BIOMARKERS OF PEDIATRIC OBSTRUCTIVE SLEEP APNEA SYNDROME AND THE ASSESSMENT OF QUALITY OF LIFE BEFORE AND AFTER ADENOTONSILLECTOMY
INTRODUCTION
Upper airway obstruction during sleep is the hallmark of obstructive sleep apnea. In this condition, nasal obstruction accompanied by mouth breathing is caused by local anatomic features like septum deviation, maxillofacial deformities, chronic nasal inflammation due to allergic or non-allergic causes, or by obesity (1, 2). In children, adenoid and tonsillar hypertrophy are the most frequent causes for upper airways obstruction which often develops after recurrent respiratory tract infections (3, 4) with inflammation and proliferation of adenoid and tonsil lymphoid tissue (5). If left untreated, it can contribute to the onset or worsening of sleep disorders, including obstructive sleep apnea (6), so, adenotonsillectomy (AT) is the first line treatment for pediatric obstructive sleep apnea syndrome (OSAS).
OSAS affects up to 4% of all children (7), and is characterized by repeated episodes of partial (hypopnea) or complete (apnea) collapse of the upper airways during sleep, associated with episodes of hypoxia and hypercapnia, with gas exchange abnormalities which lead to sleep fragmentation through repeated awakenings to restore the upper airway permeability (7, 8). The cycles of hypoxia/reoxigenation promote oxidative stress with generation of reactive oxygen species (ROS) which, besides the persistent local inflammation, induce a systemic inflammatory response with the production of pro-inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-1 (9). In addition, persistent inflammation can further contribute to oxidative stress via the increased release of ROS (9, 10), by activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) expression, one of the most important sources of free reactive radicals (11). NOX2 expression and ROS production have been shown to be increased in children with OSAS, with a significant decrease in their level after adenotonsillectomy (11). NOX2 is also responsible for endothelial dysfunction, thus contributing to the development of cardiovascular complication of OSAS (11). ROS initiates lipid peroxidation with degradation of polyunsaturated fatty acids to malondialdehyde (MDA), the most known end product of lipid peroxidation (10). Also, an altered expression of matrix metalloproteases (MMPs) and their tissue inhibitors (TIMPs) was observed in subjects with OSAS, MMP-2 and MMP-9 being involved in the atherosclerotic lesion development and progression (12).
It was demonstrated that cytokines such as TNF-α, IL-6 and IL-1 have an important role in sleep regulation (13, 14). Also, elevated levels of pro-inflammatory cytokines and free radicals were found in patients with OSAS in a severity-dependent manner, with a reduction of their levels in patients undergoing continuous positive airway pressure therapy (CPAP) (15-17). Besides children, adults with OSAS have other comorbidities associated, such as: obesity, diabetes, hypertension, some being smokers. These can contribute, independently or simultaneously, to systemic inflammation (18) and the generation of ROS (10).
Sleep fragmentation with obstructive events, systemic inflammation, and oxidative stress will have negative consequences on patient health, including pulmonary, cardiovascular, metabolic dysfunctions (19, 20), and even neurocognitive and behavioral disorders (21). Therefore, early diagnosis of OSAS in children and appropriate treatment is necessary to prevent complications and favor their growth and harmonious development.
Recognition of sleep-disordered breathing can often be difficult in children, as the clinical manifestations differ depending on age. Usually, these children are brought to the medical consult due to breathing difficulties during sleep, revealed by snoring, persistent mouth breathing, episodes of apnea, restless sleep with frequent awakenings, sweating, and even nightmares (21, 22). Children with OSAS often have difficulty waking up, with morning headaches and xerostomia and, additionally, due to upper airways obstruction, they usually have persistent mouth breathing (21). During the daytime, the symptoms are nonspecific and are manifested by behavioral disorders, which can range from hyperactivity, aggression, irritability, low tolerance to frustration, attention deficit and decreased school performance (21-23). Many times, children with OSAS may be misdiagnosed with attention-deficit/hyperactivity disorder (ADHD) (22, 24). In a meta-analysis, published in 2011, Youssef et al. highlighted the specific symptoms of ADHD in children with OSAS and the improvement of the symptoms after the specific treatment (25).
Based on these data, the present study aimed to analyze the association between the pro-inflammatory cytokines’ levels and oxidative stress markers in children with OSAS triggered by adenoids and tonsillar hypertrophy, and evaluate the influence of surgical treatment on them.
The study also assesses the existence of neurocognitive and behavioral disorders in OSAS patients, before and after adenoid and/or tonsils removal surgery, using a validated questionnaire on childhood sleep-disordered breathing.
MATERIALS AND METHODS
Study participants
The study was approved by the Ethical Committee of Emergency Hospital for Children Cluj-Napoca, Romania (no. 68SC/2019) and by the Clinical Research Ethics Committee of University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania (no. 64/2019). Written informed consent was obtained from both parents of all participants at the beginning of the assessment.
Thirty children, aged 3 to 13 years of both genders with sleep-disordered breathing due to adenoids and/or tonsillar hypertrophy, scheduled for AT at the Ear, Nose and Throat (ENT) Department of Emergency Hospital for Children, Cluj-Napoca, Romania, were recruited prospectively from 2019 to 2020. Children with acute respiratory diseases, allergies, or chronic medical conditions like diabetes, cardiac diseases, hypertension, chronic inflammatory diseases, psychiatric diseases or any genetic, neuromuscular and craniofacial syndrome were excluded from the study.
An additional 20 children without any complaints of upper airways obstruction sleep disturbances, acute or chronic infections or diseases, was considered the control group. They were tested for blood cytokine and oxidative stress markers, but did not undergo cardio-respiratory polygraphy.
