RAPID BLOOD PRESSURE INCREASE AFTER RENAL DENERVATION
IN ANAESTHETIZED RATS: INTERACTION OF OXIDATIVE STRESS
AND NEURONAL NITRIC OXIDE SYNTHASE
2Institute of Physiology, Academy of Sciences of the Czech Republic, Prague, Czech Republic
INTRODUCTION
Both hormonal and neural stimuli generated in the kidneys contribute to the control of arterial blood pressure (BP). Long-term effects of renal denervation (DNX) commonly include a decrease in the blood pressure observed in normotensive animals, various models of hypertension and in patients with resistant form of the disease (1-4). On the other hand, we showed previously that in anaesthetized Wistar rats acute noninvasive renal denervation (DNX) induced an early increase in BP, which was highly pronounced in animals on HS diet (5). Remarkably, selective afferent renal nerve ablation was repeatedly shown to evoke BP elevation in Sprague-Dawley rats on high sodium diet (6, 7). The demonstration in our earlier study that hypertensive response to DNX was prevented by scavenging reactive oxygen species (ROS) with Tempol focused our attention on the possible critical mediatory role of the oxidative stress. A similar preventive effectiveness of nNOS blockade with N(omega)-propyl-L-arginine (L-NPA) might also be compatible with this role, given the known ROS generation by the uncoupled form of this isoform (8, 9). However, considering the inhibitory action of nNOS-derived NO on the sympatho-excitatory neurons of the hypothalamic paraventricular nucleus (PVN) and the rostral ventrolateral medulla (RVLM), brain centers controlling the systemic vascular tone (10), more direct NO actions, unrelated to the control of the oxidative stress level are also probable.
In this study we aimed to explore more thoroughly the role of oxidative stress in the hypertensive response to DNX by testing the action thereon of 4-hydroxy-3-methoxyacetophenone (apocynin, APO), a drug which inhibits NADPH oxidase, the major source of ROS (11, 12). To explore in more depth the mechanisms of different DNX effects on STD and HS diets (which would determine the renin-angiotensin system status) we decided to eliminate NADPH oxidases, the only source of ROS that is known to be stimulated by angiotensin II (13, 14). Notably, APO acts also as an antioxidant via NADPH-independent pathways (15) and anti-hypertensive properties of APO were recently reviewed in detail (16). We hypothesized also that a combination of APO and L-NPA pretreatment would have at least partially additive effect and reverse the post-DNX response to result in a distinct BP decrease, perhaps irrespective of the diet. Given the vast evidence that the state of the oxidative stress and the activity of nNOS-derived nitric oxide in the kidney can influence kidney microcirculation, especially in the medulla (17-19), in addition to blood pressure and laser-Doppler measurements of intrarenal haemodynamics, we determined effects of nNOS inhibition on the renal NO and ROS content.
MATERIALS AND METODS
Animals and diet
The experimental procedures were approved by the First Ethical Committee for Animal Experimentation, Faculty of Biology University Warsaw (No. 454/2017), which follows the European Directive 2010/63/EU on the protection of animals used for scientific purposes. Male Wistar rats (283 – 317 g) bred and housed in our animal facility (the Wistar stock bred in Mossakowski Medical Research Centre since 1996). The rats allowed free access to food and tap water were kept on standard (STD, 0.25% Na w/w) or on high-sodium diet (HS, 4% Na, w/w, SSNIFF GmbH, Soest, Germany) for 21 days.
For two days preceding the acute experiments, the rats of groups 2, 4 and 6 (see below) were pre-treated with apocynin (acetovanillon, Sigma-Aldrich, Basel, Switzerland), an inhibitor of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, given daily as intraperitoneal injections at 20 mg/kg.
Surgical preparations
Rats were anaesthetized with intraperitoneal sodium thiopental (Sandoz GmbH, Kundl, Austria), 80 mg/kg. A heated surgery table enabled the maintenance of rectal temperature at about 37ºC. A tracheal tube ensured free airways. The jugular vein and carotid artery were cannulated for fluid infusions and mean arterial blood pressure (MABP) measurement (Stoelting blood pressure meter and transducers, Wood Dale, Illinois, USA), respectively. During surgery, 3% bovine serum albumin solution was infused intravenously (i.v.) at 3 ml/h to sustain plasma volume.
The right kidney was accessed by a flank incision. A thin stainless steel wire loop was placed around the fat and connective tissue located cephalad to the right renal artery-aortic junction; it encompassed most of renal nerve fibres travelling from the coeliac ganglion to the kidney. The loop was later used for right-side denervation (DNX). Similarly, another wire loop was prepared for the left kidney denervation.
