LOCALIZATION DEPENDENT SENSITIVITY OF CEREBRAL Na,K-ATPase
TO IRRADIATION INDUCED OXIDATIVE IMBALANCE IN RATS
INTRODUCTION
Recently, an increasing interest has been directed towards the investigation of brain changes after ionizing radiation. It was suggested that the final effect is the result of a blending of atherosclerotic, cardiovascular, cerebrovascular and neurodegenerative processes (1-3). Previous study of relationship between cognitive dysfunction and histological changes in the brain following the whole brain irradiation (25 Gy/single dose) showed lowering of cognitive functions together with histological and immunohistochemical alterations in brains one year after irradiation in rats. These findings were interpreted as similar to those observable in clinically accelerated brain aging, e.g. in conditions of Alzheimer’s disease, Binswanger’s disease, and multiple sclerosis (4). Besides, additional studies reported impairment of cognitive dysfunction after radiotherapy even when such therapy was not directed to brain areas (5). Patients with breast cancer exposed to adjuvant regional radiotherapy might have cognitive impairment even several months after their treatment (6).
It is widely accepted that in the mechanism induced by radiotherapy generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is involved (7, 8). However, the increase of radioresistance in cancer cells and radiation toxicity to normal tissues are severe concerns. The increased generation of ROS/RNS leads to DNA damage via strand breaks and base alterations (9). Beside the effect on DNA, it was documented that radiation can induce injury also to extranuclear targets such as plasma membrane, mitochondria and endoplasmic reticulum and induce alterations in various signaling pathways (10).
The effect of irradiation on various systems throughout the organism is intensively studied in the case of whole body treatment or using in situ approach when only selected parts of the body were irradiated. There is still insufficient information concerning the radiation-induced alterations in outlying parts of the body. Our previous study showed that irradiation of rats in mediastinal area was followed by hypertrophy of the right ventricle. Concerning the molecular alterations, decreased functionality of cardiac Na,K-ATPase, a key system responsible for maintenance of intracellular homeostasis of sodium ions, was documented (11). In the same experimental model, this enzyme showed lowered functionality in renal tissue, the most probably as a consequence of worsened oxidative status in blood plasma. It is widely accepted that Na,K-ATPase is sensitive to oxidative stress induced by various impulses (12-15). Concerning the mechanism behind the deterioration of Na,K-ATPase function due to worsened oxidative status induced by irradiation, the altered properties of binding sites for sodium and ATP together with worsened assembly of the enzyme into the surface cell membrane were documented (14). The brain represents another organ where the proper functionality of Na,K-ATPase is crucial.
The brain tissue has high rate of oxidative metabolic activity, high concentration of polyunsaturated fatty acids in membrane lipids, presence of iron ions and low capacity of antioxidant enzymes, which makes the brain very susceptible to ROS action (16). It was shown that radiation in lethal and sublethal doses on the sodium-potassium transport systems in the fractions enriched of neural and glial cells and in cortex slices from rat brain resulted in marked disturbances in the activity of Na,K-ATPase in both, neurons and glial cells (17). There is a lack of information concerning the remote effect of the radiotherapy directed to one specific part of the body on the brain. Based on our previous study with renal enzyme, we hypothesized that irradiation of rats in mediastinal area may be also followed by alterations of Na,K-ATPase in brain tissue. Thus, the present study was oriented to remote effect of mediastinal irradiation on this enzyme in two selected regions of the brain, in the cortex and in the cerebellum. For detailed description of the mechanism behind alteration of the Na,K-ATPase in consequence of irradiation, studies of enzyme kinetics under different assay conditions and expression of catalytic subunits a1-3 were used as tools.
MATERIALS AND METHODS
Animal model and radiation
All procedures in this study were approved by the Institutional Animal Care Committee and their correspondence to IACUC was attested by State Veterinary and Food Administration of the Slovak Republic.
Male Wistar rats were obtained from Velaz Praha (Czech Republic) and maintained in our animal care facility on a 12:12-hour light/dark cycle with free access to food and water. At the age of 14 weeks, animals (n = 8) were anesthetized with thiopental (65 mg·kg–1 body weight). Rats were irradiated with 5 MeV/1kW electron linear accelerator UELR 5-1S with tungsten converter to X-rays at the dose rate 10 Gy/min (Producer NIIEFA St. Petersburg, RF). A single dose of 25 Gy was given locally on mediastinal area. Irradiation was directed transversally, crossing the chest of rat at the heart level, while the rest of animal was shielded with 20 cm thick lead plates. The schematic presentation of irradiation procedure is shown in our previous paper (14). Despite the shielding of rats by lead plates the mortality amounted 17% in the group of irradiated animals. A single dose of 25 Gy to the heart corresponds to the cumulative dose of irradiation commonly used in patients. Six weeks after irradiation, the rats were anesthetized with thiopental (65 mg·kg–1 body weight) and euthanized by heart excision. Age-matched Wistar male rats from the same breeding facility served as controls (n = 8). Cerebral cortex and cerebellum were quickly removed, rapidly rinsed with ice-cold physiological saline, frozen in liquid nitrogen and stored at –60°C until use.