Questionnaire evaluation
The parent of each subject completed a sleep-disordered breathing questionnaire containing questions about nighttime and daytime symptoms of OSAS before AT and 6 weeks after the surgery. The questionnaire developed by Prof. Dr. Ronald Chervin and his colleagues at the University of Michigan - „Pediatric Sleep Questionnaire: validity and reliability of scales for sleep-disordered breathing, snoring and behavioral problems“ was used (26). To be used in Romania, in 2014, a group of experts from pneumology, somnology, and pediatrics, translated the questionnaire and validate it (27). Before the study start, the consent of the author was obtained. The questionnaire has high sensitivity and specificity (81% and 87%, respectively) and is easy to apply. It contains 22 queries grouped on 3 sub-scales: snoring (9 questions), drowsiness (7 questions), and behavior (6 questions) which will be answered by “yes”, “no” and “don’t know”. The answers are marked as follows: yes = 1; no = 0; do not know = missing. The total score is calculated taking into account only the answers „yes“ and „no“. The numbers of “yes “answers are divided by the total number of ”yes” and “no” answers and the result is a ratio between 0.0 and 1.0. Subjects with a score of more than 0.33 were considered to have sleep-disordered breathing (27).
Anthropometry and physical assessment
A complete physical examination was performed to all subjects included in the study. The height and the weight of the subjects were measured with a calibrated stadiometer and a weighing scale. An ear, nose and throat examination was performed to assess the size of the adenoids (percentage occupying post-nasal space) and tonsils (Brodsky scale: 0 - tonsils located inside the tonsillar fossa, 1 - tonsils occupying less than 25% of the oropharynx, 2 - tonsils occupying from 25 – 50% of the oropharynx, 3 - tonsils occupying from 50 – 75% of the oropharynx, and 4 - tonsils occupying > 75% of the oropharynx) (28).
Sleep study
Overnight cardio-respiratory polygraphy was performed before and 6 weeks after AT, using Nox T3 polygraphy device (Nox Medical, USA). Respiratory events were scored using the standard criteria of the American Academy of Sleep Medicine. Obstructive apnea was defined as a reduction in airflow greater than 90% for at least two breath duration, with increased abdominal and thoracic movements, while hypopnea was defined as a reduction of airflow by 30%, associated with oxygen desaturation ≥ 3% (29). The diagnosis of OSAS was based on the index of apnea-hypopnea (AHI) results. AHI was calculated as the total number of episodes of apnea and hypopnea per hour of sleep of the total sleep time (TST). According to consensus guidelines (29), in children, an AHI ≥ 1/hTST is diagnostic of OSAS. An AHI ≥ 1 to < 5 indicated a mild OSAS, ≥ 5 to < 10 indicated moderate OSAS, and ≥ 10 indicated severe OSAS (29, 30). According to AHI value, patients were divided in three groups: in the first group, patients with mild OSAS, in the second group, patients with moderate OSAS, and in the third group, patients with severe OSAS.
Six weeks after adenotonsillectomy, a cardio-respiratory polygraphy was performed in OSAS patients in order to monitor the clinical evolution and to evaluate residual OSAS.
Blood and tissue collection
Blood samples of all patients and controls were obtained from the antecubital vein after topical anesthesia with lidocaine-prilocaine cream and stored into tubes containing ethylenediaminetetraacetic acid (EDTA). All samples were collected between 8.00 and 11.00 a.m. The blood was immediately centrifuged (2000 rpm) for 10 minutes, and stored at –20°C until determination. In patients’ group, the blood samples were collected before AT and 6 weeks after the surgery. Adenoidectomy, tonsillectomy or adeno-tonsillectomy was performed under general anesthesia in an operating room of ENT Department of Emergency Hospital for Children. After surgical removal, the tissue specimens were stored in a container and immediately frozen at –80°C until determination.
Detection of the inflammatory markers in the blood, adenoid and tonsils tissues
The pro-inflammatory cytokines TNF-α and IL-6 levels in the blood, adenoid and tonsils tissues were evaluated by ELISA assay according to the manufacturer’s protocol. ELISA tests were purchased from Elabscience (Houston, TX, USA). All chemicals and reagents were of high-grade purity. Results are expressed as pg/ml in serum or pg/mg protein in tissue homogenates.
The levels of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were also evaluated in the blood of all patients and were analyzed in the hospitals ‘central laboratories.
Oxidative stress markers
To quantify the oxidative stress, malondialdehyde levels were assessed in the blood, adenoid and tonsils tissues. Malondialdehyde was determined by the fluorimetric method with 2-thiobarbituric acid described by Conti (31). 2-thiobarbituric acid and Bradford reagent were purchased from Merck KGaA (Darmstadt, Germany). Malondialdehyde values are expressed as nmoles/ml in serum, or nmoles/mg protein in tissue homogenates. The systemic antioxidant defenses were evaluated in the blood by catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPX). SOD was determined using the cytochrome C reduction analysis (32) and catalase by method described by Pippenger et al. (33). GPX activity was evaluated by using the method published by Flohe and Gunzler (34) and protein levels with Bradford method (35). The results were expressed in U/mg protein.
Statistical analysis
Quantitative data were reported as median and IQR (interquartile range defined as the value of the first quartile to the value of the third quartile) since the groups (cases and controls) have up to most 30 subjects. Comparison between cases (OSAS) and controls was made with Mann-Whitney test while the comparison in the case group between before (baseline, variables identified with ‘bVariableAbb’ where Abb is the abbreviation) and after intervention was made with Wilcoxon matched pairs test. Kruskal-Wallis test was used to assess the differences between children with severe, moderate and mild OSAS. Qualitative data were reported as numbers and percentages and the differences between cases and controls were tested with Chi-Squared test or Fisher exact test. Spearman’s rank correlation coefficient was calculated to test the correlation between quantitative variables. Data analysis was made with Statistic program (v. 13, USA). A P-value less than 0.05 was considered statistically significant when cases were compared to controls and an adjustment was applied when OSAS severity classes were compared when a P-value less than 0.017 was considered statistically significant.
RESULTS
Fifty patients aged 3 to 13 years of both genders were included in this study: 30 children in the OSAS group and 20 children in the control group. Less than half children were girls and were similar in terms of age (Table 1). The children were younger in the mild OSAS group as compared to those with severe OSAS, but no statistically significant difference was observed between OSAS patients and the control group in terms of age (Kruskal-Wallis test: Statistics = 4.3; P-value = 0.1191). The groups were similar in terms of demographic characteristics as well as in regards of the presence of obesity (Table 1).
