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. 1997; 100: 808-813.
A c c e p t e d : August 28, 2019