Biochemical analysis of oxidative status
Markers of protein oxidation were measured by spectrophotometric analysis of advanced oxidation protein products (AOPP) according to Witko-Sarsat et al. (18). Briefly, 200 µL of diluted plasma (1:4 in phosphate buffer saline (PBS), pH = 7.2) were mixed with 20 µL of glacial acetic acid. Chloramine T with potassium iodide was used for the calibration curve construction. The absorbance was measured at 340 nm.
Two markers of carbonyl stress were estimated. The advanced glycation end products (AGEs) were determined according to Munch et al. (19). In this assay, 20 µL of plasma were diluted with PBS (pH = 7.2). Fluorescence was measured at λex. = 370 nm and λem. = 440 nm. In the calibration curve, AGE-modified bovine serum albumin was used as standard. For fructosamine concentration, 20 µL of samples and standards (1-deoxy-morpholino-D-fructose) were put to the microtiter plate. Thereafter, nitro blue tetrazolium was added, content was shortly mixed, and incubated at 37°C for 15 minutes. The absorbance was measured at 530 nm (20).
Two markers of antioxidant status were measured: total antioxidant capacity (TAC) and ferric reducing antioxidant power (FRAP). TAC was measured according to Erel (21). Briefly, the plasma samples were mixed with acetate buffer (pH = 5.8). As blank, the initial absorbance was measured at 660 nm. When ABTS solution (2.2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid with acetate buffer) was added, the absorbance (660 nm) was measured again. FRAP was measured according to Benzie and Strain (22). FRAP reagent (warmed to 37°C, composed of acetate buffer (pH = 3.6), tripyridyls-triazine, FeCl3 × 6H2O and water) was put to the microtiter plate and initial absorbance was measured as blank. The samples were added to reagent, and the absorbance measured again at 593 nm.
The concentration of proteins was measured by bicinchoninic acid kit (Sigma-Aldrich, Munich, Germany), according to the manufacturer’s instructions. The bovine serum albumin was used as standard. All measurements were performed on a Tecan Sapphire II Instrument (Grodig, Austria) and reagents used for these measurements were obtained from Sigma-Aldrich (Munich, Germany).
Assay of Na,K-ATPase activity
The plasmalemmal membrane fractions from cerebral cortexes and cerebellums were isolated according to Jorgensen (23). Amount of proteins was determined by the procedure of Lowry et al. (24) using bovine serum albumin as a standard.
All assays of Na,K-ATPase activity were described previously (25). Briefly, all steps were performed at 37°C using 10 µg·ml–1 of membrane protein in an assay buffer containing (in mmol·l–1): 4 MgCl2, 100 NaCl, 10 KCl and 50 TRIS (pH = 7.4). The samples were pre-incubated for 20 min in substrate-free medium. The enzyme reaction was initiated by addition of increasing amount of TRIS-ATP in the range of 0.16 – 8.00 mmol·l–1. The reaction was stopped after 20 min by adding 12% ice-cold trichloracetic acid. The inorganic phosphorus generated from ATP hydrolysis was estimated according to the method of Taussky and Shorr (26). In order to establish the Na,K-ATPase activity exclusively, the ATP hydrolysis occurring in the presence of Mg2+ only, was subtracted. The enzyme kinetics for sodium activation was determined by the same way. The concentration of NaCl varied in the range of 2 – 100 mmol·l–1 and the amount of ATP was constant (8 mmol·l–1). The kinetic parameters of Na,K-ATPase - Vmax, Km and KNa values were evaluated by direct non-linear regression of the obtained data.
Preparation of tissue fractions for electrophoresis and immunochemical Western blot analysis
The tissue samples from rat cerebral cortex and cerebellum were re-suspended in ice-cold homogenizing buffer containing (in mmol·l–1): 50 Tris-HCl, 250 sucrose, 1.0 dithiothreitol, 1.0 phenylmethylsulfonylfluoride (pH 7.4) and homogenized with a glass-teflon homogenizer. The homogenates were centrifuged at 800 × g for 5 min at 4°C, pellets were discarded after this centrifugation and the supernatants were centrifuged again at 16100 × g for 30 min. Following this second centrifugation, the supernatants were discarded again and the pellets were re-suspended in homogenizing buffer supplemented with 0.2% Triton X-100 and centrifuged at 16100 × g for 1 min. The Triton X-100 soluble supernatants represented the particulate fraction of the samples. The protein concentrations were estimated by the method of Bradford (27).