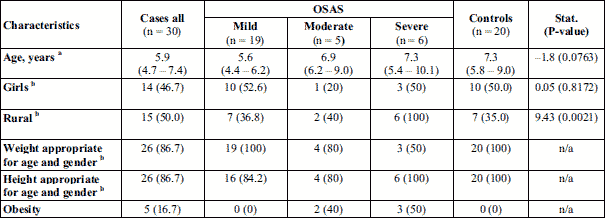
Adenoid hypertrophy was observed in 26 of all 30 patients with OSAS, 17 cases with mild OSAS, 5 with moderate, and 4 with severe OSAS (Table 2). Tonsillar hypertrophy was observed on 22 out of 30 patients in the case group, with 12 cases among those with mild OSAS, 4 with moderate, and 6 with severe OSAS (Table 2). Eighteen children presented both adenoid and tonsillar hypertrophy, 10 cases with mild OSAS, 4 with moderate, and 4 with severe OSAS (Table 2).
In children with adenoid or tonsillar hypertrophy the preoperative AHI score ranged between 1.9 and 14/hTST, while in children with adenotonsillar hypertrophy, AHI score ranged between 1.2 and 18.8/hTST (Fig. 1). A significant decrease in AHI score was observed in both groups of patients regardless the intervention (Fig. 1).
Regardless of the OSAS class, AHI decreases significantly after the intervention in most patients with OSAS, but they were part of the mild and moderate OSAS subgroups. In patients with severe OSAS, a slightly higher AHI value was maintained postoperatively, but much lower compared to the initial value (Fig. 2).
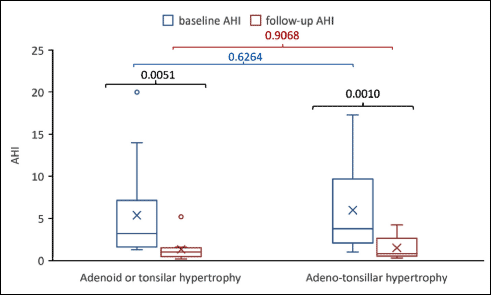 |
Fig. 1. Changes in AHI by intervention: baseline vs. follow-up. The P-values in black reflect the comparisons between baseline and follow-up in the same group. The P-value in blue compares the baseline value. The comparison of those with adenoid or tonsilar hypertrophy vs. those with adeno-tonsillar hypertrophy is given in blue for the baseline values and red for the follow-up values. The x in the graph is given by the mean value, the line in the box is given by the median value, the extreme of the box are given by the first and third quartile and the whiskers are the minimum and the maximum value. The ○ is an extreme value. |
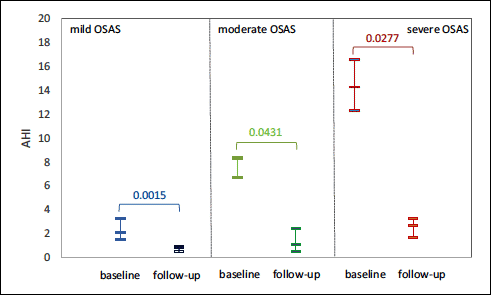 |
Fig. 2. Changes in AHI score after surgical intervention. The P-values correspond to the Wilcoxon matched pairs test (13 cases with baseline mild OSA, 5 with moderate OSA and 6 with severe OSA). The mid line is equal to the median and the extreme lines are given by the value of the first and third quartile. |
Serum oxidative stress assessment
Oxidative stress biomarkers (malondialdehyde, superoxide dismutase, catalase and glutathione peroxidase activities) were evaluated in all subjects included in the study. Malondialdehyde levels increased in patients compared to controls (median = 2.31 nmoles/ml, IQR = (1.94 to 2.92) versus median = 1.28 nmoles/ml, IQR = (1.03 to 1.47); P-value < 0.0001), and among them, higher values were recorded in patients with severe OSAS, suggesting the involvement of oxidative stress process is aggravating the disease (Fig. 3).
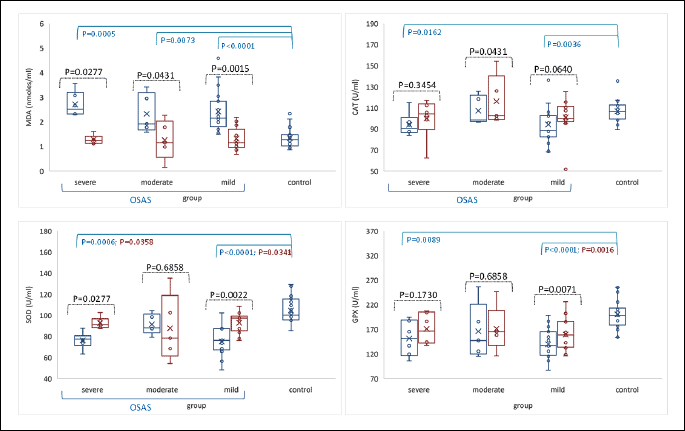
MDA, malondialdehyde; CAT, catalase; SOD, superoxide dismutase; GPX, glutathione peroxidase.
Antioxidant defense was quantified by the activity of CAT, SOD and GPX enzymes in erythrocyte lysate. CAT activity was significantly lower in patients than in controls (95.9, IQR = (83.59 to 102.53) versus median = 106.8 U/ml (99.4 to 112.03); P-value 0.0040). SOD (median = 78.29 U/ml, IQR = (68.59 to 87.54) versus median =100.17 U/ml, IQR = (96.4 to 114.87); P-value < 0.0001). GPX activity (median = 139.25 U/ml, IQR = (117.61 to 172.87) versus 198.45 U/ml, IQR = (184.53 to 211.35); P-value < 0.0001) decreased in OSAS patients compared to controls. These data suggest that antioxidant defense is impaired in patients with OSAS (Fig. 3). The analysis of oxidative stress markers in patients’ subgroups (mild, moderate and severe) showed that in mild OSAS the CAT, SOD and GPX activities were lower than in moderate and severe forms.
Six weeks postoperatively, a significant decrease of malondialdehyde level was observed (median = 1.18 nmoles/ml, IQR = (1.09 to 1.56), n = 24; P-value = 0.0029), in parallel with significant improvement of antioxidant enzymes activities (CAT: median = 102.31 U/ml, IQR = (98.52 to 112.37), n = 24; P-value = 0.0061; SOD: median = 92.77 U/ml, IQR = (86.32 to 99.01), n = 24; P-value = 0.0072, and GPX: 158.53 U/ml, IQR = (141.36 to 186.28), n = 24; P-value < 0.0001), (Fig. 3).