In the immobilized left kidney laser-Doppler probes (Perimed AB, Jarfalla, Sweden) were placed for the measurement of blood perfusion of the renal cortex (CBF), and outer and inner medulla (OMBF, IMBF). The details of this measurement technique were described previously (5).
Protocols of acute experiments
After the placement of the flow probes in the left kidney and stabilization of the parameters, control values were recorded for 30 min. In the groups 3, 4 and 7 (see below), after the usual control, the rats received N(omega)-propyl-L-arginine (L-NPA, Tocris, UK), a selective inhibitor of neuronal nitric oxide synthase (nNOS). Intravenous L-NPA infusion was started 30 min before the first denervation and continued till the end of the experiment, infused i.v. at 1 mg/kg/h (5, 18).
Subsequently, the right-side DNX was performed. Briefly, the wire loop was tightened and the tissue within was cauterized using a 5 MHz current generated for 5 – 10 s using a electrosurgery device (BonART, New Taipei City, Taiwan). Five minutes later a 30-min experimental and measurement period was made. Thereafter left DNX was performed, followed by another 30-min observation period. The effectiveness of this denervation technique was repeatedly confirmed previously by demonstration of the depletion of renal tissue noradrenaline content to < 5% of control and by demonstration of an acute rapid increase in cortical blood flow and of distinct denervation natriuresis (2, 20). The extent of NA decrease was found similar with our methodology (< 5%) and the classical renal denervation method (cutting renal nerves running along the renal artery plus application of phenol onto the artery wall) (20).
This basic protocol was applied in eight experimental groups:
- untreated rats maintained on standard diet (STD, n = 8);
- apocynin-pretreated rats maintained on standard diet (STD + APO, n = 7);
- rats receiving NPA, maintained on standard diet (STD + L-NPA, n = 7);
- apocynin-pretreated rats, maintained on standard diet and receiving NPA in acute experiment (STD + APO + L-NPA, n = 5);
- untreated rats maintained on high sodium diet (n = 9);
- apocynin-pretreated rats maintained on high sodium diet (HS + APO, n = 9);
- rats receiving NPA, maintained on high sodium diet (HS + L-NPA, n = 9);
- apocynin-pretreated rats, maintained on high sodium diet and receiving NPA in acute experiment, (HS + APO + L-NPA, n = 6).
Nitric oxide and reactive oxygen species measurements in the renal medulla
For measurement of the medullary NO and ROS (H202) signals, a needle-shaped ISO-NOP 200 sensor (0.2 mm in diameter) and HPO 100 sensor (0.1 mm diameter, calibrated by the manufacturer), connected with Free Radical Analyzer TBR4100 (World Precision Instruments, Sarasota, FL, USA), were inserted to the depth of 5 mm. To verify in vitro the responsiveness of the NO sensor, calibration curves relating the readings (nA) to the known increasing concentrations of NO released from S-nitroso-N-acetyl-D,L-penicillamine (SNAP) were established as recommended by the manufacturer of the equipment and described in detail by Zhang and Broderick (21). In vivo studies confirmed a dose-dependent decrease in tissue NO signal (expressed in nA), equivalent to NO bioavailability, in response to intravenous administration of L-NAME, and an increase in NO after renal artery infusion of SNAP, in agreement with an earlier report from this laboratory (22).
Statistical analysis
Data are expressed as means ± SEM. Significance of changes within one group over time was first evaluated by the repeated measures analysis of variance (ANOVA), followed by Student t-test for dependent variables. Differences between groups were first analyzed by the classical one-way ANOVA followed by two-sided modified Student t-test for independent variables, using Bonferroni correction for multiple comparisons. P ≤ 0.05 was taken to indicate significance of differences.
RESULTS
Preliminary evaluation of the baseline data (pooled from control periods of all groups) showed that APO pretreatment affected blood pressure. On standard diet (STD) MABP was significantly lower in APO-pretreated rats (110 ± 2 mmHg, n = 16) compared to 119 ± 2 mmHg measured in untreated rats (n = 12, P = 0.003). On high-salt diet (HS) the respective values were 118 ± 4 (n = 15) in the APO and 123 ± 5 mmHg in the untreated group (n = 16, difference NS, P = 0.42). The slight HS-dependent blood pressure elevation in untreated Wistar rats was not very surprising, indeed, high salt intake was recently reported to result in hypertension in this strain (19, 23).