Samples of particulate protein fractions (for α1-3 Na,K-ATPase subunits detection) containing equivalent amounts of proteins (25 µg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel (10%) electrophoresis. For Western blot assays, separated proteins were transferred from gel to a nitrocellulose membrane overnight at 4°C. The quality of the transfer was controlled by Ponceau S staining of nitrocellulose membranes after the transfer. Specific antibodies against α1 (mouse monoclonal antibody from Sigma; product number A-277, in dilution 1:250), α2 (rabbit polyclonal antibody from Milipore; #07-674, in dilution 1:1000) and a3 (rabbit polyclonal antibody from Milipore; #06-172, in dilution 1:1000) subunits of Na,K-ATPase were used for the primary immunodetection. Peroxidase-labelled anti-mouse (from Cell Signaling; #7076, in dilution 1:1000) and anti-rabbit (from Cell Signaling; #7074S, in dilution 1:1000) immunoglobulins were used as the secondary antibodies. Bound antibodies were detected by the enhanced chemiluminescence detection method using Amersham Imager 600. Densitometric quantification of protein levels was performed by comparison to loading control β-actin (mouse monoclonal antibody (AC-15) from Abcam; ab6276, in dilution 1:1000 and corresponding anti-mouse secondary antibody) using an ImageJ program.
Statistical analysis
All investigated parameters are expressed as means ± standard errors of mean (SEM). For statistical analysis t-test with Mann-Whitney rank sum test was applied. The differences were considered to be significant when the P-value was less than 0.05.
RESULTS
Plasma protein concentration and biochemical analysis of oxidative status
Total plasma protein concentration was lower in irradiated rats when compared with control animals (in g/l: 84 ± 5 control versus 59 ± 5 irradiated, P = 0.004). Regarding the biochemical analysis of oxidative status, following parameters of oxidative stress and antioxidant status were statistically different between the control and irradiated group: AGEs (in relative fluorescence units/l, 5.15 ± 0.38 control versus 9.77 ± 0.97 irradiated, P = 0.001), TAC (in µmol/l, 530 ± 23 control versus 264 ± 28 irradiated, P < 0.0001) and FRAP (in µmol/l, 670 µ 75 control versus 345 ± 57 irradiated, P = 0.005). The experimental groups were not significantly different in AOPP (in mmol/l, 56.8 ± 7.9 control versus 46.3 ± 7.7 irradiated, P = 0.36) and fructosamine plasma levels (in µmol/l, 0.46 ± 0.05 control versus 0.49 ± 0.05 irradiated, P = 0.66). The relative ratios of values in irradiated rats when compared with controls are summarized in the Fig. 1.
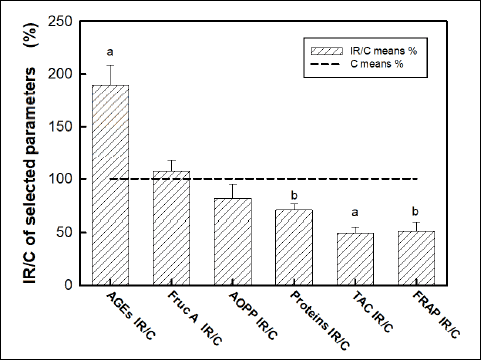 |
Fig. 1. Comparison of plasma protein concentration and oxidative status in blood plasma of irradiated rats (IR) versus control rats (C). Concentrations of: advanced glycation end products (AGEs), fructosamine (Fruc A), advanced oxidation protein products (AOPP), total antioxidant capacity (TAC), ferric reducing antioxidant power (FRAP). Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. Statistical significance: aP < 0.001, bP < 0.005. |
Na,K-ATPase kinetics
Cerebral cortex
in vitro activation of the Na,K-ATPase with increasing concentrations of the substrate (ATP) in isolated plasmalemmal membrane fraction from rat cerebral cortex revealed lower activity of this enzyme (by 12%) throughout the investigated concentration range in irradiated rats when comparing with control group (Fig. 2). Evaluation of kinetic parameters resulted in decreased Vmax value (by 14%) together with unchanged value of Km in irradiated rats (Fig. 3).
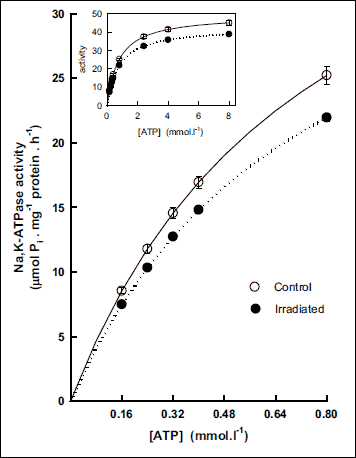 |
Fig. 2. Activation of Na,K-ATPase from cerebral cortex by low concentrations of substrate ATP in control and irradiated (25 Gy) Wistar rats. Insert: Activation of the enzyme in the whole investigated concentration range of ATP. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
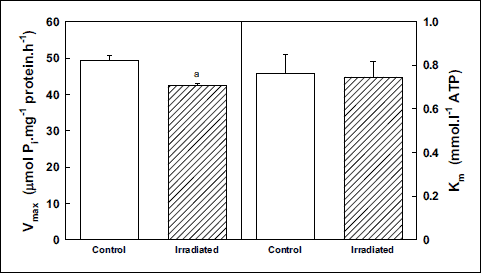 |
Fig. 3. Kinetic parameters of Na,K-ATPase from cerebral cortex during activation with ATP in control and irradiated (25 Gy) Wistar rats. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. Statistical significance: aP < 0.001. |
Activation of the Na,K-ATPase with increasing concentration of sodium ions showed a biphasic effect. In the concentration range below 8 mmol·l–1, the enzyme activity was higher and above this concentration, the activity decreased in irradiated rats as compared with controls (Fig. 4). Evaluation of kinetic parameters resulted in statistically significant decrease of the Vmax value (by 13%) together with decrease of the KNa value (by 25%) in irradiated rats (Fig. 5).