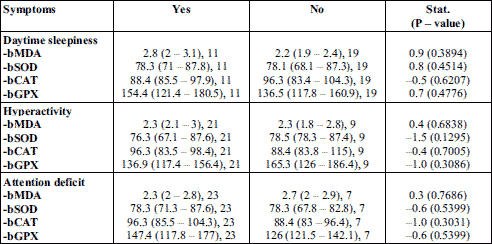
The baseline oxidative stress markers levels were compared with clinical parameters such as: daytime sleepiness, hyperactivity, and attention deficit. Malondialdehyde was increased in OSAS patients, higher values being observed in patients with daytime sleepiness. CAT activity was lower in patients having daytime sleepiness, but increased in those with hyperactivity and attention deficit. SOD activity did not change in both patient groups, except for a slightly decreased value in patients having hyperactivity. GPX activity increased in patients with daytime sleepiness and attention deficit, and decreased in group of patients having hyperactivity (Table 3).
Serum inflammatory markers assessment
Fig. 4 presents the comparison of preoperative and postoperative levels of pro-inflammatory markers of the study group and control subjects.
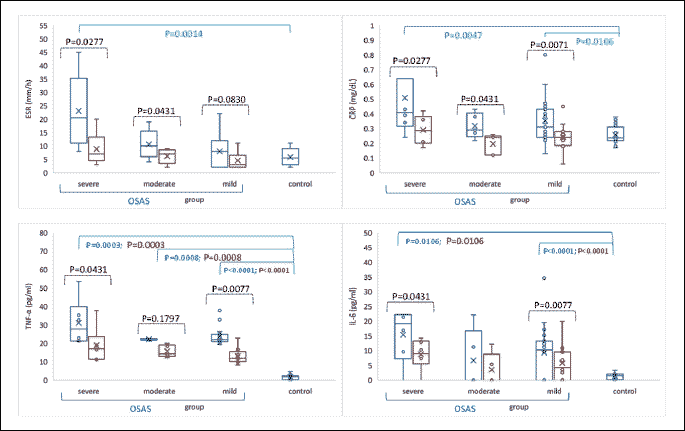
ESR levels increased significantly in OSAS patients as compared with the control group (median = 9.5 mm/h, IQR = (4 to 14.8), n = 30 versus median = 5.5 mm/h (3 to 9), n = 20; P-value = 0.0266). Children with severe OSAS had significantly higher ESR levels as compared to those with mild or moderate OSAS (Kruskal-Wallis test: Statistics = 7.6 P-value = 0.0222), (Fig. 4). A monotonic association has been identified between age and post-intervention ESR (n = 24, r = 0.45, P-value = 0.02802). Regarding CRP, the values were also significantly increased in OSAS patients compared to the control group (median = 0.34 mg/dl, IQR = (0.26 to 0.43) versus 0.24 mg/dl, IQR = (0.22 to 0.29); P-value = 0.0013), with higher values in severe OSAS patients (Fig. 4). TNF-α and IL-6 concentrations were significantly increased in OSAS patients (median = 22.35 pg/ml, IQR = (21.28 to 24.46) and median = 10.7 pg/ml (0 to 16.44), respectively) compared with the control group (median = 1.77 pg/ml, IQR = (0.67 to 2.2), P-value < 0.0001 and median = 1.44 pg/ml, IQR = (0.39 to 2.09), P-value < 0.0001), (Fig. 4). The analysis of cytokines in subgroups, mild, moderate and severe OSAS, showed elevated TNF-α and IL-6 level in patients with severe form of disease (Fig. 4).
Children with OSAS were reevaluated 6 weeks after AT. ESR and CRP values were significantly reduced in the study group (P-value = 0.0001) and also between different OSA classes. TNF-α and IL-6 levels also decreased significantly in OSAS patients after surgical treatment (P-value < 0.0001, respectively < 0.0004), (Fig. 4).
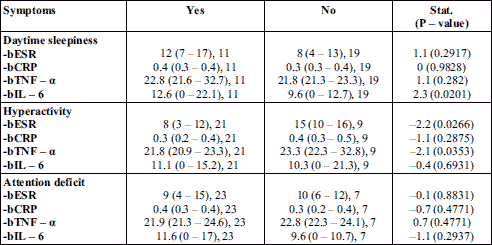
When comparing the baseline inflammatory markers levels with clinical parameters such as: daytime sleepiness, hyperactivity, and attention deficit, an increased IL-6 value was observed in patients with daytime sleepiness and attention deficit, as well as elevated TNF-α and ESR level in patients with daytime somnolence (Table 4).
The assessment of inflammatory cytokines and oxidative stress in adenoids and tonsil tissue
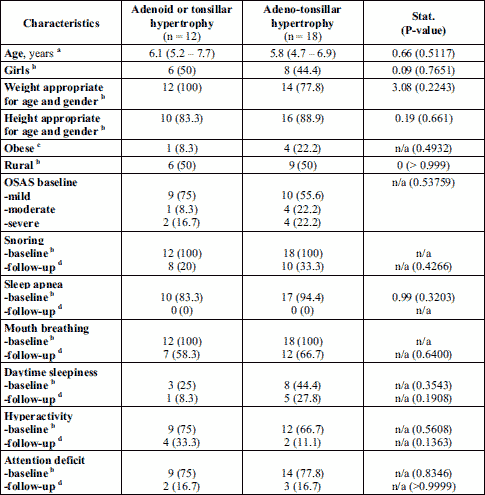
Of all the 30 children with OSAS, 18 children were operated for adenotonsillar hypertrophy and 12 children for adenoid or tonsillar hypertrophy.
The pro-inflammatory cytokines, TNF-α and IL-6 levels in tissue homogenates increased significantly in children with severe OSAS compared to children with mild and moderate disease, but without statistical significance (Table 5). Similarly, in tonsils tissue, TNF-α and IL-6 levels were also increased, but without statistical significance in children with severe OSAS than in patients with mild and moderate OSAS, (Table 5).