There were no consistent or meaningful between-group differences in baseline renal haemodynamic parameters.
Effects of L-NPA in rats on STD and HS diet, untreated or pretreated with APO, are shown in Table 1. It will be noticed that baseline MABP (pooled data for control and L-NPA treatment) was not significantly higher on HS than on STD diet (123 ± 5 versus –119 ± 2 mmHg (NS). On STD diet baseline (before L-NPA) renal haemodynamic parameters (CBF, OMBF, IMBF) at least tended to be lower in APO-pretreated rats (–21, –44 and –21%, respectively). By contrast, on HS diet these parameters tended to be higher after APO treatment (+14, +6 and +37%, respectively). Thus, on the standard diet APO tended to decrease while on high-salt diet it tended to increase renal perfusion, which indicates that vasomotor responses to APO may depend on body sodium and fluid status. L-NPA significantly increased CBF only in rats on STD diet, this was seen in both untreated (+7%) and APO-pretreated animals (+5%). In rats untreated with APO on STD and HS diet L-NPA increased IMBF, by 7% and 14%, respectively.
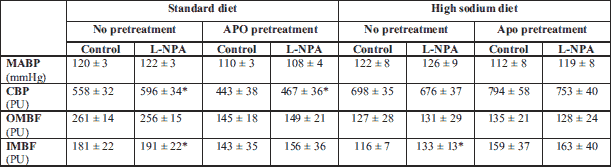
Effects of sequential right and left kidney denervation on MABP in rats maintained on standard (STD) or high salt diet (HS) are shown in Fig. 1. In untreated rats on STD, bilateral DNX caused a 6% blood pressure increase (P = 0.0001) compared to a 15% increase (P = 0.01) on HS diet; in the latter group the increase was significant already after unilateral (right-side) denervation. The HS versus STD group difference in MABP increases was significant (P = 0.002).
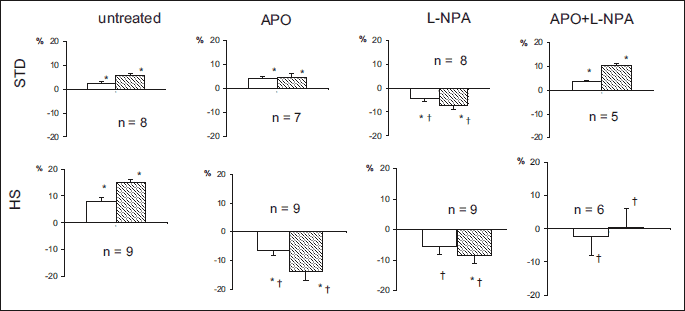
The effects of treatments (APO, L-NPA) on MABP responses were different in STD and HS groups. In STD rats APO or combined APO and L-NPA treatment did not significantly alter post-DNX MABP increases. In contrast, the treatment with L-NPA alone reversed the response and a significant 7% decrease in MABP occurred. Interestingly, in parallel, CBF tended to increase, from 596 ± 34 to 630 ± 36 perfusion units (PU; +6%, P = 0.05, data not shown).
In HS rats each of the three treatment regimes (APO, L-NPA, L-NPA + APO) abolished post-DNX increase in MABP; APO or L-NPA given alone reversed the response and significant decreases of 14% and 8%, respectively, were seen (Fig. 1). The changes in CBF, OMBF and IMBF observed on either diet in the four experimental groups were not sufficiently robust and consistent (even though some were significant) to allow the reliable interpretation.
Effects of a separate experimental series designed to explore the effects of L-NPA on NO and ROS content in the renal medulla of rats on STD or HS diet (n = 5 in either group) are shown in the Fig. 2. Under baseline conditions NO tended to be slightly lower (–7%) in HS than in STD rats whereas ROS were about 16% higher in the former group (differences not significant). In STD rats the renal NO bioavailability increased after L-NPA (in four out of 5 animals); in the HS group the delta values were greatly scattered and inconsistent. L-NPA failed to visibly affect renal tissue ROS levels.