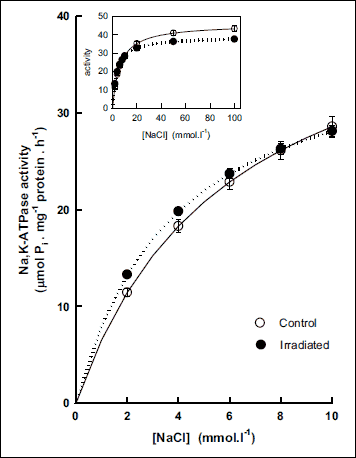 |
Fig. 4. Activation of Na,K-ATPase from cerebral cortex by low concentrations of NaCl in control and irradiated (25 Gy) Wistar rats. Insert: Activation of the enzyme in the whole investigated concentration range of NaCl. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
 |
Fig. 5. Kinetic parameters of Na,K-ATPase from cerebral cortex during activation with NaCl in control and irradiated (25 Gy) Wistar rats. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. Statistical significance: aP < 0.05. |
Cerebellum
Studying the impact of irradiation on the Na,K-ATPase kinetics in cerebellum, we observed different effects from those documented in cerebral cortex. Activation of samples from cerebellum with increasing concentrations of ATP resulted in slight increase of enzyme activity. The effect was higher at lower presence of ATP representing 11% at 0.16 mmol·l–1. With increasing concentrations of ATP, the effect decreased stepwise to similar activities observed in the presence of 8 mmol·l–1 (Fig. 6) resulting in slight but statistically insignificant decrease of the Km value when compared with control group. The Vmax value remained also unaltered 6 weeks after irradiation (Fig. 7).
Activation of the Na,K-ATPase from cerebellum with increasing concentration of sodium showed continual increase of its activity in the whole concentration range in irradiated rats when comparing with controls. The effect increased stepwise with increasing concentrations of Na+ from 4% observed in the presence of 2 mmol.l–1 to 9% observed in the presence of 100 mmol.l–1 of NaCl (Fig. 8) resulting in slight but statistically insignificant increase of the KNa value in irradiated rats when compared with controls (Fig. 9).
 |
Fig. 6. Activation of Na,K-ATPase from cerebellum by low concentrations of substrate ATP in control and irradiated (25 Gy) Wistar rats. Insert: Activation of the enzyme in the whole investigated concentration range of ATP. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
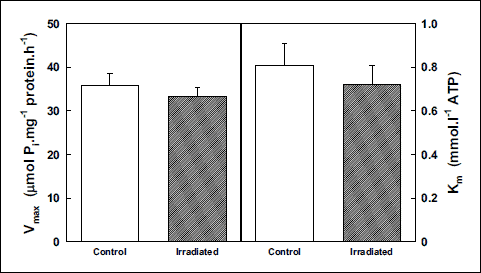 |
Fig. 7. Kinetic parameters of Na,K-ATPase from cerebellum during activation with ATP in control and irradiated (25 Gy) Wistar rats. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
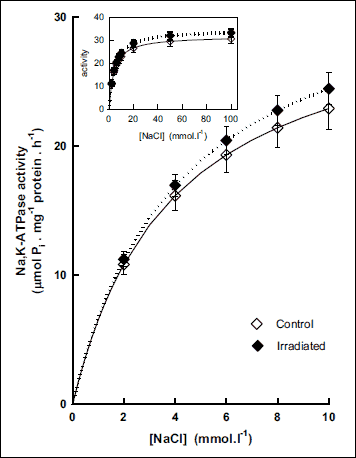 |
Fig. 8. Activation of Na,K-ATPase from cerebellum by low concentrations of NaCl in control and irradiated (25 Gy) Wistar rats. Insert: Activation of the enzyme in the whole investigated concentration range of NaCl. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
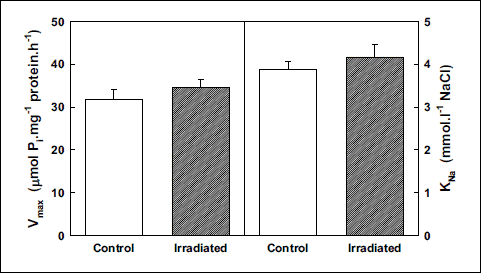 |
Fig. 9. Kinetic parameters of Na,K-ATPase from cerebellum during activation with NaCl in control and irradiated (25 Gy) Wistar rats. Data represent means ± SEM, n = 8 in control, n = 8 in irradiated group. |
Western blot analysis of Na,K-ATPase isoforms
Cerebral cortex
Analysis of Na,K-ATPase catalytic a-subunits by Western blot (Fig. 10) showed unchanged level of α1 subunit in irradiated rats when compared with controls. The presence of α2 and α3 was significantly lower after irradiation - approximately by 25% and 30% respectively.