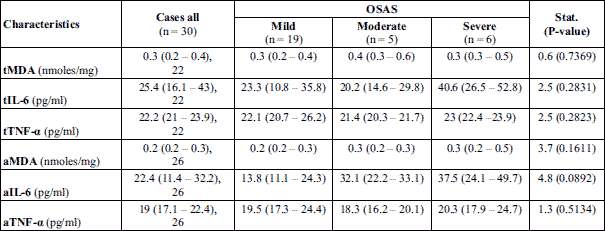
Malondialdehyde levels evaluated in tonsils and adenoid tissues in patients with OSAS showed an increased values, but without statistical significance between OSAS groups (Table 5).
Child’s behavior
Regarding the symptoms caused by OSAS, it was observed that snoring was present in all 30 patients included in the study, with significant improvement postoperatively. Agitated sleep due to episodes of apnea during sleep was seen in 27 of the 30 patients (Table 6). Postoperatively, no patient had sleep apnea with agitated sleep.
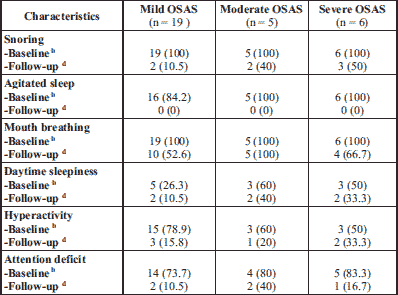
Oral breathing due to adenoids and tonsillar hypertrophy was present in all 30 patients. Postoperatively, oral breathing persisted in all patients with moderate OSAS. Ten patients with mild OSAS and 6 patients in the severe OSAS group still had mouth breathing.
Behavioral changes such as daytime sleepiness, hyperactivity and attention deficit were also assessed in patients with OSAS. Of these, hyperactivity (n = 21) and attention deficit (n = 23) were more frequently present than daytime sleepiness (n = 11). Postoperatively, a significant reduction in the number of patients with hyperactivity was observed (n = 15), most of them being in the group of patients with mild OSAS (n = 11). A significant reduction in the number of patients with attention deficit was also observed (n = 18) in all the three subgroups.
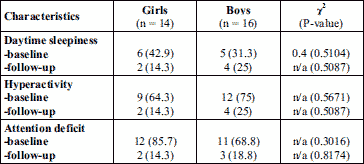
The preoperative symptoms as daytime sleepiness, hyperactivity and attention deficit of boys and girls in the patient group were compared. Thus, the boys were more hyperactive compared to girls, whereas the presence of daytime sleepiness and attention deficit of both sexes was similar (Table 7). Postoperatively, there were no significant differences between sexes.
DISCUSSIONS
Adenotonsillar hypertrophy is the main cause for obstructive sleep apnea syndrome (OSAS) in children. Polysomnography is the “gold standard” diagnostic test for OSAS, but because it is an expensive and a difficult method to apply to children, cardio-respiratory polygraphy could be a simple and a useful method for OSAS diagnosis (36). OSAS is characterized by recurrent hypoxemic episodes, overproduction of reactive oxygen species (ROS) and consecutive redox misbalance. Oxidative stress amplify the systemic inflammation trough up-regulation of pro-inflammatory cytokines with negative consequences on cardiovascular and pulmonary functions, and also on child’s growth with occurrence of neurocognitive impairment (19, 20).
The first aim of our pilot study was to evaluate the oxidative stress in childhood OSAS by measuring malondialdehyde (MDA) level, a lipid peroxidation marker, and the antioxidant enzymes activities. The redox imbalance was already demonstrated in adults with OSAS in a severity dependent manner (10, 37, 38). However, adults with OSAS have associated different comorbidities, such as obesity, diabetes, hypertension, which may contribute, independently or simultaneously, to systemic inflammation (18) and ROS generation (10).
Several studies investigated the relationship between OSAS and oxidative stress in children. Dogruer et al. found higher malondialdehyde levels in serum and increased antioxidant activity (SOD and GPX) in children with OSAS before AT, with a decrease of their value after AT (39). Tauman et al. showed an increased level of plasma oxidized low-density lipoprotein, a product of lipid peroxidation, in OSAS in children, in correlation with the severity of disease (10). Verhulst et al. measured uric acid, as a marker of tissue hypoxia in overweight and obese OSAS children, and found a significant correlation between uric acid levels and OSAS severity, independently of adiposity (40). Other results confirmed a significant correlation between uric acid concentration and the degree of hypoxia in obese children with OSAS, with a reduction in its levels following weight loss (41).
In our study, serum malondialdehyde levels increased significantly in OSAS patients compared to controls, especially in severe OSAS disease (Fig. 3), suggesting the involvement of oxidative stress in OSAS, even in the absence of associated comorbidities. High malondialdehyde levels has also been detected in adenoids and tonsils tissues, but with lower values than those in serum, suggesting that although adenotonsillar hypertrophy is the major cause of OSAS in children, the systemic changes in redox status have an important contribution to the onset of disease.
Antioxidant status quantified by CAT, SOD, and GPX enzymes activities was also evaluated (Fig. 3). SOD is the first line of enzymatic defense that catalysis the dismutation of the highly reactive superoxide anion (O) to hydrogen peroxide (H2O2), which further will be destroyed by CAT and GPX (42). The lower antioxidant enzymes activity in OSAS patients compared to healthy subjects suggested an impaired antioxidant defense in OSAS. The antioxidant enzymes activity in subgroups of OSAS displayed higher activity of SOD, CAT and GPX in patients with moderate and severe OSAS compared with those of mild form of disease. It seems that lipid peroxidation plays an important role in the pathogenesis of OSAS, and overproduction of ROS promotes oxidative stress that decreases the activity of antioxidant enzymes. In patients with severe hypoxia and high levels of free radicals, the antioxidant activity increase adaptively as a protection mechanism, to prevent important damages (43). Therefore, more and deeply studies are needed to clarify these aspects.