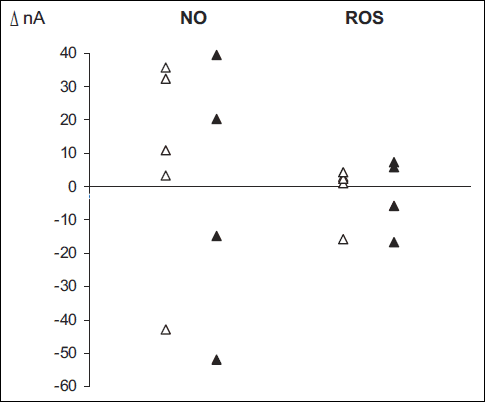 |
Fig. 2. The changes (Δ, nA) in NO and ROS in the response to L-NPA as observed in rats on standard (blank symbols) or high salt diet (black symbols). N = 5 in either diet group. The measurements were taken after i.v. L-NPA infusion (1 mg/kg/h). |
DISCUSSION
We showed previously that in anaesthetized Wistar rats acute noninvasive renal denervation (DNX) induced an early increase in MABP, which was minor on STD but substantially pronounced in animals on HS diet (5). Both on HS and STD diet the effect of DNX was abolished by blockade of nNOS whereas reduction of body oxidative stress using Tempol did that in HS group only. Given the vast evidence that DNX usually induces a long term decrease in MABP (1, 2, 24), these findings might appear surprising and counter-intuitive, and since their interpretation was not entirely straightforward, we decided to extend the studies by applying a presumably more powerful pharmacological measure, intended to block the oxidative stress-related MABP increase after DNX.
In the present study using the same basic experimental set-up we were able, first, to reproduce the phenomenon of the early paradoxical post-DNX increase in MABP, most conspicuous on HS diet, and, second, we confirmed the blocking of this effect by nNOS inhibition, in both the HS and STD group.
More important was the present novel finding that the blocking effect of APO, a powerful inhibitor of superoxide anion generation by NADH(P) oxidase complex (NOX), on post-DNX pressure increase was, as was earlier the case with Tempol (5), demonstrable in HS rats only. Remarkably, this was so even though our present pooled baseline data (before DNX) showed that APO per se decreased MABP more and significantly so in STD diet rats. However, it will be noticed that on STD diet the circulating angiotensin II level was higher, and so was the NADPH oxidase activity and systemic ROS levels, therefore inhibition of the oxidase activity and elimination of vasoconstrictor ROS might lead to a blood pressure decrease. Moreover, it can be speculated that the decrease was due to the well recognized vasodepressor and hypotensive APO action unrelated to inhibition of NADPH oxidase (12, 15, 16). Taken together, the earlier and the novel data suggest very strongly that the major mechanisms underlying the modest post-DNX increase in MABP in STD rats and the respective pronounced increase in HS rats may not be the same.
Apparently, in HS rats with presumably more pronounced baseline oxidative stress a reduction of ROS, either by their scavenging using Tempol or by inhibiting their generation using APO, was a measure sufficient to prevent and even to reverse post-DNX increase in MABP. A similar effect of the pretreatments with two drugs with a different mechanism of action but a similar final result indicates that in HS rats the necessary background for the MABP increase was the state of oxidative stress. As discussed by us earlier (5) there is a prevailing experimental evidence that, under baseline conditions of systemic and also intrarenal oxidative stress (as on HS diet), the impulse traffic ascending along renal afferents inhibits the brain cardiovascular centres; admittedly, some evidence for the stimulatory action has also been reported (10).
The afferent neural inhibition of the brain centres prevents their excessive sympatho-excitatory activity which would normally cause vasoconstriction and blood pressure elevation. Total DNX and especially the de-afferentation component eliminates the described inhibitory traffic: this results in an increased sympatho-excitation of systemic resistance vessels and MABP elevation. Thus, the baseline conditions necessary for the post-DNX pressor response is the systemic and intrarenal oxidative stress, a consequence of high salt intake. Why was L-NPA a “universal” blocker of post-DNX pressor response, effective both on HS and STD diet, in the latter case perhaps in the absence of major oxidative stress?
On the whole, it is known that oxidative stress (as on HS diet) stimulates sympathetic vasoconstrictor outflow. Moreover, effects of nNOS are different under normal conditions as compared with the state of oxidative stress. We propose that in the former situation nNOS-derived NO inhibits the vasomotor centres whereas in oxidative stress, due to uncoupling of nNOS molecule, it can aggravate the stress and increase the vasoconstrictor outflow from the brain centres. Therefore, in the latter situation, earlier inhibition of nNOS would decrease or abolish the usual vasopressor effect of DNX observed without the treatment. Notably, as recently pointed out (15), in the absence of detailed in vivo data on the activity of reactive oxygen and nitrogen species at functionally critical sites in the body, due to their variable intracellular localization and their instability, the exact nature and direction of the reactions are hardly predictable.