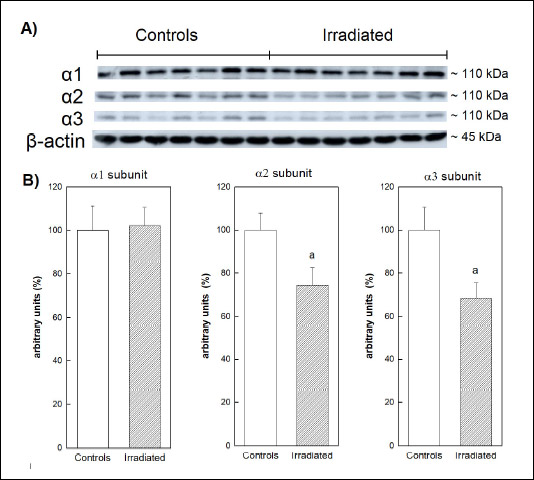 |
Fig. 10. (A): Immunoblot analysis of a1–3 subunits of Na,K-ATPase from cerebral cortex 6 weeks after irradiation of rats by 25 Gy in the mediastinal area. (B): Relative abundance of a1–3 subunits of Na,K-ATPase. Data represent mean ± SEM, n = 7 in each group. Statistical significance: aP < 0.05. |
Cerebellum
The expression of all 3 isoforms of a-subunits remained unaltered after irradiation (Fig. 11).
 |
Fig. 11. (A): Immunoblot analysis of α1–3 subunits of Na,K-ATPase from cerebellum 6 weeks after irradiation of rats by 25 Gy in the mediastinal area. (B): Relative abundance of α1–3 subunits of Na,K-ATPase. Data represent mean ± SEM, n = 7 in control group and n = 6 in irradiated group. |
DISCUSSION
In the present study we tried to bring more insight into the effect of imbalanced oxidative status on the mechanism of Na,K-ATPase alterations in cerebral cortex and cerebellum. In the pathogenesis of various encephalopathies deterioration of Na,K-ATPase in consequence of increased level of ROS as well as RNS is involved as documented by numerous studies exploiting experimental animal models. Using iron-induced model of traumatic epilepsy in rats, increased lipid peroxidation together with decreased Na,K-ATPase activity in cortex was observed (28). Model of barium-induced toxicity provoked oxidative stress, cellular injury and decrease of Na,K-ATPase activity in cerebellum of rats (29). Experimental model of memory impairment revealed increased concentration of thiobarbituric acid reactive substances and decreased activity of Na,K-ATPase in the cerebral cortex, hippocampus and hypothalamus (30). In a rat model of diabetic encephalopathy induced by streptozotocin, the oxidative stress was accompanied with lowered activity of Na,K-ATPase in hippocampus (31). In disruption of oxidative status and the activity of Na,K-ATPase in the brain, the ageing of experimental animals also contributes, as synaptosomes from 18-months old rats were more sensitive to hyperglycemic conditions as compared with 3-months old rats (32). Results from another study showed that alcohol administration to rats decreased the Na,K-ATPase activity in brain cortex what was ascribed to ROS/RNS-induced damage (33). Therefore the present study was oriented to bring new information concerning the influence of imbalanced oxidative status on the mechanism of Na,K-ATPase alterations in cerebral cortex and cerebellum studying its expression and its binding properties for energy substrate ATP and for cofactor Na+ six weeks after mediastinal irradiation of rats.
Plasma protein concentration and biochemical analysis of oxidative status
The hypoproteinemia as the consequence of irradiation was already observed in animal experiments (34, 35) and also in humans (36). The measurement of post-radiation plasma protein concentration is clinically significant, as hypoalbuminemia was reported as an independent predictor of poor outcome of selected malignities (37, 38). High-resolution proteomics used to analyze plasma from non-human primates showed time- and radiation-dose dependent changes in the plasma proteome after whole body irradiation (39). Since the liver is major contributor of plasma proteins, the observed hypoproteinemia after irradiation may be the consequence of liver injury (40). The hepatic functions could be affected also indirectly via production of AGEs which levels were increased in our study post irradiation. The AGEs was shown to elicit oxidative stress and consequently inflammatory responses in various types of cells including hepatocytes (41, 42). In addition to altered protein concentration and increased AGEs levels, the negative impact of irradiation was also exhibited in antioxidant status in plasma. Post-radiation decrease in TAC and FRAP of plasma was shown in human (43) as well as animal (44) studies. The reduced quality of antioxidant defense can be a consequence of increased production of ROS/RNS, the most probable cause of acute and chronic toxicity from irradiation (8, 45, 46).
Na,K-ATPase
Direct irradiation of the brain, when the rats were subjected to whole body irradiation, lowered the activity of Na,K-ATPase by 40% in the homogenate of whole brain (47). Basing on the results of present study oriented to remote effect of mediastinal irradiation on this enzyme in two selected regions of the brain, we were able to differentiate between the response of Na,K-ATPase in cortex and in cerebellum to worsened oxidative status of blood plasma. To obtain new and better insight into the mechanism of radiation-induced alterations of this enzyme molecule the determination of enzyme kinetics and Western blot analysis were applied.