Several studies found high levels of cytokines in adult OSAS (14, 44) and pediatric OSAS patients (45-47). Therefore, the inflammatory markers (TNF-α, IL-6, ESR and CRP) were assessed in children with OSAS before and after AT (Fig. 4). Our findings are in agreement with other literature data (46, 47), which confirmed high secretion of IL-6 and TNF-α, as well as increase ESR and CRP, in OSAS patients. The inflammatory markers measured in subgroups of OSAS showed their growth in severe OSAS, accordingly to the study by Friedman et al. (48). These findings bring arguments for involvement of inflammation in aggravation of hypoxia during sleep.
In children with OSAS, high levels of IL-1β, IL-6, IL-8, TNF-α were found in tonsillar tissue (47, 49). Recent studies have demonstrated significant differences of cytokine levels from the upper airway tissues, between the mild and severe OSAS (50, 51). Thus, in our study, TNF-α and IL-6 increased significantly in severe OSAS subgroup (Table 5). Beside this, a systemic inflammation generated by OSAS is also associated, and together with local changes, increase the proliferation of upper airway lymphoid tissue and amplify the local inflammation inducing upper airway collapsibility (49). Our results confirmed the local and systemic activation of inflammatory response in OSAS patients, and the relationship between chronic nocturnal hypoxia and adenoid and/or tonsillar hypertrophy.
Six weeks after AT, children were reevaluated. The assessment of AHI is a useful criterion in evaluating the effectiveness of OSAS treatment (4, 36). AHI decreased significantly in patients with OSAS, most patients being in the mild and moderate OSAS subgroups (Fig. 2). In severe OSAS, a slightly higher AHI value was maintained postoperatively, but much lower compared to the initial. In addition, our results showed a significant reduction of lipid peroxidation after AT, with increased antioxidant activity, suggesting the implication of redox imbalance in OSAS (Fig. 3). Elevated results of OSAS-related inflammatory markers returned to the reference range of healthy subjects (P-value < 0.05), with the exception of TNF-α which remained slightly increased (Fig. 4). The explanation is related to the persistence of intermittent hypoxia during sleep in those with residual OSAS, which maintain chronic inflammation (47). In conclusion, AT can enhance significant healing of hypoxia and decrease the degree of systemic inflammation, and consequently diminish the risk of developing cardiovascular and pulmonary diseases.
Another objective of the study is to evaluate the symptoms related to adenotonsillar hypertrophy and OSAS, before and after adenoid and/or tonsils removal surgery.
In children, adenotonsillar hypertrophy is a risk factor for sleep-disordered breathing, with snoring, difficulty breathing, episodes of apnea, and persistent mouth breathing, which ranges from primary snoring, through upper airways resistance syndrome, and finally to OSAS (52). In the study, all children included in OSAS group had snoring, episodes of apnea with agitated sleep, and persistent mouth breathing (Table 2). Beside this, poor quality of sleep can trigger daytime behavioral problems, daytime sleepiness, emotional stress, hyperactivity and attention deficit with poor performance at kindergarten or at school (21, 52, 53). In children with adenotonsillar hypertrophy, these symptoms were more frequent compared to those with adenoids or tonsillar hypertrophy only.
It was demonstrated that oxidative stress is involved in the occurrence of some nervous system diseases (38, 54). Repeated processes of airway obstruction with apnea episodes during sleep of OSAS lead to nocturnal chronic intermittent hypoxia with overproduction of ROS, decline of antioxidant capacity, which promotes oxidative stress and inflammation with endothelial dysfunction (38, 54). Inflammatory cytokines, such as IL-1 and IL-6 can go through the blood-brain barrier, increasing the vagal activity and affect the central nervous system functionality (47). Moreover, chronic inflammation could induce and aggravate the neuroinflammation (47). So, the cerebral cortex and hippocampus are vulnerable to oxidative stress and inflammation, which induce brain dysfunction and neurocognitive alteration (38, 54).
The baseline oxidative stress and inflammatory markers levels were compared with daytime sleepiness, hyperactivity, and attention deficit. It was noticed a lower antioxidant activity (CAT and GPX) especially in patient with hyperactivity, in parallel with high values of inflammatory markers (ESR, TNF-α, and IL-6), and malondialdehyde in daytime sleepiness (Table 3 and 4). The association between daytime sleepiness and inflammatory cytokines, particularly TNF-α, has been demonstrated in OSAS patients (46, 55, 56). The results of our study confirmed that oxidative stress and inflammation can contribute to neurocognitive dysfunctions in OSAS.
Generally, the daytime sleepiness is a common clinical feature of OSAS in adults (55), but in our study, this symptom was present in 11 patients, 5 of them with mild form of disease. It seems that daytime sleepiness in children has different clinical manifestations compared to adults. Children may have hyperactivity, irritability, attention problems rather than taking naps or having unintentional sleep episodes (57).
Our study revealed the presence of hyperactivity and attention problems in children with OSAS, even in those with mild OSAS (Table 6). Boys were more hyperactive compared to girls, whereas the presence of daytime sleepiness and attention deficit of both sexes were very similar. Due to the behavioral problems related to sleep-disordered breathing, children are sometimes misdiagnosed with attention deficit/hyperactivity disorder (ADHD). Previous studies have shown that hypoxia in OSAS is related to ADHD, and that symptoms of ADHD are improved after OSAS treatment (25, 58).
Among the frequent symptoms caused by OSAS, snoring and persistent mouth breathing were present in all OSAS patients, and 27 of the 30 OSAS patients had agitated sleep due to repeated awakenings during night sleep to restore airways permeability. Six weeks after AT, no patient had episodes of sleep apnea or agitated sleep, and significant improvement, regarding behavioral was observed. The results were in agreement with other studies and confirmed a significant improvement in obstructive behavioral problems in children after AT (59). In a study that evaluated the changes in the quality of life of 63 children, aged 4 – 13 years, after AT for obstructive sleep apnea, significant improvement was reported regarding hyperactivity, attention deficit, and impulsivity (60). A study by Wei et al. included 117 with sleep-disordered breathing who underwent AT (61). They also used the Pediatric Sleep Questionnaire and another form, Conners’ Parent Rating Scale-Revised Short Form that were completed before and 6 months after AT. Both, sleep and behavior improved after the surgery. The authors concluded that both forms used are useful measures for screening and following children after AT (61).