Furthermore, why Tempol in the earlier and APO in the present study reversed the pressure response to DNX (a decrease was seen) in HS rats only whereas in both studies L-NPA clearly and significantly reversed this response both in STD and HS rats, thus, in the former group presumably in the absence of major oxidative stress? Our finding is in agreement with the observation that in Wistar rats the blockade of nNOS was found to increase the renal sympathetic nerve activity both on STD and HS diet, by 16 and 24%, respectively (our unpublished data) so it can be expected that under L-NPA treatment the DNX would induce a pressure decrease.
The earlier and present data jointly suggest that the L-NPA dependent reversal of pressure response to DNX is not directly related to the alleviation of intrarenal, brain or systemic oxidative stress. It will be noticed that, at least for the kidney we failed to show here any consistent effect of L-NPA on tissue ROS content (Fig. 2). More probably, the cause of the “reversal phenomenon” was elimination of the inhibitory action of NO on the vasomotor activity of brain vasomotor centres, such as rostral ventrolateral medulla (RVLM), and of the consequent increased vasomotor output. Notably, nNOS is the main source of NO in the brain (25), in contrast to the prevalence of eNOS at the peripheral vasculature. Briefly, we provide here good evidence suggesting that L-NPA reduced NO release in the brain centres, which largely eliminated their blocking action on the sympathetic output responsible for peripheral vasoconstriction. This was translated into an increased (perhaps close to maximal) baseline tone of resistance vessels. The problem with this interpretation is that the MABP increase seen after L-NPA was very slight (Table 1). It will be suspected, however, that within only 30 min of observation the L-NPA dependent changes may have not yet developed. An obvious limitation of this functional study is the lack of information on the actual expression and activity of nNOS (and the degree of its uncoupling) and NADPH oxidase at critical sites, such as the kidney and brain centres. The novelty of the present study is the observation that APO blockade was as effective as general ROS scavenging, which indicates that their generation by NADPH oxidase was responsible for the role of the oxidative stress in the paradoxical response to DNX.
The reason why the actual reversal (not just abolishment) of the post-DNX pressure change (a significant decrease) was seen in both STD and HS rats treated with L-NPA, and only in HS rats pretreated with APO is unclear. It will be recalled, however, that individual afferent renal fibers might conduct either inhibitory or excitatory signals to brain centers (5, 10) so that the final resultant effect of de-afferentation is difficult to predict.
Given the presumably different mechanisms of prevention of the post-DNX pressure increase by APO and L-NPA, it was expected that combination of the two drugs would enhance the preventive action and actually alter the response and cause a major pressure decrease. This did not occur: in STD rats the combined treatment did not affect the pressure increase at all and in HS rats the increase was abolished but no reversal of the response occurred, in contrast to what was seen with APO or
L-NPA given alone. Apparently, the drugs in some way opposed each other’s action. In search for the explanation of this puzzling finding, some pharmacological or pharmacodynamic interference of the two drugs cannot be dismissed. However, a functional mechanistic background of the opposed effects of L-NPA and APO should also be considered, especially in view of the evidence on the antioxidant action of APO independent of inhibition of ROS generation by NOX (15, 16). It was also recently reported that in peripheral vasculature of spontaneously hypertensive rats, APO increased NO bioavailability via direct increasing of local eNOS expression (26). We could assume that the puzzling antagonism of APO and L-NPA action is based on their interaction at the level of the brain. The nNOS-derived NO normally inhibits the sympathoexcitatory vasomotor brain activity (27, 28) which would therefore increase after L-NPA. The other determinant of the vasomotor activity of the brain centres is the local oxidative stress, therefore APO treatment would decrease this activity: a change opposite to that of L-NPA. Possibly, in STD rats the two opposed effects are in balance, hence the post-DNX pressure response is not altered. On HS diet an imbalance of the two effects might occur, especially on the kidney level, followed by a modification of the afferent pulse traffic, which ultimately resulted in an abolishment of the post-DNX pressure increase.
The complexity of the results and interpretation is further visualized by our measurements of renal tissue NO responses to the inhibition of nNOS. While L-NPA treatment should be followed by a decrease in medullary tissue NO bioavalibility, a tendency to an increase was actually observed. This may have been due to the phenomenon of nNOS uncoupling, likely to occur in the hypoxic renal medulla. The uncoupled synthase generates O2– (superoxide) instead of NO. This one, in turn can combine with NO generated to yield ONOO– (peroxynitrite). Therefore, the blockade of nNOS can indeed increase the amount of NO. On the other hand, against expectation, we failed to show here any consistent effect of L-NPA on tissue ROS content.