In central nervous system, 3 isoforms of the catalytic subunit of Na,K-ATPase (α1, α2, α3) were recognized. The isoform α1 is expressed ubiquitously in the brain tissue while the α2 isoform is expressed primarily in astrocytes and developing neurons, and α3 isoform is restricted to neurons (48-50). These isoforms differ in the ability to bind intracellular sodium as their KNa value increases in the sequence α1 < α2 < α3. The increase in KNa value corresponds to decrease in affinity of enzyme to bind Na+. The α3 isoform has approximately threefold lower affinity to Na+ compared with α1 (51). It was hypothesized that the ubiquitously expressed α1 isoform maintains neuronal basal functions while the α3 isoform serves as reserve pump that becomes activated in condition of high intracellular sodium concentration (52).
Our study revealed that in rats subjected to irradiation in mediastinal area the number of active Na,K-ATPase molecules was lowered in cerebral cortex as indicated by decreased Vmax values for both types of enzyme activations - with substrate ATP and cofactor Na+. It has to be mentioned that estimated kinetic parameters represent a cumulative contribution of all 3 isoforms present in the brain tissue. This finding seems to be in agreement with decrease of the Na,K-ATPase activity via oxidative damage in the frontal cortex of the rats as a consequence of repeated restraint stress (12). On the other hand, in cerebral cortex of irradiated rats the enzyme binds better Na+ as indicated by lowered KNa value. This effect observed especially in lower concentrations of NaCl (corresponding to physiological concentrations of intracellular sodium) may represent a compensatory effect against the lowered number of active enzyme molecules. This fact seems to be a consequence of relatively lower presence of α2 and α3 subunits in cerebral cortex of irradiated rats (Fig. 12). Thus, the proportion of α1 which was not affected by irradiation relatively increased. Because this subunit has the best binding properties for Na+ the higher relative presence of α1 resulted in lowered cumulative KNa value.

Depression of protein expression of α2 and α3 subunits of Na,K-ATPase in the cerebral cortex seems to be a considerable consequence of irradiation because their function is crucial for maintenance of proper function of neural cells (53). The α2 isoform is accepted as an important player in neurobiology and development of migraine, in correlations between primary brain dysfunction and mechanisms of headache pain generation (54). Malfunction of this isoform is also involved in impairment of learning (55). Concerning the decrease of α3 protein expression, it may be also clinically significant because dysfunction of this isoform is assumed as a key player in neurodevelopmental, neuropsychiatric, motor, cognitive and neurodegenerative disorders (50, 56).
It is worthy to add that occurrence of neurodegenerative complications after irradiation is time dependent. At 6 and 9 months after irradiation, there were no significant differences between the control and irradiated groups of rats in passive avoidance and water maze tests. However, at 12 months after irradiation, the passive avoidance task revealed a deterioration of cognitive function in the irradiated group (57). So, our data indicating the decrease in the amount of active Na,K-ATPase molecules (namely the α2 and α3 subunits in cerebral cortex) six weeks after single dose irradiation suggest that this enzymatic system localized in the surface membrane of cells may represent one of the first systems injured by irradiation preceding alterations of other intracellular systems eventuating in subsequent pathological alterations of the brain.
On the other hand, the Na,K-ATPase in cerebellum seems to be more resistant to imbalanced oxidative status of blood plasma after irradiation, because the number of active enzyme molecules was not altered as indicated by similar values of Vmax in both experimental groups. Binding properties of the enzyme for energy substrate ATP as well as for sodium ions remained also unaltered as indicated by similarity of Km and KNa values in both experimental groups. These findings obtained by studies of enzyme kinetics were supported by analysis of the protein expression of a subunits of Na,K-ATPase showing no change between the experimental groups regarding the presence of all three investigated isoforms.
Our observation of different response of Na,K-ATPase in cerebral cortex and cerebellum to irradiation induced worsened oxidative status is in agreement with previous data documenting, that the response of neurons to overload of ROS is not uniform in the brain (58). Overload of ROS induces limited damage in cerebellum while in hippocampus and cerebral cortex it may induce neuronal death (59).
In summary, the irradiation by 25 Gy in the mediastinal area of rats induced remote deteriorating effects in cerebral cortex despite the protection of unexposed parts of the body whose were covered by lead shield. In the mediation of this process, the most probably ROS and RNS are involved, as documented by worsened oxidative status of blood plasma. The functionality of the main consumer of the intracellular ATP in cerebral cortex - the Na,K-ATPase was also disturbed as documented by lowered number of active enzyme molecules namely the α2 and α3 subunits. According to our findings and considering the data available in databases, it may be hypothesized that the lowered functionality of α2 and α3 subunits of Na,K-ATPase may represent a predisposition to neurodegenerative disorders after irradiation. The observed effect seems to be localization dependent as the enzyme in cerebellum was resisted to irradiation.
Acknowledgments: The study was supported by Slovak Grant Agency: VEGA-2/0166/17, VEGA 2/0063/18, VEGA 1/0234/18 and ITMS-26230120006.
Conflict of interests: None declared.