Although we demonstrated significant improvements in obstructive symptoms in most of the children after AT, seven children continued to snore irregularly and half of all children continued to have residual mouth breathing. Data are showing that chronic mouth breathing may have an impact on orofacial growth with impairment of maxillomandibular development (62, 63). Breathing re-education and myofunctional therapy through daily exercise involving orofacial muscles and stimulation of sensory pathways helps to establish normal orofacial muscle tone in order to eliminate mouth breathing and reestablish nasal breathing (62, 63). In young school-aged children, oropharyngeal exercises performed after AT improved residual OSAS symptoms (62).
Although, our study included a small number of subjects, the data showed significant improvement of inflammatory cytokines, oxidative stress, sleep quality and behavior in patients undergoing adenotonsillectomy. However, the data at the limit of significance would probably have been significant if the group of patients had been larger. Therefore, more data collection and long-term follow-up are needed.
Our study demonstrated the increase of systemic nonspecific inflammation in children with OSAS, and the association of severe disease with important inflammation in adenoids and tonsils tissues and redox misbalance. AT diminished the systemic inflammatory response and oxidative stress and increased the antioxidant enzymes activities. The inflammation and oxidative stress associated with OSAS are reversible processes, and their recognition and treatment would help to reduce the risks and complications associated with OSAS.
Beside this, AT is associated with significant improvements of sleep quality and behavior in children with OSAS, even when the condition is mild. Although, our study includes a small number of patients and evaluated only few parameters for quantification of inflammation and oxidative stress, it brings arguments for the use of these markers in the preoperative and postoperative evaluation of OSAS patients.
Authors’ contributions: Study conception and design: C.N.T.-S. and G.A.F. Acquisition of data: C.N.T.-S. Analysis and interpretation of data: S.D.B and C.N.T.-S. Drafting of manuscript: C.N.T.-S. Critical revision: G.A.F., S.C.M., D.I. and P.C.P. Carrying out laboratory tests: N.D. Carrying out surgical intervention: G.M. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding: This study was supported by the financing project within the PhD School of “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Conflict of interests: None declared.
REFERENCES
- Magliulo G, Iannella G, Ciofalo A, et al. Nasal pathologies in patients with obstructive sleep apnoea. Acta Otorhinolaryngol Ital 2019; 39: 250-256.
- Cao Y, Wu S, Zhang L, Yang Y, Cao S, Li Q. Association of allergic rhinitis with obstructive sleep anea: a meta-analysis. Medicine (Baltimore) 2018; 97: e13783. doi: 10.1097/MD.0000000000013783
- Tagaya M, Nakata S, Yasuma F, et al. Relationship between adenoid size and severity of obstructive sleep apnea in preschool children. Int J Pediatr Otorhinolaryngol 2012; 76: 1827-1830.
- Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 2016; 47: 69-94.
- Bulfamante AM, Saibene AM, Felisati G, Rosso C, Pipolo C. Adenoidal disease and chronic rhinosinusitis in children - is there a link? J Clin Med 2019; 8: 1528-1540.
- Naclerio RM, Bachert C, Baraniuk JN. Pathophysiology of nasal congestion. Int J Gen Med 2010; 3: 47-57.
- Kadmon G, Shapiro CM, Chung SA, Gozal D. Validation of pediatric obstructive sleep apnea screening tool. Int J Pediatr Otorhinolaryngol 2013; 77: 1461-1464.
- Deflandre E, Bonhomme V, Courtois AC, Degey S, Poirrier R, Brichant JF. Influence of premedication with alprazolam on the occurence of obstructive apneas. A prospective randomized double-blind study. J Physiol Pharmacol 2016; 67: 617-624.
- Lavie L. Obstructive sleep apnoea syndrome-an oxidative stress disorder. Sleep Med Rev 2003; 7: 35-51.
- Tauman R, Lavie L, Greenfeld M, Sivan Y. Oxidative stress in children with obstructive sleep apnea syndrome. J Clin Sleep Med 2014; 10: 677-681.
- Loffredo L, Zicari AM, Occasi F, et al. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: role of NADPH oxidase. Atherosclerosis 2015; 240: 222-227.
- Hopps E, Lo Presti R, Montana M, Canino B, Calandrino V, Caimi G. Analysis of the correlations between oxidative stress, gelatinases and their tissue inhibitors in the human subjects with obstructive sleep apnea syndrome. J Physiol Pharmacol 2015; 66: 803-810.
- Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des 2008; 14: 3408-3416.
- Constantinidis J, Ereliadis S, Angouridakis N, Konstantinidis I, Vital V, Angouridaki C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol 2008; 265: 1275-1279.
- Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004; 27: 123-128.
- Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest 2005; 127: 1674-1679.
- Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003; 107: 1129-1134.
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 2009; 51: 294-302.
- Suzuki YI, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 2006; 40: 1683-1692.
- Lavie L. Oxidative stress-a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis 2009; 51: 303-312.
- Dehlink E, Tan UL. Update on paediatric obstructive sleep apnoea. J Thorac Dis 2016; 8: 224-235.
- Sterni LM, Tunkel DE. Obstructive sleep apnea in children: an update. Pediatr Clin North Am 2003; 50: 427-443.
- Kaemingk KL, Pasvogel AE, Goodwin JL, et al. Learning in children and sleep disordered breathing: findings of the Tucson Children’s Assessment of Sleep Apnea (tuCASA) prospective cohort study. J Int Neuropsychol Soc 2003; 9: 1016-1026.
- Chervin RD, Archbold KH, Dillo, JE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics 2002; 109: 449-456.
- Youssef NA, Ege M, Angly SS, Strauss JL, Marx CE. Is obstructive sleep apnea associated with ADHD? Ann Clin Psychiatry 2011; 23: 213-224.
- Cherevin R. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep disordered breathing, snoring, sleepiness and behavioral problems. Sleep Medicine 2000; 1: 21-32.
- Oros M, Mihaltan F. Chestionar pentru identificarea riscului de apnee in somn la copii, varianta prelucrata in limba romana - studiu preliminar. Internal Medicine 2015; 12: 7-13.
- Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989; 36: 1551-1569.
- Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med 2017; 13: 665-666.