The findings provide new insights and a starting point for a more detailed research needed to explain the complex mechanisms of interplay of NO availability and oxidative stress, especially in the brain centres engaged in the control of systemic vascular resistance and blood pressure. Examination of the vascular and pressure response to DNX provides here a useful in vivo model. It will perhaps be worthwhile to watch for possible early increase in blood pressure in patients with resistant hypertension subjected to catheter-based renal denervation treatment procedure (29). One should be aware that the early pressure increase might present an additional risk of such treatment.
Acknowledgements: The study was supported in part by the Polish-Czech Joint Research Project (Mobility Project PAN-17-13 financed by Polish Academy of Sciences and the Czech Academy of Sciences).
Conflict of interests: None declared.
REFERENCES
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997; 77: 75-197.
- Kompanowska-Jezierska E, Wolff H, Kuczeriszka M, Gramsbergen JB, Walkowska A, Johns EJ, Bie P. Renal nerves and nNOS: Role in natriuresis of acute sodium loading in conscious rats. Am J Physiol Regul Integr Comp Physiol 2008; 94: 1130-1139.
- Kline RL. Renal nerves and experimental hypertension: evidence and controversy. Can J Physiol Pharmacol 1987; 65: 1540-1547.
- Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61: 457-464.
- Walkowska A, Sadowski J, Kompanowska-Jezierska E. Oxidative stress and neuronal NOS activity: putative determinants of rapid blood pressure increase after renal denervation in anesthetized rats. Physiol Res 2013; 62: 257-266.
- Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 2003; 42: 968-973.
- Wainford R, Carmichael C, Walsh K. Afferent renal nerve modulation of sodium homeostasis and blood pressure: a sodium sensitive PVN Gαi2 protein dependent mechanism countering the development of salt-sensitive hypertension? [abstract] J Hypertens 2016; 34 (Suppl. 1): 74.
- Sun, J, Druhan, LJ, Zweiter, JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys 2008; 41: 126-133.
- Ihara H, Kitamura A, Kasamatsu S, et al. Superoxide generation from nNOS splice variants and its potential involvement in redox signal regulation. Biochem J 2017; 474: 1149-1162.
- Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev 1991; 71: 659-682.
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86: 494-501.
- Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 2010; 342: 325-339.
- Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 2016; 25: 119-146.
- Chen F, Haigh S, Barman S, Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol 2012; 3: 412. doi: 10.3389/fphys.2012.00412
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008; 51: 211-217.
- Virdis A, Gesi M, Taddei S. Impact of apocynin on vascular disease in hypertension. Vascul Pharmacol 2016; 87: 1-5. doi: 10.1016/j.vph.2016.08.006
- Cowley AW Jr., Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 2015; 308: F179-F197.
- Kakoki M, Zou AP, Mattson DL. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am J Physiol Regul Integr Comp Physiol 2001; 281: R91-R97.
- Kuczeriszka M, Walkowska A, Olszynski KH, Rafalowska J, Sadowski J, Kompanowska-Jezierska E. Arginine and tetrahydrobiopterin supplementation in rats with salt-induced blood pressure increase: minor hypotensive effect but improvement of renal haemodynamics. J Physiol Pharmacol 2019; 70: 219-227.
- Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: natriuresis and increased cortical blood flow. J Physiol 2001; 531: 527-534.
- Zhang X, Broderick M. Amperometric detection of nitric oxide. Mod Asp Immunobiol 2000; 1: 160-165.
- Grzelec-Mojzesowicz M, Sadowski J. Renal tissue NO and intrarenal haemodynamics during experimental variations of NO content in anaesthetised rats. J Physiol Pharmacol 2007; 58: 149-163.
- Stepien M, Kujawska-Luczak M, Szulinska M, et al. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in an NaCl-induced hypertensive rat model. J Physiol Pharmacol 2018; 69: 275-282.
- Jacob F, LaBine, BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: role of beta1-adrenoceptor activity. Clin Exp Pharmacol Physiol 2005; 32: 255-262.
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990; 347: 768-770.
- Perassa LA, Graton ME, Potje SR, et al. Apocynin reduces blood pressure and restores the proper function of vascular endothelium in SHR. Vascul Pharmacol 2016; 87: 38-48.
- Horn T, Smith PM, McLaughlin BE, et al. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol 1994; 266: R306-R313.
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol 1998; 275: R728-R734.
- Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; 391(10137): 2346-2355.
A c c e p t e d : August 28, 2019