REFERENCES
- Loganovsky K, Perchuk I, Marazziti D. Workers on transformation of the shelter object of the Chernobyl nuclear power plant into an ecologically-safe system show qEEG abnormalities and cognitive dysfunctions: a follow-up study. World J Biol Psychiatry 2016; 17: 600-607.
- Rong X, Yin J, Wang H, Zhang X, Peng Y. Statin treatment may lower the risk of postradiation epilepsy in patients with nasopharyngeal carcinoma. Epilepsia 2017; 58: 2172-2177.
- Marazziti D, Piccinni A, Mucci F, Baroni S, Loganovsky K, Loganovskaja T. Ionizing radiation: brain effects and related neuropsychiatric manifestations. Probl Radiac Med Radiobiol 2016; 21: 64-90.
- Akiyama K, Tanaka R, Sato M, Takeda N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 2001; 41: 590-598.
- Dietrich J, Kaiser J. Cancer, chemotherapy and cognitive dysfunction. Eur Neurol Rev 2016; 12: 43-45.
- Shibayama O, Yoshiuchi K, Inagaki M, et al. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med 2014; 3: 702-709.
- Jiang H, Wang H, De Ridder M. Targeting antioxidant enzymes as a radiosensitizing strategy. Cancer Lett 2018; 438: 154-164.
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012; 327: 48-60.
- Fischer N, Seo EJ, Efferth T. Prevention from radiation damage by natural products. Phytomedicine 2018; 47: 192-200.
- Corre I, Niaudet C, Paris F. Plasma membrane signaling induced by ionizing radiation. Mutat Res 2010; 704: 61-67.
- Mezesova L, Vlkovicova J, Kalocayova B, et al. Effects of g-irradiation on Na,K-ATPase in cardiac sarcolemma. Mol Cell Biochem 2014; 388: 241-247.
- Novaes LS, dos Santos NB, Dragunas G, et al. Repeated restraint stress decreases Na,K-ATPase activity via oxidative and nitrosative damage in the frontal cortex of rats. Neuroscience 2018; 393: 273-283.
- Bogdanova A, Petrushanko IY, Hernansanz-Agustin P, Martinez-Ruiz A. “Oxygen Sensing” by Na,K-ATPase: these miraculous thiols. Front Physiol 2016; 7: 314. doi: 10.3389/fphys.2016.00314
- Kalocayova B, Kovacicova I, Radosinska J, et al. Alteration of renal Na,K-ATPase in rats following the mediastinal g-irradiation. Physiol Rep 2019; 7: e13969. doi: 10.14814/phy2.13969
- Xie Z, Jack-Hays M, Wang Y, et al. Different oxidant sensitivities of the alpha 1 and alpha 2 isoforms of Na+/K(+)-ATPase expressed in baculovirus-infected insect cells. Biochem Biophys Res Commun 1995; 207: 155-159.
- Rauchova H, Vokurkova M, Koudelova J. Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol Res 2012; 61 (Suppl. 1): S89-S101.
- Shainskaya AM, Dvoretsky AI. Mechanism of radiation injury in the ion transport systems of nervous tissue. Acta Physiol Hung 1990; 76: 295-299.
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996; 49: 1304-1313.
- Munch G, Keis R, Webels A, et al. Determination of advanced glycation endproducts in serum by fluorescence spectroscopy and competitive ELISA. Mail React Foods Med 1997; 35: 669-677.
- San-Gil F, Schier GM, Moses RG, Gan IE. Improved estimation of fructosamine, as a measure of glycated serum protein, with the Technicon RA-1000 analyzer. Clin Chem 1985; 31: 2005-2006.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004; 37: 277-285.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239: 70-76.
- Jorgensen PL. Purification and characterization of (Na+ plus K+)-ATPase III. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochem Biophys Acta 1974; 356: 36-52.
- Lowry HO, Rosebrough NJ, Farr LA, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Jagmasevic-Mezesova L, Svitok P, Kalocayova B, Zeman M, Vrbjar N. Sex-specific response of renal Na,K-ATPase to prenatal angiotensin 2 exposure and increased salt intake in offspring. J Physiol Pharmacol 2018; 69: 83-90.
- Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 1953; 202: 675-685.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
- Das J, Singh R, Sharma D. Antiepileptic effect of fisetin in iron-induced experimental model of traumatic epilepsy in rats in the light of electrophysiological, biochemical, and behavioral observations. Nutr Neurosci 2017; 20: 255-264.
- Elwej A, Ghorbel I, Chaabane M, et al. Zinc and selenium modulate barium-induced oxidative stress, cellular injury and membrane-bound ATPase in the cerebellum of adult rats and their offspring during late pregnancy and early postnatal periods. Arch Physiol Biochem 2018; 124: 237-246.
- Abdalla FH, Schmatz R, Cardoso AM, et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na+,K+-ATPase activities. Physiol Behav 2014; 135: 152-167.
- Liu JP, Feng L, Zhang MH, et al. Neuroprotective effect of Liuwei Dihuang decoction on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. J Ethnopharmacol 2013; 150: 371-381.