- Tsai CM, Kang CH, Su MC, et al. Usefulness of desaturation index for the assessment of obstructive sleep apnea syndrome in children. Int J Pediatr Otorhinolaryngol 2013; 77: 1286-1290.
- Conti M, Morand PC, Levillain P, Lemonnier A. Improved fluorimetric determination of malondaldehyde. Clin Chem 1991; 37: 1273-1275.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971; 44: 276-287.
- Pippenger CE, Browne RW, Armstrong D. Regulatory antioxidant enzymes. Methods Mol Biol (Clifton, NJ) 1998; 108: 299-313.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984; 105: 114-121.
- Noble JE, Bailey MJ. Quantitation of protein. Methods Enzymol 2009; 463: 73-95.
- Tiboc (Schnell) CN, Man SC, Sas V, Filip GA. Home respiratory polygraphy-an alternative for the diagnosis of pediatric obstructive sleep apnea syndrome. J Pediatr Perinatol Child Health 2019; 3: 163-173.
- Mancuso M, Bonanni E, Logerfo A, et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med 2012; 13: 632-636.
- Zhou L, Chen P, Peng Y, Ouyang R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid Med Cell Longev 2016; 2016: 9626831. doi: 10.1155/2016/9626831
- Dogruer ZN, Unal M, Eskandari G, et al. Malondialdehyde and antioxidant enzymes in children with obstructive adenotonsillar hypertrophy. Clin Biochem 2004; 37: 718-721.
- Verhulst SL, Hoeck KV, Schrauwen N, et al. Sleep-disordered breathing and uric acid in overweight and obese children and adolescents. Chest 2007; 132: 76-80.
- Van Hoorenbeeck K, Franckx H, Debode P, et al. Weight loss and sleepdisordered breathing in childhood obesity: effects on inflammation and uric acid. Obesity 2012; 20: 172-177.
- Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol 2011; 25: 287-299.
- Ekinci A, Dagistan H, Aksoy A, Ekinci H. Comparison of preoperative and postoperative antioxidant levels in patients with adenotonsillar hypertrophy. Eur J Rhinol Allergy 2020; 3: 34-38.
- Oh J-T, Chung J-W. Inflammatory cytokine level in patients with obstructive sleep apnea and treatment outcome of oral appliance therapy. J Oral Med Pain 2016; 41: 126-132.
- Huang YS, Guilleminault C, Hwang FM, et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (Baltimore) 2016; 95: e4944. doi: 10.1097/MD.0000000000004944
- Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci 2019; 20: 459. doi: 10.3390/ijms20030459
- Huang YS, Chin WC, Guilleminault C, Chu KC, Lin CH, Li HY. Inflammatory factors: nonobese pediatric obstructive sleep apnea and adenotonsillectomy. J Clin Med 2020; 9: 1028. doi: 10.3390/jcm9041028
- Friedman M, Lin HC, Gurpinar B, Joseph NJ. Minimally invasive single-stage multilevel treatment for obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2007; 117: 1859-1863.
- Chen VG, Fonseca V, Amaral JB, et al. Inflammatory markers in palatine tonsils of children with obstructive sleep apnea syndrome. Br J Otorhinolaryngol 2020; 86: 23-29.
- Loubaki L, Jacques E, Semlali A, Biardel S, Chakir J, Series F. Tumor necrosis factor-alpha expression in uvular tissues differs between snorers and apneic patients. Chest 2008; 134: 911-918.
- Kimoff RJ, Hamid Q, Divangahi M, et al. Increased upper airway cytokines and oxidative stress in severe obstructive sleep apnoea. Eur Respir J 2011; 38: 89-97.
- Galland BC, Dawes PJ, Tripp EG, Taylor BJ. Changes in behavior and attentional capacity after adenotonsillectomy. Pediatr Res 2006; 59: 711-716.
- Aggarwal K, Akhtar N, Mallick H. Sleep quality mediates the relationship between risk of obstructive sleep apnea and acute stress in young adults. J Physiol Pharmacol 2021; 72: 105-110.
- Daulatzai MA. Pathogenesis of cognitive dysfunction in patients with obstructive sleep apnea: a hypothesis with emphasis on the nucleus tractus solitarius. Sleep Disord 2012; 2012: 251096. doi: 10.1155/2012/251096
- He K, Kapur VK. Sleep-disordered breathing and excessive daytime sleepiness. Sleep Med Clin 2017; 12: 369-382.
- Cao Y, Song Y, Ning P, et al. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: a meta-analysis update. BMC Pulm Med 2020; 20: 215. doi: 10.1186/s12890-020-01253-0
- Trosman I, Trosman SJ. Cognitive and behavioral consequences of sleep disordered breathing in children. Med Sci (Basel) 2017; 5: 30. doi: 10.3390/medsci5040030
- Wu J, Gu M, Chen S, et al. Factors related to pediatric obstructive sleep apnea-hypopnea syndrome in children with attention deficit hyperactivit disorder in different age groups. Medicine (Baltimore) 2017; 96: e8281. doi: 10.1097/MD.0000000000008281
- Orji FT, Ezeanolue BC. Outcome of adenotonsillectomy for sleep and breathing difficulties in Nigerian children with obstructive adenotonsillar enlargement. Indian J Otolaryngol Head Neck Surg 2012; 64: 131-136.
- Ayral M, Baylan MY, Kinis V, et al. Evaluation of hyperactivity, attention deficit, and impulsivity before and after adenoidectomy/adenotonsillectomy surgery. J Craniofac Surg 2013; 24: 731-734.
- Zojaji R, Mirzadeh M, Mazloum Farsi Baf M, Khorashadizadeh M, Sabeti HR. The effect of adenotonsillectomy on children’s quality of life. Iranian J Otorhinolaryngol 2014; 26: 199-205.
- Lee SY, Guilleminault C, Chiu HY, Sullivan SS. Mouth breathing, „nasal disuse,“ and pediatric sleep-disordered breathing. Sleep Breath 2015; 19: 1257-1264.
- McKeown P, O’Connor-Reina C. Breathing re-education and phenotypes of sleep apnea: a review. J Clin Med 2021; 10: 471. doi: 10.3390/jcm10030471
A c c e p t e d : August 30, 2021