- Torlinska T, Grochowalska A, Kupsz J, Skoracka J, Kojo S. in vivo and in vitro effects of hyperglycemia on Na+-K +, Ca+2, Mg+2- dependent ATPases activity in brain synaptosomes of aging rats. J Physiol Pharmacol 2006; 57: 145-158.
- Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N. Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties. Mol Cell Biochem 2013; 375: 39-47.
- El-Gazzar MG, Zaher NH, El-Hossary EM, Ismail AF. Radio-protective effect of some new curcumin analogues. J Photochem Photobiol B Biol 2016; 162: 694-702.
- Nandchahal KK, Routh J, Mathur S, Bhartiya HC. Radiation response of plasma protein and albumin of peripheral blood and its modification by MPG (2-mercaptopropionylglycine) in mice. Strahlenther Onkol 1990; 166: 306-309.
- Bartlett EK, Roses RE, Meise C, Fraker DL, Kelz RR, Karakousis GC. Preoperative radiation for retroperitoneal sarcoma is not associated with increased early postoperative morbidity. J Surg Oncol 2014; 109: 606-611.
- Kang HS, Roh JL, Lee SW, et al. Noncancer-related health events and mortality in head and neck cancer patients after definitive radiotherapy: a prospective study. Medicine (Baltimore) 2016; 95: e3403. doi: 10.1097/MD.0000000000003403
- Wang CY, Hsieh MJ, Chiu YC, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol 2009; 92: 270-275.
- Byrum SD, Burdine MS, Orr L, et al. Time-and radiation-dose dependent changes in the plasma proteome after total body irradiation of non-human primates: implications for biomarker selection. PLoS One 2017; 12: e0174771. doi: 10.1371/journal.pone.0174771
- Furuse J, Ishii H, Nagase M, Kawashima M, Ogino T, Yoshino M. Adverse hepatic events caused by radiotherapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol 2005; 20: 1512-1518.
- Hyogo H, Yamagishi S. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr Pharm Des 2008; 14: 969-972.
- Zhuang A, Yap FY, Bruce C, et al. Increased liver AGEs induce hepatic injury mediated through an OST48 pathway. Sci Rep 2017; 7: 12292. doi: 10.1038/s41598-017-12548-4
- Khalil Arjmandi M, Moslemi D, Sadati Zarrini A, et al. Pre and post radiotherapy serum oxidant/antioxidant status in breast cancer patients: impact of age, BMI and clinical stage of the disease. Rep Pract Oncol Radiother 2016; 21: 141-148.
- Manda K, Ueno M, Moritake T, Anzai K. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: Protective effect of a-lipoic acid. Behav Brain Res 2007; 177: 7-14.
- Alikhani M, MacLellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Physiol 2006; 292: C850-C856.
- Yang L, Meng H, Yang M. Autophagy protects osteoblasts from advanced glycation end products-induced apoptosis through intracellular reactive oxygen species. J Mol Endocrinol 2016; 56: 291-300.
- Abou-Seif MA, El-Naggar MM, El-Far M, Ramadan M, Salah N. Amelioration of radiation-induced oxidative stress and biochemical alteration by SOD model compounds in pre-treated γ-irradiated rats. Clin Chim Acta 2003; 337: 23-33.
- McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci 1991; 11: 381-391.
- Bottger P, Tracz Z, Heuck A, Nissen P, Romero-Ramos M, Lykke-Hartmann K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol 2011; 519: 376-404.
- Holm TH, Lykke-Hartmann K. Insights into the pathology of the a3 Na+/K+-ATPase ion pump in neurological disorders; lessons from animal models. Front Physiol 2016; 7: 209. doi: 10.3389/fphys.2016.00209
- Zahler R, Zhang Z-T, Manor M, Boron WF. Sodium kinetics of Na,K-ATPase a isoforms in intact transfected HeLa cells. J Gen Physiol 1997; 110: 201-213.
- Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci 2005; 10: 2373-2396.
- De Lores Arnaiz GR, Ordieres MG. Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 2014; 10: 85-102.
- Friedrich T, Tavraz NN, Junghans C. ATP1A2 mutations in migraine: seeing through the facets of an ion pump onto the neurobiology of disease. Front Physiol 2016; 7: 239. doi: 10.3389/fphys.2016.00239
- Hertz L, Chen Y. Importance of astrocytes for potassium ion (K+) homeostasis in brain and glial effects of K+ and its transporters on learning. Neurosci Biobehav Rev 2016; 71: 484-505.
- Shrivastava AN, Triller A, Melki R. Cell biology and dynamics of neuronal Na+/K+-ATPase in health and diseases. Neuropharmacology 2018; Dec 11: S0028-3908(18)30907-9. doi: 10.1016/j.neuropharm.2018.12.008
- Yoneoka Y, Satoh M, Akiyama K, Sano K, Fujii Y, Tanaka R. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol 1999; 72: 1196-1201.
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2010; 2: 12. doi: 10.3389/fnagi.2010.00012
- Beckhauser TF, Francis-Oliveira J, de Pasquale R. Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. J Exp Neurosci 2106; 10 (Suppl. 1): 23-48.
A c c e p t e d : August 28, 2019